Q.1.
Calcium phosphate is present in tooth enamel. Its nature is __________.
-
42%
basic
-
33%
acidic
-
17%
neutral
-
8%
amphoteric
Q.2.
Which one of the following nutrient is not available in fertilizers?
-
27%
Nitrogen
-
0%
Phosphorus
-
64%
Iron
-
9%
Potassium
Q.3.
Materials which can be drawn into wires are called ductile. Which of the following is not used as a ductile material?
-
9%
Silver
-
9%
Copper
-
82%
Sulphur
-
0%
Aluminium
Q.4.
Which of the following is used for dissolution of gold?
-
17%
Hydrochloric acid
-
8%
Sulphuric acid
-
17%
Nitric acid
-
58%
Aqua regia
Q.5.
Metal that exists in the liquid state at room temperature is :
-
80%
Mercury
-
20%
Silver
-
0%
Palladium
-
0%
Platinum
Q.6.
An element $$A$$ is soft and can be cut with a knife. This is very reactive with air and cannot be kept open in the air. It reacts vigorously with water. The element $$A$$ is:
-
0%
Magnesium
-
100%
Sodium
-
0%
Gold
-
0%
Calcium
Q.7.
Boojho has learned that non-metals on beating with a hammer are generally broken into pieces. Which of the following can be broken into pieces?
-
0%
Iron nail
-
0%
Aluminium wire
-
0%
Copper plate
-
100%
Piece of coal
Q.8.
Magnesium ribbon on burning in air produces:
-
0%
magnesium oxide, water and light
-
0%
magnesium oxide and heat
-
100%
magnesium oxide, heat and light
-
0%
magnesium oxide, water and heat
Q.9.
Which of the following observations most strongly suggest that a solid element X is a non- metal?
-
70%
X forms an acidic oxide.
-
30%
X has a high melting point
-
0%
X is a conductor of electricity.
-
0%
X reacts vigorously with chlorine.
Q.10.
Which of the following non-metals react and catches fire on exposure to air?
-
87%
Phosphorus
-
0%
Nitrogen
-
7%
Sulphur
-
7%
Hydrogen
Q.11.
Soft metal(s) which can be cut with a knife is(are) :
-
0%
Lithium
-
0%
Potassium
-
29%
Sodium
-
71%
A, B and C
Q.12.
Generally, metallic oxides are basic and non-metallic oxides are acidic in nature. Solution of which of the following oxides in water will change the colour of blue litmus to red?
-
67%
Sulphur dioxide
-
17%
Magnesium oxide
-
0%
Iron oxide
-
17%
Copper oxide
Q.13.
Paheli prepared a blue coloured solution of copper sulphate in beaker A and placed an iron nail in it. Boojho prepared a yellowish green solution of ferrous sulphate in beaker B and placed a copper wire in it. Changes they observe in the two beakers after an hour will be :
-
38%
in beaker A, no change ; in beaker B, change to green
-
12%
in beaker A, no change ; in beaker B, no change
-
38%
in beaker A, changes to green ; in beaker B, no change
-
12%
in beaker A, changes to green ; in beaker B, change to blue
Q.14.
Non-metals which are present in fertilizers and enhance the growth of plants is:
-
0%
nitrogen
-
0%
phosphorus
-
0%
potassium
-
100%
both $$A$$ and $$B$$
Q.15.
Sulphur is a poor conductor of electricity.
-
86%
True
-
14%
False
Q.16.
Which of the following are properties of non-metals?
-
0%
Lustre
-
14%
Can form alloys
-
0%
Amorphous
-
86%
Non-conductive towards electricity
Q.17.
A student added zinc granules to copper sulphate solution taken in a test tube. Out of the following, the correct observation (s) made by the student will be:
-
0%
zinc granules have no regular shape
-
0%
zinc granules are silvery grey coloured
-
0%
colour of zinc granules changed to brownish black
-
100%
$$CuSO_{4}$$ change into $$ZnSO_{4}$$
Q.18.
Which of the following is an insulator?
-
20%
Copper
-
0%
Iron
-
60%
Sulphur
-
20%
Graphite
Q.19.
State true or false.
Diamond and iodine are non-metals which are lustrous.
-
80%
True
-
20%
False
Q.20.
A non-metallic oxide dissolves in water to give :
-
20%
alkali
-
80%
acid
-
0%
base
-
0%
none of these
Q.21.
Metals can be hammered into thin sheets. This property is called:
-
0%
density
-
100%
malleability
-
0%
ductility
-
0%
strength
Q.22.
Which of the following elements produce basic oxides upon reacting with oxygen?
-
17%
Chorium
-
0%
Sulphur
-
33%
Sodium
-
50%
Magnesium
Q.23.
The oxide of sodium is _______ in nature.
-
40%
acidic
-
60%
basic
-
0%
amphoteric
-
0%
none of these
Q.24.
Which of the following is liquid at ordinary temperature ?
-
0%
Germanium
-
80%
Gallium
-
0%
Gold
-
20%
Galena
Q.25.
An alloy which is sonorous is:
-
20%
duralumin
-
60%
bell metal
-
20%
type metal
-
0%
gun metal
Q.26.
Compound can be reduced to copper when heated with coke is
-
25%
Copper hydroxide
-
50%
Copper oxide
-
0%
Copper sulphide
-
25%
All of these
Q.27.
Which of the following statements is correct about the physical properties of metals and non-metals?
-
0%
Both are sonorous.
-
0%
Both are poor conductors of heat.
-
100%
Most metals are hard and lustrous, while most non-metals are soft and dull.
-
0%
Metals do not conduct electricity while non-metals do.
Q.28.
X is an element in the form of a powder. X burns in oxygen and the product is soluble in water. The solution is tested with litmus. If X is a non metal, then the litmus will turn:
-
0%
Red to blue
-
50%
Blue to red
-
25%
No change on red litmus
-
25%
Both $$B$$ and $$C$$
Q.29.
Compare the properties of a typical metal and a non-metal on the basis of the following.
-
100%
Metals are good conductor of heat and electricity.
-
0%
Non metals are good conductor of heat and electricity.
-
0%
Metals are bad conductor of heat and electricity.
-
0%
Both B and C
Q.30.
A student takes Cu, Al, Fe and Zn strips separately in four test-tubes labelled as I, II, III and IV respectively. He adds 10 ml of freshly prepared ferrous sulphate solution to each test-tube and observes the colour of the metal in each case. He would observe a black residue in:
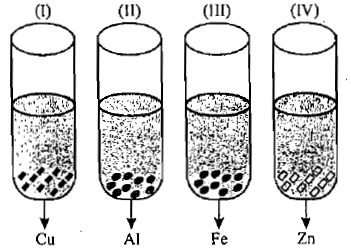

-
0%
I and II
-
50%
I and III
-
0%
II and III
-
50%
II and IV