Q.1.
Which of the following is more reactive?
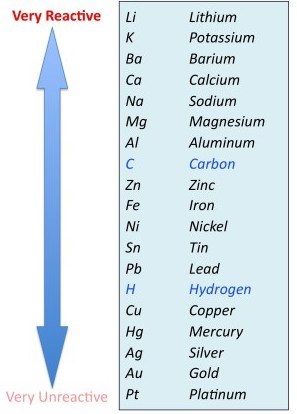

-
100%
Calcium
-
0%
Magnesium
-
0%
Zinc
-
0%
Magnesium
Q.2.
Will the following reaction take place?Ni + NaCl ->
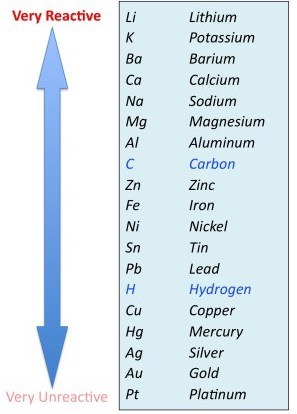

-
77%
NO
-
23%
YES
Q.3.
Which one of the following combinations result in a displacement reaction?
-
11%
Iron with magnesium chloride
-
56%
magnesium with iron chloride
-
22%
Iron with Zinc Sulphate
-
11%
gold with silver nitrate
Q.4.
A displacement reaction will occur when...
-
100%
a more reactive metal displaces a less reactive metal from its compound.
-
0%
A less reactive metal displaces a more reactive metal from its compound
-
0%
Displacement only occurs when two of the same metals are reacted
-
0%
Displacement reactions will only occur in metals above iron in the reactivity series
Q.5.
What will the products of this reaction be? calcium + zinc nitrate --------->
-
9%
Calcium + zinc nitrate
-
82%
Zinc + calcium nitrate
-
9%
there will be no reaction
-
0%
Zinc + calcium chloride
Q.6.
What are the products when magnesium reacts with hydrochloric acid?
-
12%
magnesium nitrate and hydrogen gas
-
12%
magnesium sulfate and hydrogen gas
-
12%
magnesium oxide and oxygen gas
-
62%
magnesium chloride and hydrogen gas
Q.7.
Which of the following will not undergo reaction?
-
67%
zinc metal + zinc oxide
-
17%
iron metal + copper oxide
-
0%
zinc metal + iron oxide
-
17%
magnesium + iron oxide