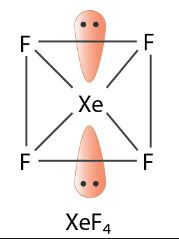
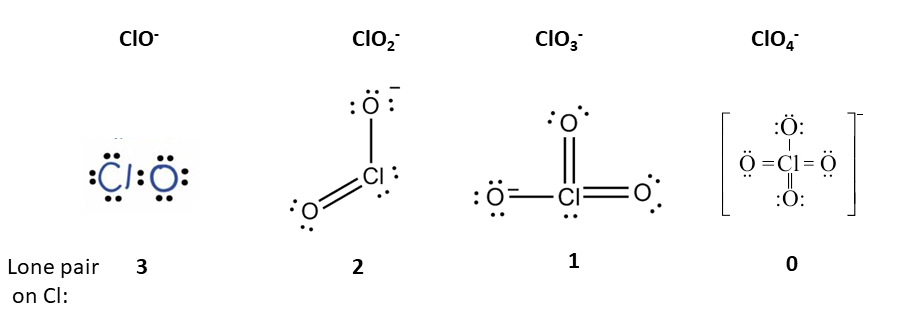
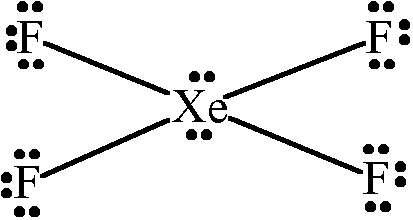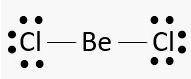Q.1.
The strength of sigma bonds formed by axial overlap of $$s-$$ or $$p-$$ orbitals of $$2nd$$ shell of participating atom decreases as:
-
38%
$$s-s>p-s>p-p$$
-
24%
$$s-s>p-p>s-p$$
-
9%
$$p-s>s-s>p-p$$
-
29%
$$p-p>s-p>s-s$$
Q.2.
Which one of the of the following molecules is expected to have zero dipole moment?
-
29%
$$H_2O$$
-
29%
$$CO_2$$
-
21%
$$SO_2$$
-
21%
$$CaF_2$$
Q.3.
The number of P-O-P and P-O-H bonds present respectively in pyrophosphoric acid molecule are_____
-
10%
1,2
-
67%
2,2
-
24%
1,4
-
0%
1,8
Q.4.
Which formulae does not correctly represents the bonding capacity of the atom involved?
-
14%
-
21%
-
50%
-
14%
Q.5.
The number of electron pairs in $$Xe{F}_{4}$$ at the corners of the square are _______.
-
7%
2
-
14%
3
-
71%
4
-
7%
6
Q.6.
What is the electronic configuration of elements of $$III$$ rd group
-
0%
$$1{ s }^{ 2 },2{ s }^{ 2 }2p{ s }^{ 3 }$$
-
79%
$$1{ s }^{ 2 },2{ s }^{ 2 }2{ p }^{ 6 },3{ s }^{ 2 }3{ p }^{ 1 }$$
-
21%
$$1{ s }^{ 2 },2{ s }^{ 2 }2{ p }^{ 6 }$$
-
0%
$$1{ s }^{ 2 },2{ s }^{ 2 }2{ p }^{ 6 },3{ s }^{ 1 }\quad $$
Q.7.
For which of the following molecule significant $$ \mu \neq 0?$$


-
13%
Only $$ (c)$$
-
47%
$$ (c)\: and\:(d)$$
-
27%
Only $$ (a)$$
-
13%
$$ (a)\: and\:(b)$$
Q.8.
Identify the pair in which the geometry of the species is T-shape and square pyramidal, respectively.
-
30%
$$ClF_3$$ and $$IO^-_4$$
-
20%
$$ICl^-_2$$ and $$ICl_5$$
-
30%
$$IO^-_3$$ and $$IO_2F^-_2$$
-
20%
$$XeOF_2$$ and $$XeOF_4$$
Q.9.
Boron cannot form which one of the following anions?
-
0%
$$\mathrm{B}\mathrm{F}_{6}^{3-}$$
-
62%
$$\mathrm{B}\mathrm{H}_{4}^{-}$$
-
25%
$$\mathrm{B}(\mathrm{O}\mathrm{H})_{4}^{-}$$
-
12%
$$\mathrm{B}\mathrm{O}_{2}^{-}$$
Q.10.
The number and type of bonds in $$C_{2}^{2-}$$ ion in $$CaC_2$$ are :
-
33%
one $$\sigma$$ bond and one $$\pi$$-bond
-
33%
one $$\sigma$$ bond and two $$\pi$$-bonds
-
33%
two $$\sigma$$ bonds and two $$\pi$$ -bonds
-
0%
two $$\sigma$$ bonds and one $$\pi$$ - bond
Q.11.
Which of the following molecules has two sigma $$(\sigma)$$ and two pi $$(\pi)$$ bonds?
-
38%
$$C_2H_4$$
-
25%
$$N_2F_2$$
-
25%
$$C_2H_2Cl_2$$
-
12%
$$HCN$$
Q.12.
Total number of lone pair of electrons in $$I_3{^-}$$ ion is :
-
14%
$$9$$
-
14%
$$12$$
-
57%
$$3$$
-
14%
$$6$$
Q.13.
Among the following, the molecule with the highest dipole moment is:
-
33%
$$\mathrm{C}\mathrm{H}_3 {Cl}$$
-
0%
$$\mathrm{C}\mathrm{H}{}_{2}\mathrm{C}l_{2}$$
-
50%
$$\mathrm{C}\mathrm{H}\mathrm{C}l_{3}$$
-
17%
$$\mathrm{C}\mathrm{C}l_{4}$$
Q.14.
Which of the following pairs of ions are isoelectronic and isostructural?
-
0%
$$SO_3^{2-}, NO_3^-$$
-
50%
$$ClO_3^-, CO_3^{2-}$$
-
50%
$$CO_3^{2-}, NO_3 {^-}$$
-
0%
$$ClO_3 {^-}, SO_3^{2-}$$
Q.15.
$$4d,5p,5f$$ and $$6p$$ orbitals are arranged in the order of decreasing energy.The correct option is:
-
80%
$$5f>6p>5p>4d$$
-
20%
$$6p>5f>5p>4d$$
-
0%
$$6p>5f>4d>5p$$
-
0%
$$5f>6p>4d>5p$$
Q.16.
Which one of the following pairs of species have this same bond order?
-
25%
$$N_{2}, {O}_{2}^{-}$$
-
50%
$$CO, NO$$
-
0%
$$O_{2}, NO^{+}$$
-
25%
$$CN^{-}, CO$$
Q.17.
-
0%
Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
-
50%
Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
-
50%
Assertion is correct but Reason is incorrect
-
0%
Both Assertion and Reason are incorrect
Q.18.
MnO is?
-
67%
Ferromagnetic
-
0%
Antiferromagnetic
-
0%
Ferrimagnetic
-
33%
Dimagnetic
Q.19.
Which of the following phenomena will occur when two atoms of the elements having same spin of electron approach for bonding?
-
0%
Orbital overlap will not occur
-
33%
Bonding will not occur
-
67%
Both $$A$$ and $$B$$ are correct
-
0%
None of the above
Q.20.
Covalency of carbon in carbon dioxide is:
-
33%
$$2$$
-
67%
$$4$$
-
0%
$$1$$
-
0%
$$0$$
Q.21.
Molecule which contains only sigma bonds in it is:
-
0%
Pentene
-
0%
Pentane
-
100%
Pentadiene
-
0%
Pentyne
Q.22.
Maximum covalency of Sulphur is :
-
0%
$$2$$
-
33%
$$4$$
-
67%
$$6$$
-
0%
$$8$$
Q.23.
In the hydrocarbon, $$HC \equiv C-H$$, the covalency of carbon is :
-
0%
one
-
0%
two
-
0%
three
-
100%
four
Q.24.
Some atoms attain their inert gas configuration by sharing of electrons. This method of attaining inert gas configuration is known as :
-
25%
covalency
-
25%
electrovalency
-
25%
stabilisation
-
25%
inertness
Q.25.
The valency of an atom is equal to its combining capacity.
-
100%
True
-
0%
False
Q.26.
Which of the following species has incomplete octet?
-
33%
$$SiF_4$$
-
67%
$${ N }^{ 3- }$$
-
0%
$$Pbr5$$
-
0%
$${B}^{3-}$$
Q.27.
The number of lone pairs available on the central atom of sulphur tetrafluoride is :
-
0%
$$0$$
-
33%
$$1$$
-
0%
$$2$$
-
67%
$$3$$
Q.28.
In Lewis dot symbols dots indicates the __________.
-
67%
electrons
-
0%
neutrons
-
0%
protons
-
33%
quarks
Q.29.
The electro valency theory explains that atoms are held together by an___________that resulted due to transfer or sharing of electrons.
-
0%
repulsive forces
-
67%
attractive forces
-
33%
binding forces
-
0%
none of these
Q.30.
What is the covalency of $${O}_{2}$$ and $${N}_{2}$$ molecules?
-
33%
0,0
-
0%
1,2
-
67%
2,3
-
0%
3,3
Q.31.
Which one of these is weakest ?
-
33%
ionic bond
-
0%
covalent bond
-
33%
metallic bond
-
33%
van der Waal forces
Q.32.
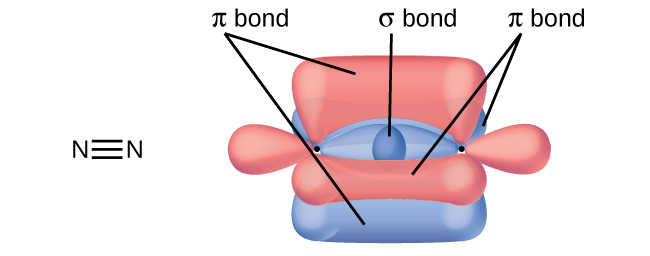
A triple bond may be best described as :
-
0%
two sigma bonds and one $$\pi$$ bond
-
33%
one sigma bond and two $$\pi$$ bonds
-
33%
two sigma bonds and two $$\pi$$ bonds
-
33%
three $$\sigma$$ bonds
-
0%
three $$\pi$$ bonds
Q.33.
The number of electron pair shared between two atoms of same or different elements during its formation of molecules is known as :
-
67%
covalency
-
33%
tetravalecy
-
0%
bivalency
-
0%
trivalency
Q.34.
A chemical bond between two atoms is:
-
0%
The tunnel in an atom through which the other one can pass
-
100%
The energy of the individual atoms
-
0%
The link between them
-
0%
A third, connecting atom
Q.35.
What is Bond formation?
-
0%
always exothermic
-
33%
always endothermic
-
33%
neither exothermic nor endothermic
-
33%
Sometimes exothermic and sometimes endothermic
Q.36.
An _____ bond is a bond in which electrons are transferred.
-
67%
Ionic
-
33%
Covalent
-
0%
Double
-
0%
Triple
Q.37.
The number of sigma and $$\pi$$ bonds in 1-butene-3yne are-
-
33%
$$5$$ sigma and $$5$$ pi
-
33%
$$7$$ sigma and $$3$$ pi
-
0%
$$8$$ sigma and $$2$$ pi
-
33%
$$6$$ sigma and $$4$$ pi
Q.38.
Covalency of nitrogen in ammonium ion is:
-
0%
$$2$$
-
67%
$$3$$
-
33%
$$4$$
-
0%
$$5$$
Q.39.
Molecule which contains only bonded pairs of electrons on the central atom is:
-
33%
$$H_{2}O$$
-
33%
$$NH_{3}$$
-
0%
$$BeCl_{2}$$
-
33%
$$BF_{3}$$
Q.40.
Number of bonded electrons in ethane molecule are:
-
50%
$$7$$
-
50%
$$12$$
-
0%
$$10$$
-
0%
$$14$$
Q.41.
In which of the following molecule, the central atom has three lone pairs of electrons?
-
50%
Ammonia
-
0%
Xenon difluoride
-
50%
Chlorine trifluoride
-
0%
Hydrogen sulphide
Q.42.
Molecule which contains $$4$$ bonded pairs and $$2$$ lone pairs of electrons on the central atom is:
-
33%
$$XeF_{2}$$
-
33%
$$CO_{2}$$
-
33%
$$XeF_{4}$$
-
0%
$$SF_{6}$$
Q.43.
Chemical bond implies ___________.
-
0%
repulsion
-
0%
attraction
-
100%
attraction and repulsion balanced at a particular distance
-
0%
attraction and repulsion
Q.44.
$$Cl+Cl\rightarrow Cl_{2}$$, this is an example for __________.
-
100%
Endothermic reaction
-
0%
Exothermic reaction
-
0%
Either exothermic or endothermic
-
0%
Neither exothermic nor endothermic
Q.45.
Molecule having maximum number of covalent bonds is:
-
0%
$$NH_{4}OH$$
-
50%
$$NH_{4}Cl$$
-
50%
$$CO(NH_{2})_{2}$$
-
0%
$$CH_{3}OH$$
Q.46.
Maximum covalency of nitrogen is:
-
50%
$$4$$
-
0%
$$5$$
-
0%
$$3$$
-
50%
$$6$$
Q.47.
The number of lone pairs on chlorine atom in $$\mathrm{Cl}\mathrm{O}^{-},$$ $$\mathrm{Cl}\mathrm{O}^{-}_{2},\ \mathrm{Cl}\mathrm{O}^{-}_{3},\ \mathrm{C}\mathrm{l}\mathrm{O}^{-}_{4}$$ ions are:
-
50%
$$0, 1, 2, 3$$
-
50%
$$1, 2, 3, 4$$
-
0%
$$4, 3, 2, 1$$
-
0%
$$3, 2, 1, 0$$
Q.48.
According to VSEPR theory, the shape of the water molecule is :
-
0%
linear
-
50%
planar triangle
-
0%
angular
-
50%
octahedral
Q.49.
The covalency of central atom is maximum in:
-
0%
$$HCN$$
-
50%
$$NH_{4}^+$$
-
50%
$$PCl_{5}$$
-
0%
$$H_{2}O$$
Q.50.
Compound having maximum number of bonded pairs of electrons in its molecule is:
-
0%
Ethane
-
0%
Ammonia
-
50%
Sulphur hexafluoride
-
50%
Bromine Pentafluoride