Q.1.
The hottest region of Bunsen flame shown in the figure below is:
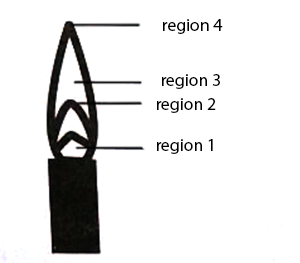

-
0%
region 2
-
0%
region 3
-
0%
region 4
-
0%
region 1
Q.2.
Which chemical substance is emitted in car exhaust and is a cause of acid rain?
-
0%
Nitrogen oxides
-
0%
Lead
-
0%
Carbon dioxide
-
0%
DDT
-
0%
Nitrates
Q.3.
-
0%
Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
-
0%
Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
-
0%
Assertion is correct but Reason is incorrect
-
0%
Assertion is incorrect but reason is correct
Q.4.
$$I$$. The most significant way in which humans are exposed to mercury is through drinking water.
$$II$$. A great deal of mercury enters the atmosphere because of coal-burning power plants.
$$II$$. A great deal of mercury enters the atmosphere because of coal-burning power plants.
-
0%
Statement $$I$$ is true, Statement $$II$$ is true
-
0%
Statement $$I$$ is true, Statement $$II$$ is false
-
0%
Statement $$I$$ is false, Statement $$II$$ is true
-
0%
Statement $$I$$ is false, Statement $$II$$ is false
-
0%
Statement $$I$$ is true, Statement $$II$$ is true and is a correct explanation of the phenomena described in $$I$$
Q.5.
State whether true or false:
We can use water as a fire extinguisher for fires involving petrol.
-
0%
True
-
0%
False
Q.6.
A good fuel has these qualities:
-
0%
should have high calorific value
-
0%
should be costly
-
0%
should cause any pollution
-
0%
should be easy to transport
Q.7.
How water helps in extinguishing fire?
-
0%
Water cools the combustible material so that its temperature is brought below the ignition temperature.
-
0%
Water heats the combustible material so that its temperature is brought below the ignition temperature.
-
0%
Water cools the combustible material so that its temperature is brought above the ignition temperature.
-
0%
None of these
Q.8.
The elements that are combustible in the fuel are:
-
0%
carbon and hydrogen
-
0%
carbon, hydrogen and ash
-
0%
carbon, hydrogen and sulphur
-
0%
carbon, nitrogen and ash
Q.9.
During burning of candle, different zones of combustion in the flame are listed below:-
The correct order of temperature of zones is :
a) Outermost zone
b) Innermost zone
c) Middle zone
b) Innermost zone
c) Middle zone
The correct order of temperature of zones is :
-
0%
$$A>B>C$$
-
0%
$$C>B>A$$
-
0%
$$B>C>A$$
-
0%
$$A>C>B$$
Q.10.
What reactant must be present for a reaction to be classified as combustion?
-
0%
$${Cl}_{2}$$
-
0%
$${CH}_{4}$$
-
0%
$${O}_{2}$$
-
0%
$${CO}$$
Q.11.
Which kind of material is extinguished by water on burning?
-
0%
Wood
-
0%
Paper
-
0%
Petrol
-
0%
Both a and b
Q.12.
- What is geothermal energy ?
-
0%
Ocean thermal energy
-
0%
Interior heat of the earth.
-
0%
Energy obtained from the heat of hot dry rocks
-
0%
None of these
Q.13.
Fire extinguishers containing $$CO_2$$ are the best extinguishers for fires involving electrical equipment:
-
0%
because $$CO_2$$ being heavier than oxygen covers the fire like a blanket.
-
0%
because $$CO_2$$ being heavier than oxygen increases fire.
-
0%
because $$CO_2$$ being lighter than oxygen covers the fire like a blanket.
-
0%
None of these
Q.14.
How carbon dioxide is stored in the house to extinguish fire?
-
0%
It can store in the bottles
-
0%
It can store in the cylinders under high pressure
-
0%
It can store in canes
-
0%
It can not store in any form
Q.15.
The unit of calorific value is:
-
0%
kJ/kg
-
0%
kg/kJ
-
0%
mg/kJ
-
0%
g/kJ
Q.16.
A thin paper cup filled with water does not catch fire when placed over a flame. This is because:
-
0%
the water cuts off oxygen supply to the paper cup
-
0%
water is an excellent conductor of heat
-
0%
the paper cup does not reach to ignition temperature
-
0%
paper is a poor conductor of heat
Q.17.
The cooking gas burns rapidly and produces heat and light. Such combustion is called _________.
-
0%
rapid combustion
-
0%
incomplete combustion
-
0%
regular combustion
-
0%
none of these
Q.18.
Choose the correct answer from the alternatives given.
In an atomic explosion, enormous energy is released due to _______________.
In an atomic explosion, enormous energy is released due to _______________.
-
0%
Conversion of light energy into hear energy
-
0%
Conversion of heat energy into light energy
-
0%
Conversion of mechanical energy into nuclear energy
-
0%
Conversion of mass into energy
Q.19.
Which of the following example represents spontaneous combustion?
-
0%
Burning of matchstick.
-
0%
Burning of cooking gas.
-
0%
Ignition of a cracker.
-
0%
Phosphorus burns in air at room temperature
Q.20.
Which of the following example(s) represents rapid combustion?
-
0%
Burning of matchstick.
-
0%
Burning of cooking gas.
-
0%
Phosphorus burns in air at room temperature.
-
0%
Ignition of a cracker.
Q.21.
Which of the following examples represents explosion?
-
0%
Burning of matchstick
-
0%
Burning of cooking gas
-
0%
Ignition of a cracker
-
0%
Burning of phosphorus in air at room temperature
Q.22.
Explosion of crackers takes place due to application of _________ on them.
-
0%
temperature
-
0%
pressure
-
0%
heat
-
0%
none of these
Q.23.
When fuel is added to the exhaust (burnt) gases in a jet engine combustion takes place. Some facts are mentioned here about this combustion. Pick the false one?
-
0%
It increases the intake of oxygen from the air
-
0%
It reduces fuel consumption
-
0%
It gives additional thrust
-
0%
It is known as 'after burning'
Q.24.
A physical process in which a substance reacts with oxygen to give off heat is called combustion.
-
0%
True
-
0%
False
Q.25.
A white solid $$X$$ reacts with dil.$$HCl$$ to give colourless gas which is used in fire extinguishers. The solid $$X$$ is:
-
0%
$$NaCl$$
-
0%
$$CH_3COONa$$
-
0%
$$Na_2CO_3$$
-
0%
$$NaHCO_3$$
Q.26.
If butane on combustion gives carbon monoxide, find the number of $$O_ 2$$ molecules required.
-
0%
6
-
0%
5.5
-
0%
4.5
-
0%
4
Q.27.
Gobar gas contains mainly:
-
0%
methane
-
0%
ethane
-
0%
butane
-
0%
carbon monoxide
Q.28.
If you watch a burning candle carefully, you will find different colurs in the flame. Which is the hottest part of the candle flame?
-
0%
The middle orange part
-
0%
The middle, yellow part
-
0%
The outermost portion that is blue
-
0%
The innermost black zone
Q.29.
State true or false.
Combustion can take place at all temperatures less than an ignition temperature.
-
0%
True
-
0%
False
Q.30.
The heat liberated per kilogram of a fuel is called its:
-
0%
calorific value
-
0%
mass value
-
0%
weight value
-
0%
fuel value