MCQ Questions for Class 11 Medical Chemistry Quiz with Answers Chapter Wise PDF Download
CBSE Multiple Choice Type Questions for 11th Class Medical Chemistry PDF formatted study resources are available for free download. These Grade 11 Medical Chemistry CBSE MCQ Mock Test helps you learn & practice the concepts in a fun learning way.
Class 11 Medical Chemistry MCQs Multiple Choice Questions with Answers
Here are the chapterwise CBSE MCQ Quiz Test Questions for Class 11th Medical Chemistry in pdf format that helps you access & download so that you can practice online/offline easily.
- Chemical Bonding And Molecular Structure Class 11 Medical Chemistry MCQ Questions
- Classification Of Elements And Periodicity In Properties Class 11 Medical Chemistry MCQ Questions
- Environmental Chemistry Class 11 Medical Chemistry MCQ Questions
- Equilibrium Class 11 Medical Chemistry MCQ Questions
- Hydrocarbons Class 11 Medical Chemistry MCQ Questions
- Hydrogen Class 11 Medical Chemistry MCQ Questions
- Organic Chemistry Some Basic Principles And Techniques Class 11 Medical Chemistry MCQ Questions
- Redox Reactions Class 11 Medical Chemistry MCQ Questions
- Some Basic Concepts Of Chemistry Class 11 Medical Chemistry MCQ Questions
- States Of Matter Gases And Liquids Class 11 Medical Chemistry MCQ Questions
- Structure Of Atom Class 11 Medical Chemistry MCQ Questions
- The P-Block Elements Class 11 Medical Chemistry MCQ Questions
- Thermodynamics Class 11 Medical Chemistry MCQ Questions
- The S-Block Elements Class 11 Medical Chemistry MCQ Questions
Chemical Bonding And Molecular Structure Class 11 Medical Chemistry MCQ Quiz



Chemical Bonding And Molecular Structure Questions and Answers
| Chemical Bonding And Molecular Structure Quiz Question | Answer |
|---|---|
| The strength of sigma bonds formed by axial overlap of s− or p− orbitals of 2nd shell of participating atom decreases as: | p−p>s−p>s−s |
| Which one of the of the following molecules is expected to have zero dipole moment? | CO2 |
| The number of P-O-P and P-O-H bonds present respectively in pyrophosphoric acid molecule are_____ | 1,4 |
| Which formulae does not correctly represents the bonding capacity of the atom involved? | |
| The number of electron pairs in XeF4 at the corners of the square are _______. | 4 |
| What is the electronic configuration of elements of III rd group | 1s2,2s22p6,3s23p1 |
For which of the following molecule significant μ≠0? |
(c)and(d) |
| Identify the pair in which the geometry of the species is T-shape and square pyramidal, respectively. | XeOF2 and XeOF4 |
| Boron cannot form which one of the following anions? | BF3−6 |
| The number and type of bonds in C2−2 ion in CaC2 are : | one σ bond and two π-bonds |
Classification Of Elements And Periodicity In Properties Class 11 Medical Chemistry MCQ Quiz



Classification Of Elements And Periodicity In Properties Questions and Answers
| Classification Of Elements And Periodicity In Properties Quiz Question | Answer |
|---|---|
| The element with configuration 1s22s22p63s2 would be | A metal |
| The basic character of MgO,SrO,K2O and NiO increases in the order: |
NiO<MgO<SrO<K2O |
| IUPAC symbol of atomic number 119. | Uue |
| The electronic configuration with the highest ionization enthalpy is: | [Ne]3s23p3 |
| Aqueous solution of which salt will not contain ions with the electronic configuration 1s22s22p63s23p6? | NaF |
| The atomic numbers of elements A,B,C and D are Z−1,Z,Z+1 and Z+2, respectively. If ′B′ is a noble gas, choose the correct answers from the following statements (1) ′A′ has higher electron affinity (2) ′C′ exists in +2 oxidation state (3) ′D′ is an alkaline earth metal |
(1) and (3) |
| The halogen with highest ionisation potential is : |
F |
Identify the element with atomic number 10. |
Neon |
| The metal having highest ionisation potential is : |
lithium |
| Diagonal relationship is shown by : |
elements of third period |
Environmental Chemistry Class 11 Medical Chemistry MCQ Quiz



Environmental Chemistry Questions and Answers
| Environmental Chemistry Quiz Question | Answer |
|---|---|
| The layer of atmosphere between 10 km to 50 km above the sea level is called as: | stratosphere |
| The regions of the atmosphere, where clouds form and where we live respectively, are: | Troposphere and Troposphere |
| Biochemical Oxygen Demand (BOD) is the amount of oxygen required (in ppm) : |
by bacteria to break-down organic waste in a certain volume of a water sample. |
| Biochemical Oxygen Demand(BOD) value can be a measure of water pollution caused by the organic matter. Which of the following statements is correct? | Polluted water has BOD value higher than 10 ppm |
| Which is wrong with respect to our responsibility as a human being to protect our environment? | Using plastic bags |
| Depletion of which gas in the atmosphere can lead to an increase incidence of skin cancers? | Ozone |
| World ozone day is celebrated on __________ | 16th September |
| Ozone in stratosphere extends | 20-25km |
| The stratospheric ozone depletion leads to? | All of the above |
| Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion. |
Equilibrium Class 11 Medical Chemistry MCQ Quiz



Equilibrium Questions and Answers
| Equilibrium Quiz Question | Answer |
|---|---|
| Which of the following is strongest conjugate base | P - |
| Which of the following combination will act as a buffer? | CH3COOH+NaOH (Molar ratio 2:1) |
| Number of buffers can be made by reaction of H3PO4 and NaOH is | 3 |
| HCOO−+H2O⇌HCOOH+OH− is related:- | h=√KhC |
| Percentage dissociation of a 0.024M solution of a weak acid HA is: (Ka=2×10−3) |
≈29% |
| Which of the following is the correct order of hydrated radii? | Cs+ < Rb+<K+<Na+ < Li+ |
| For an acidic indicator has Ka value of 10−5. The % basic form when pH equal to 5.3 is : [Given log2=0.3] |
66.66 |
| When a strong base NaOH is added to the acidic buffer containing CH3COOH+CH3COONa then which of the following reaction occurs? | CH3COOH+NaOH→CH3COONa+H2O |
| Calculate the ratio of solubility of AgCl in 0.1 M AgNO3 and in pure water. Given : Ksp(AgCl)=1.8×10−10 |
1.34×10−4M |
| What is the pH of a solution of 0.28 M acid and 0.84 M of its conjugate base if the ionztion constant of acid is 4×10−4? | 3.88 |
Hydrocarbons Class 11 Medical Chemistry MCQ Quiz



Hydrocarbons Questions and Answers
| Hydrocarbons Quiz Question | Answer |
|---|---|
| Acetylene reacts with excess of hypochiorous acid to produce |
Dichloroacetaldehyde. |
| In which case butane is formed ? | 2C2H5−CI+Na |
| CH3CH=CH−CH2−CO2H→Δ(X) (major); Product (X) is : | CH3CH=CH−CH3 |
| Electromagnetic radiation with maximum wavelength is | radio waves |
IUPAC name of the following compound is: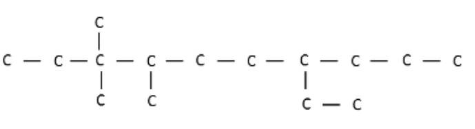 |
7- ethyl- 3,3, 4- trimethyl decane |
| Which of the following alkane gives only two mono chloro-derivatives? | propane |
| Alkanes can be obtained by the hydrolysis of | Al4C3 |
| Methane is obtained from among the following: | Both A and B |
| When the given compound undergoes Hoffmann methylation, the major product of the reaction: | |
| Which of the following is not a pair of isomers: |
Propene and Cyclopropene |
Hydrogen Class 11 Medical Chemistry MCQ Quiz



Hydrogen Questions and Answers
| Hydrogen Quiz Question | Answer |
|---|---|
| The reaction of water with ammonia is given the following equation, H2O+NH3⇌NH+4+OH−. In this reaction water behaves as : | acid |
| Assertion: H2O is the only hydride of the chalcogens which is liquid. Reasons:In ice, each O atom is surrounded by 4H atoms. |
Both A and R are correct but R is not the correct explanation of A |
| The photolysis of water gives the substance | OH−+H+ |
| Hydrogen can be put in halogen group because | It forms hydrides like chlorides |
| In the reaction 2H2O⇄H3O++OH−, water is: | Both a weak acid and a weak base |
| Only one element of ________ forms hydride. | group 6 |
| Sodium on reaction with cold water produces a residue. The nature of residue is: | colourless, soapy and slightly warm |
| Which of the following combination will produce H2 gas? | Zn metal and NaOH(aq) |
| Assertion: The O−O bond length in H2O2 is shorter than that of O2F2. Reason: H2O2 is an ionic compound. |
If both the assertion and reason are false |
| Which of the following process is not used in the concentration of H2O2? |
Sublimation |
Organic Chemistry Some Basic Principles And Techniques Class 11 Medical Chemistry MCQ Quiz



Organic Chemistry Some Basic Principles And Techniques Questions and Answers
| Organic Chemistry Some Basic Principles And Techniques Quiz Question | Answer |
|---|---|
| What is the prefix if the number of carbon atoms is 3? | Prop- |
| Number of π-bods in the following compound CH3C≡CN3 | none |
| Correctly match is : | σPx -- ψ (gerade) |
| Prefix used for −COOH functional group is : | Carboxy |
Above Gauche form is stable when Z is: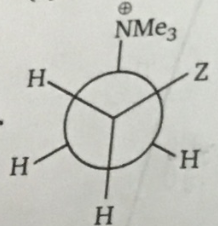 |
|
| IUPAC name of [VO(O2)(acac)2] is | bis(acetylacetonato)oxoperoxonanadium(VI) |
| Urea was first organic compounds to be synthesised by: | wohlar |
The functional groups which is not present in the given compound is: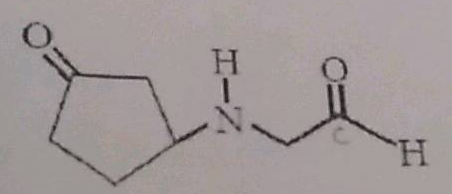 |
Amide |
| Which functional group is present in a molecule of CH3OCH2CH3? | Ether |
| Select the option having incorrect named compound(s) : (I) 3, 3-dimethyl-1-hydroxycyclohexane (II) 4-ethoxy-2-methoxypentan-3-one (III) 2-ethyl-1, 1-dimethylcyclopentane (IV) 4-amino-2-hydroxy-4-oxobutanal |
II and IV |
Redox Reactions Class 11 Medical Chemistry MCQ Quiz



Redox Reactions Questions and Answers
| Redox Reactions Quiz Question | Answer |
|---|---|
| The oxidation number of sulphur is -4 in | Na2SO3 |
| What are the oxidation numbers of 'N' in NH4NO3? | -3, +5 |
| Is the compounds is according to decreasing order of the oxidation state of nitrogen? HNO3>NO>N2> NH4Cl |
True |
| In the reaction : xBrO−3+yCr3++zH2O→Br2+CrO2−4+H+ The coefficient x, y and z are: |
6,10,22 |
| CrO2−4pH=x→Cr2O2−7pH=y→CrO2−4. x and y can be |
3 and 8 |
| Oxygen exhibit −1 oxidation state in: | H2O2 |
| Which is not redox change ? | CaCO3→CaO+CO2 |
| (i) Br2+2NaOH[cold]→NaBr+(X)+H2O (ii) 2Br2+6NaOH[hot]→NaBr+(Y)+H2O What is the oxidation number of Br in (X) and (Y) respectively? |
+1,+5 |
| Identify the element that can have only two oxidation states. | Fluorine |
| The highest oxidation state of Mn is shown by | KMnO4 |
Some Basic Concepts Of Chemistry Class 11 Medical Chemistry MCQ Quiz



Some Basic Concepts Of Chemistry Questions and Answers
| Some Basic Concepts Of Chemistry Quiz Question | Answer |
|---|---|
| Avogadro's number of Krypton atoms (Atomic weight = 83.8u) weigh: | 83.8g |
| Which gas law relates the volume of a gas to the number of molecules of the gas? |
Avogadro's Law |
| What is a chemical reaction? | Rearrangement of electrons in a chemical bond. |
| When magnesium is burnt in air, the weight of magnesium : | increases |
| What percent of a sample of nitrogen must be allowed to escape if its temperature, pressure, and volume are to be changed from 220oC,3 atm and 1.65 L to 110oC, 0.7 atm and 1 L respectively? | 81.8% |
| A mixture of SO2 and O2 was made to react in such a way that every gram of mixture gives maximum amount of SO3. The mass % of SO2 in the mixture is | 80 % |
| What is atomic mass of He in amu (u) units? | 4 |
| What should be the weight of 50% HCl which reacts with 100g of limestone? | 50 pure |
| If Avogadro number NA is changed from 6.022×1023mol−1 to 6.022×1020mol−1, this would change : | the mass of one mole of carbon |
| A certain sample of element Z contains 60% of 69Z and 40% 71Z . What is the relative atomic mass of element Z in this sample? | 69.8 |
States Of Matter Gases And Liquids Class 11 Medical Chemistry MCQ Quiz



States Of Matter Gases And Liquids Questions and Answers
| States Of Matter Gases And Liquids Quiz Question | Answer |
|---|---|
| Which of the following gas mixture is not applicable for Dalton's law of particle pressure? | SO2 and Cl2 |
| The value of gas constant per mole is approximately- | 2 cal |
| A constant gas temperature is based on | Charles's law |
| Gay Lussac law gives relation between: | PandT |
| The three phases of water (Solid, Liquid, Gas) are: | ice, water, steam |
| Identify which of the following statements is FALSE? | During a phase transition, the temperature of a substance must be constant. |
| ΔfG∘ at 500 K for substance 'S' in liquid state and gaseous state are +100.7 kcal mol−1 respectively. Vapour pressure of liquid 'S'' at 500 K is approximately equal to: R=2calK−1mol−1 |
0.1 atm |
| All kind of matter is made up of extremely small particles called ___________. | atom |
| Which term describes the mass of 6.022×1023 representative particles? | Molar mass |
| Total pressure of a gaseous mix is equal to the sum of the Partial Pressures is: |
Dalton's law |
Structure Of Atom Class 11 Medical Chemistry MCQ Quiz



Structure Of Atom Questions and Answers
| Structure Of Atom Quiz Question | Answer |
|---|---|
| The magnetic field at the center of a hydrogen atom due to the motion of electron varies with the principal quantum number as | 1/n5 |
| The equivalent weight of a metal is 4.5 and the molecular weight of its chloride isFind the atomic weight of the metal. | 9 |
| Which of the following statements about the radioactivity of an element is incorrect? | Its rate is affected by change in temperature and/or pressure |
| The Bohr model for the spectra of a H- atom | Will not be applicable for a He - atom |
| An element 'A' emits an α -particle and forms 'B'. 'A' and 'B' are | Isodiapheres |
| Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is: | 4.5 eV |
| Which of the following has the correct combination considering List−I and List−II? |
(I),(T) |
| For which one of the following, Bohr model is not valid? |
Singly ionised neon atom (Ne+) |
| Assertion: Atoms are not electrically neutral. Reason: Number of protons and electrons are different. |
Both assertion and reason statements are wrong. |
| Both Assertion and Reason are correct and Reason is the correct explanation for Assertion. |
The P-Block Elements Class 11 Medical Chemistry MCQ Quiz



The P-Block Elements Questions and Answers
| The P-Block Elements Quiz Question | Answer |
|---|---|
| The correct B --- F bond length follows the sequence : | BF3<BF−4<BF2OH<BF2NH2 |
| Which of the following is a semiconductor? | Ge |
| Which of the following statements is correct? | Electron precise hydrides have tetrahedral geometries |
| Colemanite is a mineral of: | B |
| Which of the following forms M2O type of oxide? | Tl |
| The possible oxidation state of Tl are: | +1 and +3 |
| The species, having bond angles of 120∘ is: | BCl3 |
| In the following statements, select the correct statement : |
N(SiH3)3 shows planar arrangement. |
| Hybridisation of silicon atom in silica is : |
sp3 |
| Aluminium is usually found in +3 oxidation state. In contrast, thallium exists in +1 and +3 oxidation states. This is due to : | inert pair effect |
Thermodynamics Class 11 Medical Chemistry MCQ Quiz



Thermodynamics Questions and Answers
| Thermodynamics Quiz Question | Answer |
|---|---|
| A chemical reaction is spontaneous at 298 K but non-spontaneous at 350 K. Which one of the following is true for the reaction? | ΔGΔHΔS−−− |
| Which of the following statements regarding Gibb's energy change is correct? | If ΔG is negative (< 0), the process is spontaneous. |
△H = - 336.2 kcal. What is the value of △U approximately at 300 K for the same reaction (R = 2 cal degree−1 mol−1)?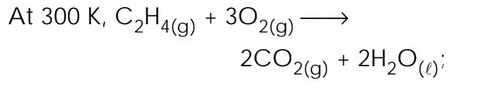 |
- 335.0 kcal |
| Properties of substances like pressure, temperature and density, in thermodynamic coordinates are | point function |
| The INCORRECT match in the following is : | ΔG0<0,K<1 |
| Which of the following property is not a thermodynamic property of the system? | heat |
| Which heat depends on the direction of current? | Peltier heat |
| An ideal gas undergoes a cyclic process as shown in Figure. ΔUBC=−5 kJ mol−1, qAB=2 kJ mol−1 WAB=−5 kJ mol−1, WCA=3 kJ mol−1 Heat absorbed by the system during process CA is: 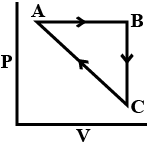 |
+5 kJ mol−1 |
| The combustions of benzene (l) gives CO2(g) and H2O(l). Given that heat of combustion of benzene at constant volume is −3263.9 kJmol−1 at 25oC, heat of combustion (in kJmol−1) of benzene at constant pressure will be: (R=8.314JK−1mol−1) |
−3267.6 |
| The standard Gibbs energy for the given cell reaction in kJ mol−1 at 298K is:Zn(s)+Cu2+(aq)→Zn2+(aq)+Cu(s) Eocell=2V at 298K (Faraday's constant, F=96000 Cmol−1) |
−384 |
The S-Block Elements Class 11 Medical Chemistry MCQ Quiz



The S-Block Elements Questions and Answers
| The S-Block Elements Quiz Question | Answer |
|---|---|
| Which of the following is organometalic compound | Methyl lithium |
Incorrect statement about given carbohydrate is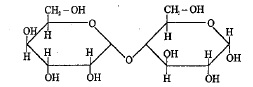 |
Above compound is a non-reducing sugar |
| Which of the following product is obtained when bleaching powder is distilled with acetone? | none of the above |
| Lithium react less vigorously with water than other metals. Give reason behind it. | Lithium has small size and very high hydration energy. |
| Diagonal relationship is found in between | A and C both |
| The alkaline earth metal ion present in chlorophyll is | Mg |
| Among the alkaline earth metals, the element forming predominantly covalent compounds is | Be |
| The ionization energy of a hydrogen atom is 13.6eV. The energy of the third-lowest electronic level in doubly ionized lithium (Z=3) is: | -122.4eV |
| Correct order is : | MgO<NiO<K2O<CS2O (basic strength ) |
| The melting point of lithium (181∘C) is just double the melting point of sodium (98∘C) because | down the group, the cohesive energy decreases |
Medical Chemistry MCQ Questions for Class 11 - Practice Test with Solutions
Do you want to overcome your drawbacks while attempting the quizzes or MCQ tests like time consumption, approaching questions, etc.? Take the advantage of practicing with MCQExams.com MCQ Questions for Standard 11 Medical Chemistry Test. As it is a time-based approach and also provides answers to all questions.
One should practice the MCQs in this way for a better assessment of their preparation level. All chapters CBSE Class 11 Medical Chemistry MCQ Quiz Questions with Solutions PDF free download links are available for easy access & quick reference.
How to Use MCQExams.com Chapterwise 11th Medical Chemistry MCQ Interactive Quiz?
Guys do you love to share your practice hacks and tips with your friends? If yes, then our 11tth standard CBSE Medical Chemistry MCQ interactive quiz help you do the same. Excited to know the process then jump into the below steps right away:
- Go with the respective chapter class 11 Medical Chemistry MCQ quiz link from the above
- Now, you will find the MCQ quiz boxes for the Thermodynamics chapter along with the interactive quiz windows.
- Click on the CBSE 11th Class Thermodynamics MCQ Interactive Quiz and it will redirect you to another window where it displays the questions with options in stories format.
- Answer the question one after another and learn the answers right away this helps you to do a quick assessment of your knowledge.
- You can also share this cool MCQ Interactive Quiz Questions of Plus One Medical Chemistry topicwise with your friends by just tapping on the send arrow located at the top left corner of the story.
- After clicking the button, you can opt for the copy link option and easily paste the link on your friend's chat or else in your whatsapp story too. Isn’t it cool!!
- Keep passing this interesting approach of practicing Plus One CBSE Medical Chemistry Thermodynamics MCQ Questions to your co-students and help them in attempting the entrance exams like JEE & NEET.