Q.1.
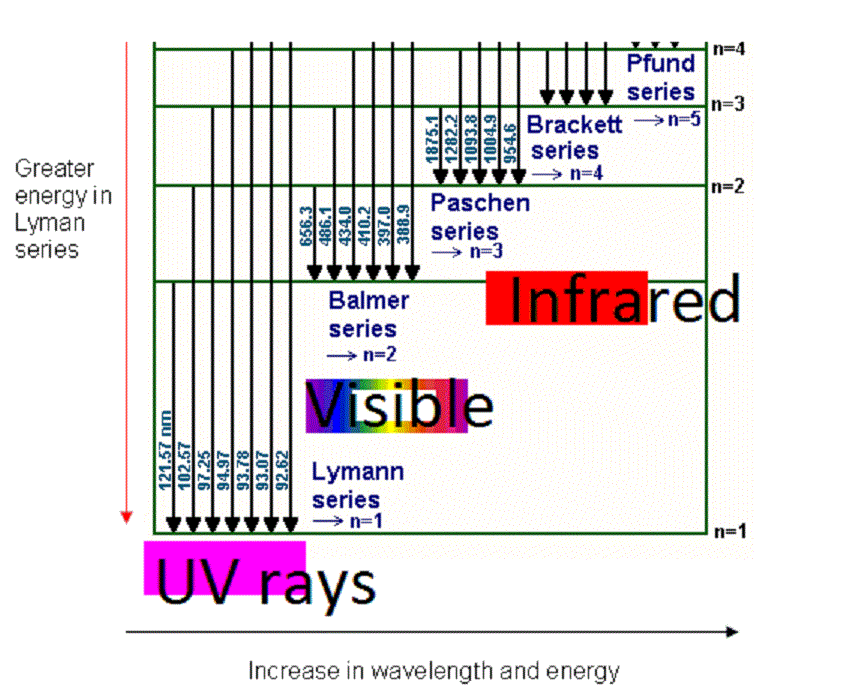
Let $$v_{1}$$ be the frequency of the series limited of the Lyman series, $$v_{2}$$ be the frequency of the first line of the Lyman series, and $$v_{3}$$ be the frequency of the series limited of the Balmer series. Then:
-
0%
$$v_{1}-v_{2}=v_{3}$$
-
0%
$$v_{2}-v_{1}=v_{3}$$
-
0%
$$v_{3}=\frac{1}{2} (v_{1}+v_{3})$$
-
0%
$$v_{1}+v_{2}=v_{3}$$
Q.2.
If $$ A_{n}$$ is the area enclosed in the $$ n$$th orbit in a hydrogen atom then the graph log $$ \left ( \frac{A_{n}}{A_{1}} \right )$$ against log $$ n$$:
-
0%
will have slope 2 (a straight line)
-
0%
will have slope 4 (a straight line)
-
0%
will be a monotonically increasing non-linear curve
-
0%
will be a circle
Q.3.
Let $$m_{P}$$ be the mass of a proton, $$m_{n}$$ the mass of a neutron, $$M_{1}$$ the mass of a $$_{10}^{20}Ne$$ nucleus and $$M_{2}$$ the mass of a $$_{20}^{40} Ca$$ nucleus. Then
-
0%
$$M_{2} = 2M_{1}$$
-
0%
$$M_{2} > 2M_{1}$$
-
0%
$$M_{2} < 2M_{1}$$
-
0%
$$M_{1} < 10(m_{n} + m_{p})$$
Q.4.
The quantum number n of the state finally populated in $$He^{+}$$ ions is
-
0%
$$2$$
-
0%
$$3$$
-
0%
$$4$$
-
0%
$$5$$
Q.5.
An electron in a hydrogen atom makes a transition from $$n=n_{1}$$ to $$n=n_{2}$$. The time period of the electron in the initial state is eight times that in the final state. The possible values of $$n_{1}$$ and $$n_{2}$$ are
-
0%
$$n_{1}=4, n_{2}=2$$
-
0%
$$n_{1}=8, n_{2}=2$$
-
0%
$$n_{1}=8, n_{2}=1$$
-
0%
$$n_{1}=6, n_{2}=3$$
Q.6.
If elements of quantum number greater than $$'n'$$ were not allowed, the number of possible elements in nature would have been
-
0%
$$\displaystyle\frac{1}{2}n(n+1)$$
-
0%
$$\left\{\displaystyle\frac{n(n+1)}{2}\right\}^2$$
-
0%
$$\displaystyle\frac{1}{6}n(n+1)(2n+1)$$
-
0%
$$\displaystyle\frac{1}{3}n(n+1)(2n+1)$$
Q.7.
Monochromatic radiations of wavelength $$\lambda$$ are incident on a hydrogen sample in the ground state. Hydrogen atom absorbs the light and subsequently emits radiations of $$10$$ different wavelengths. The value of $$\lambda$$ is nearly
-
0%
$$203\space nm$$
-
0%
$$95\space nm$$
-
0%
$$80\space nm$$
-
0%
$$73\space nm$$
Q.8.
Check the correctness of the following statements about the Bohr Model of hydrogen atom:
$$(i)$$ The acceleration of the electron in $$n=2$$ orbit is more than that in $$n=1$$ orbit.
$$(ii)$$ The angular momentum of the electron in $$n=2$$ orbit is more than that in $$n=1$$ orbit.
$$(iii)$$ The KE of the electron in $$n=2$$ orbit is less than that in $$n=1$$ orbit
$$(i)$$ The acceleration of the electron in $$n=2$$ orbit is more than that in $$n=1$$ orbit.
$$(ii)$$ The angular momentum of the electron in $$n=2$$ orbit is more than that in $$n=1$$ orbit.
$$(iii)$$ The KE of the electron in $$n=2$$ orbit is less than that in $$n=1$$ orbit
-
0%
All the statements are correct
-
0%
Only $$(i)$$ and $$(ii)$$ are correct
-
0%
Only $$(ii)$$ and $$(iii)$$ are correct
-
0%
Only $$(iii)$$ and $$(i)$$ are correct
Q.9.
Find the value of principal quantum number n corresponding 20 kg satellite.
-
0%
$$1.6\times 10^{45}$$
-
0%
$$1.6\times 10^{8}$$
-
0%
$$7.6\times 10^{5}$$
-
0%
$$8.3\times 10^{11}$$
Q.10.
Find the radius of first two allowed orbits.
-
0%
$$2.5 \times 10^{-84}m, 10^{-83}m$$
-
0%
$$400 km, 1600 km$$
-
0%
$$200 m, 800 m$$
-
0%
$$3 km, 12 km$$
-
0%
none
Q.11.
If potential energy between a proton and an electron is given by $$|U| = ke^2/2R^3$$, where $$e$$ is the charge of electron and $$R$$ is the radius of atom, then radius of Bohr's orbit is given by ($$h$$ = Planck's constant, $$k$$ = constant)
-
0%
$$\displaystyle\frac{ke^2m}{h^2}$$
-
0%
$$\displaystyle\frac{6\pi^2}{n^2} \displaystyle\frac{ke^2m}{h^2}$$
-
0%
$$\displaystyle\frac{2\pi}{n} \displaystyle\frac{ke^2m}{h^2}$$
-
0%
$$\displaystyle\frac{4\pi^2ke^2m}{n^2h^2}$$
Q.12.
Which ray/radiation can be detected by GM counter?
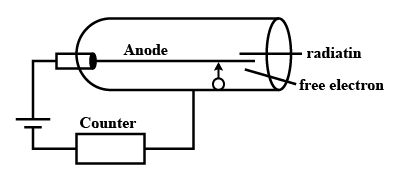

-
0%
Alpha
-
0%
Beta
-
0%
$$\gamma$$ ray
-
0%
all
Q.13.
The wavelength of $$K_\alpha$$ X-rays of two metals $$A$$ and $$B$$ are $$\dfrac{4}{1875R}$$ and $$\dfrac{1}{675R}$$, respectively, where $$\mbox{'R'}$$ is Rydberg's constant. The number of elements lying between $$A$$ and $$B$$ according to their atomic number is
-
0%
$$3$$
-
0%
$$6$$
-
0%
$$5$$
-
0%
$$4$$
Q.14.
In a hydrogen atom, electron is in the $$n^{th}$$ excited state. It comes down to the first excited state by emitting $$10$$ different wavelengths. The value of $$n^{th}$$ is
-
0%
$$6$$
-
0%
$$7$$
-
0%
$$8$$
-
0%
$$9$$
Q.15.
Which of the following statement about hydrogen spectrum are correct?
-
0%
All the lines of Lyman series lie in ultraviolet region
-
0%
All the lines of Balmer series lie in visible region
-
0%
All the lines of Paschen series lie in infrared region
-
0%
None of the above
Q.16.
If, in a hydrogen atom, radius of $$n^{th}$$ Bohr orbit is $$r_n$$, frequency of revolution of electron in $$n^{th}$$ orbit if $$f_n$$, and area enclosed by the $$nth$$ orbit is $$A_n$$, then which of the following graphs are correct?
-
0%
-
0%
-
0%
-
0%
Q.17.
According to Bohr's theory of hydrogen-atom, for the electron in the $$n^{th}$$ permissible orbit
-
0%
$$\mbox{Linear Momentum } \propto \displaystyle\frac{1}{n}$$
-
0%
$$\mbox{Radius of orbit } \propto n$$
-
0%
$$\mbox{Kinetic Energy } \propto \displaystyle\frac{1}{n^2}$$
-
0%
$$\mbox{Angular Momentum } \propto n$$
Q.18.
Which of the following products, in a hydrogen atom, are independent of the principal quantum number $$n$$? The symbols have their usual meanings.
-
0%
$$\omega^2r$$
-
0%
$$\displaystyle\frac{E}{v^2}$$
-
0%
$$v^2r$$
-
0%
$$\displaystyle\frac{E}{r}$$
Q.19.
Let $$A_n$$ be the area enclosed by the $$n^{th}$$ orbit in a hydrogen atom. The graph of $$ln(A_n/A_l)$$ against $$ln(n)$$
-
0%
Will pass through origin
-
0%
Will be a straight line with slope $$4$$
-
0%
Will be monotonically increasing nonlinear curve
-
0%
Will be circle
Q.20.
Mark out the correct statement(s).
-
0%
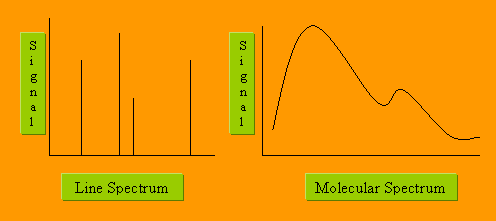
Line spectra contain information about atoms only
-
0%
Line spectra contain information about both atoms and molecules
-
0%
Band spectra contain information about both atoms and molecules
-
0%
Band spectra contain information about molecules only
Q.21.
Continuous spectrum is produced by
-
0%
Incandescent electric bulb
-
0%
Sun
-
0%
Hydrogen molecules
-
0%
Sodium vapor lamp
Q.22.
In Bohr's model of the hydrogen atom, let $$R,V,T,$$ and $$E$$ represent the radius of the orbit, speed of the electron, time period of the revolution of electron and the total energy of the electron, respectively. The quantities proportional to the quantum number $$n$$ are
-
0%
$$VR$$
-
0%
$$RE$$
-
0%
$$\displaystyle\frac{T}{R}$$
-
0%
$$\displaystyle\frac{V}{E}$$
Q.23.
Let the energy of the electron confined in the atom be $$E$$ . At what $$F_0$$ would the atom ionize?
-
0%
$$\displaystyle F_0 = \frac {E^2 \pi \varepsilon_0} {e} $$
-
0%
$$\displaystyle F_0 = \frac {E^2 \varepsilon_0} {e^3} $$
-
0%
$$\displaystyle F_0 = \frac { \pi \varepsilon_0} {e^3} $$
-
0%
$$\displaystyle F_0 = \frac {E^2 \pi \varepsilon_0} {e^3} $$
Q.24.
If the atom $$_{100} Fm^{257}$$ follows the Bohr model and the radius of $$_{100} Fm^{257}$$ is n times the Bohr radius, then find $$n$$.
-
0%
$$100$$
-
0%
$$200$$
-
0%
$$4$$
-
0%
$$1/4$$
Q.25.
The orbital radius of the first excited level of positronium atom is
Note: $$a_0$$ is the orbital radius of ground state of hydrogen atom
Note: $$a_0$$ is the orbital radius of ground state of hydrogen atom
-
0%
$$4a_0$$
-
0%
$$a_0/2$$
-
0%
$$8a_0$$
-
0%
$$2a_0$$
Q.26.
What principle is violated here?
-
0%
Laws of Motion
-
0%
Energy conservation
-
0%
Nothing is violated
-
0%
Cannot be decided
Q.27.
The ratio between Bohr radii is
-
0%
1 : 2 : 3
-
0%
2 : 4 : 6
-
0%
1 : 4 : 9
-
0%
1 : 3 : 5
Q.28.
The radius of the first Bohr orbit is
-
0%
$$0.529\times10^{-10}\space m$$
-
0%
$$0.106\times10^{-10}\space m$$
-
0%
$$0.318\times10^{-10}\space m$$
-
0%
None of these
Q.29.
In which transition is a Balmer series photon absorbed?
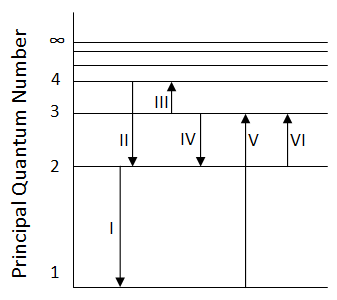

-
0%
$$II$$
-
0%
$$III$$
-
0%
$$IV$$
-
0%
$$VI$$
Q.30.
The minimum permissible radius of the orbit will be :
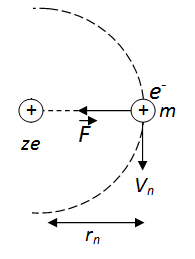

-
0%
$$\displaystyle\frac{2\epsilon_0h^2}{m\pi e^2}$$
-
0%
$$\displaystyle\frac{4\epsilon_0h^2}{m\pi e^2}$$
-
0%
$$\displaystyle\frac{\epsilon_0h^2}{m\pi e^2}$$
-
0%
$$\displaystyle\frac{\epsilon_0h^2}{2m\pi e^2}$$
Q.31.
Two smooth tunnels are dug from one side of the earths surface to the other side, one along a diameter and the other along a chord. Now two particles are dropped from one end of each of the tunnels. Both the particles oscillate simple harmonically along the tunnels. Let $${T}{1}$$ and $${T}{2}$$ be the time periods and $${v}_{1}$$ and $${v}_{2}$$ be the maximum speed of the particles in the two tunnels. Then
-
0%
$${T}_{1}={T}_{2}$$
-
0%
$${T}_{1}>{T}_{2}$$
-
0%
$${v}_{1}={v}_{2}$$
-
0%
$${v}_{1}>{v}_{2}$$
Q.32.
A donor atom in a semiconductor has a loosely bound electron. The orbit of this electron is considerably affected by the semiconductor material but behaves in many ways like an electron orbiting a hydrogen nucleus. Given that the electrons has an effective mass of $$0.07\ m_e$$ (where $$m_{e}$$ is mass of the free electron) and the space in which it moves has a permittivity $$13\epsilon_{0}$$, then the radius of the electron's lowermost energy orbit will be close to (The Bohr radius of the hydrogen atom is $$0.53\overset {\circ}{A})$$.
-
0%
$$0.53\overset {\circ}{A}$$
-
0%
$$243\overset {\circ}{A}$$
-
0%
$$10\overset {\circ}{A}$$
-
0%
$$100\overset {\circ}{A}$$
Q.33.
The radius of the first orbit of hydrogen is $$r_H$$, and the energy in the ground state is $$-13.6$$eV. Considering a $$\mu^-$$-particle with a mass $$207$$ $$m_e$$ revolving round a proton as in Hydrogen atom, the energy and radius of proton and $$\mu^-$$ combination respectively in the first orbit are:
[Assume nucleus to be stationary]
-
0%
$$-13.6\times 207\ eV$$, $$\displaystyle\frac{r_H}{207}$$
-
0%
$$-207\times 13.6\ eV$$, $$207$$ $$r_H$$
-
0%
$$\displaystyle\frac{-13.6}{207}\ eV$$, $$\displaystyle\frac{r_H}{207}$$
-
0%
$$\displaystyle\frac{-13.6}{207}\ eV$$, $$207$$ $$r_H$$
Q.34.
In the Bohr model of a hydrogen atom, the centripetal force is furnished by the coulomb attraction between the proton and the electron. If $$a_0$$ is the radius of the ground state orbit, m is the mass and e is the charge on the election and $$\varepsilon_0$$ is the vacuum permittivity, the speed of the electron is?
-
0%
$$0$$
-
0%
$$\displaystyle\frac{e}{\sqrt{\varepsilon_0a_0m}}$$
-
0%
$$\displaystyle\frac{e}{\sqrt{4\pi\varepsilon_0a_0m}}$$
-
0%
$$\sqrt{\displaystyle\frac{4\pi\varepsilon_0a_0m}{e}}$$
Q.35.
To calculate the size of a hydrogen anion using the Bohr model, we assume that its two electrons move in an orbit such that they are always on diametrically opposite sides of the nucleus. With each electron having the angular momentum $$\hbar = h/2\pi$$, and taking electron interaction into account the radius of the orbit in terms of the Bohr radius of hydrogen atom $$a_{B}= \dfrac {4\pi \epsilon_{0}\hbar^{2}}{me^{2}}$$ is
-
0%
$$a_{B}$$
-
0%
$$\dfrac {4}{3}a_{B}$$
-
0%
$$\dfrac {2}{3}a_{B}$$
-
0%
$$\dfrac {3}{2}a_{B}$$
Q.36.
The number of revolutions per second made by an electron in the first Bohr's orbit of hydrogen atom is(Given, $$r=0.53\overset{o}{A}$$).
-
0%
$$6\times 10^5$$Hz
-
0%
$$2.5\times 10^{12}$$Hz
-
0%
$$1.9\times 10^{13}$$Hz
-
0%
$$6.5\times 10^{15}$$Hz
Q.37.
Which of the following statement is wrong?
-
0%
$$\Psi_s$$ = - $$\pi$$
-
0%
DPD = -OP + TP
-
0%
$$\Psi_w$$ = $$\Psi_s$$ + $$\Psi_p$$
-
0%
$$\Psi_w$$ = -DPD
Q.38.
To calculate the size of a hydrogen anion using the Bohr model, we assume that its two electrons move in an orbit such that they are always on diametrically opposite sides of the nucleus. With each electron having the angular momentum equal to $$\dfrac{h}{ 2 \pi}$$, and taking electron interaction into account the radius of the orbit in terms of the Bohr radius of hydrogen atom $$a_B=\displaystyle\frac{4\pi \epsilon_0h^2}{me^2}$$ is?
-
0%
$$a_B$$
-
0%
$$\displaystyle\frac{4}{3}a_B$$
-
0%
$$\displaystyle\frac{2}{3}a_B$$
-
0%
$$\displaystyle\frac{3}{2}a_B$$
Q.39.
If velocity of $$\alpha - $$particle is made $$1/3$$ times, then % variation in distance of closest approach will be: (with respect to initial)
-
0%
300%
-
0%
600%
-
0%
800%
-
0%
80%
Q.40.
The radius of the smaller electron orbit in hydrogen-like ion is $$\dfrac{0.51\times 10^{-10}}{4}$$m, then it is?
-
0%
hydrogen atom
-
0%
$$He^+$$
-
0%
$$Li^+$$
-
0%
$$Be^{3+}$$
Q.41.
Given mass number of glod=197, density of gold=19.7 g per $$cm^3$$, Avogadro's number = $$6\times 10^{23}$$.The radius of the gold atom is approximately:
-
0%
$$1.5 \times 10^{-8}m$$
-
0%
$$1.5 \times 10^{-9}m$$
-
0%
$$1.5 \times 10^{-10}m$$
-
0%
$$1.5 \times 10^{-12}m$$
Q.42.
In the Bohr's model of hydrogen-like atom the force between the nucleus and the electron is modified as
$$\quad F=\cfrac { { e }^{ 2 } }{ 4\pi { \varepsilon }_{ 0 } } \left( \cfrac { 1 }{ { r }^{ 2 } } +\cfrac { \beta }{ { r }^{ 3 } } \right) $$, where $$\beta$$ is a constant. For this atom, the radius of the $$n$$th orbit in terms of the Bohr radius $$\left( { a }_{ 0 }=\cfrac { { \varepsilon }_{ 0 }{ h }^{ 2 } }{ m\pi { e }^{ 2 } } \right) $$ is:
$$\quad F=\cfrac { { e }^{ 2 } }{ 4\pi { \varepsilon }_{ 0 } } \left( \cfrac { 1 }{ { r }^{ 2 } } +\cfrac { \beta }{ { r }^{ 3 } } \right) $$, where $$\beta$$ is a constant. For this atom, the radius of the $$n$$th orbit in terms of the Bohr radius $$\left( { a }_{ 0 }=\cfrac { { \varepsilon }_{ 0 }{ h }^{ 2 } }{ m\pi { e }^{ 2 } } \right) $$ is:
-
0%
$${ r }_{ n }={ a }_{ 0 }n-\beta $$
-
0%
$${ r }_{ n }={ a }_{ 0 }{ n }^{ 2 }+\beta \quad $$
-
0%
$${ r }_{ n }={ a }_{ 0 }{ n }^{ 2 }-\beta $$
-
0%
$${ r }_{ n }={ a }_{ 0 }n+\beta $$
Q.43.
A diatomic molecules has moment of inertia $$I$$. By Bohr's quantization condition its rotational energy in the $$n^{th}$$ level $$(n = 0$$ is not allowed) is
-
0%
$$\dfrac {1}{n^{2}}\left (\dfrac {h^{2}}{8\pi^{2}I}\right )$$
-
0%
$$\dfrac {1}{n}\left (\dfrac {h^{2}}{8\pi^{2}I}\right )$$
-
0%
$$n\left (\dfrac {h^{2}}{8\pi^{2}I}\right )$$
-
0%
$$n^{2}\left (\dfrac {h^{2}}{8\pi^{2}I}\right )$$
Q.44.
Orbits of a particle moving in a circle are such that the perimeter of the orbit equals an integer number of de-Broglie wavelengths of the particle. For a charged particle moving in a plane perpendicular to a magnetic field, the radius of the $$n$$th orbital will be proportional to
-
0%
$${ n }^{ 2 }$$
-
0%
$$n$$
-
0%
$${ n }^{ 1/2 }$$
-
0%
$${ n }^{ 1/4 }$$
Q.45.
An electron in a hydrogen atom makes a transition from $$n=n_{1}$$ to $$n=n_{2}$$. The time period of the electron in the initial state is eight times that in the final state. The possible values of $$n_{1}$$ and $$n_{2}$$ are
-
0%
$$n_{1}=4, n_{2}=2$$
-
0%
$$n_{1}=8, n_{2}=2$$
-
0%
$$n_{1}=8, n_{2}=1$$
-
0%
$$n_{1}=6, n_{2}=3$$
Q.46.
A mixture of $$2$$ moles of helium gas (atomic mass $$=4$$ amu) and $$1$$ mole of argon gas (atomic mass $$=40$$ amu) is kept at $$300$$ K in a container. The ratio of the rms speeds $$\left(\dfrac{v_{rms}(helium)}{v_{rms}(argon)}\right)$$ is?
-
0%
$$0.32$$
-
0%
$$0.45$$
-
0%
$$2.24$$
-
0%
$$3.16$$
Q.47.
The wavelengths involved in the spectrum of deuterium $$\left( _{ 1 }^{ 2 }{ D } \right) $$ are slightly different from that of hydrogen spectrum, because
-
0%
size of the two nuclei are different
-
0%
nuclear forces are different in the two cases
-
0%
masses of the two nuclei are different
-
0%
attraction between the electron and the nucleus is different in the two cases
Q.48.
In certain electronic transition from quantum level n to ground state in atomic hydrogen in one or more steps no line belonging to Brackett series is observed. The wave numbers which may be observed in Balmer series is then?
-
0%
$$\dfrac{8R}{9}, \dfrac{5R}{36}$$
-
0%
$$\dfrac{3R}{16}, \dfrac{8R}{9}$$
-
0%
$$\dfrac{5R}{36}, \dfrac{3R}{16}$$
-
0%
$$\dfrac{3R}{4}, \dfrac{3R}{16}$$
Q.49.
Calculate the radius of the nth orbit
-
0%
$$\sqrt{\dfrac{nh}{2\pi e B}}$$
-
0%
$$\sqrt{\dfrac{nheB}{2\pi}}$$
-
0%
$$\sqrt{\dfrac{nhe}{2\pi B}}$$
-
0%
$$\sqrt{\dfrac{nhB}{2\pi e}}$$
Q.50.
Angular momentum in second Bohr orbit of H-atom is $$x$$. Then find out angular momentum in 1st excited state of $${Li}^{+2}$$ ion:
-
0%
$$3x$$
-
0%
$$9x$$
-
0%
$$\cfrac{x}{2}$$
-
0%
$$x$$