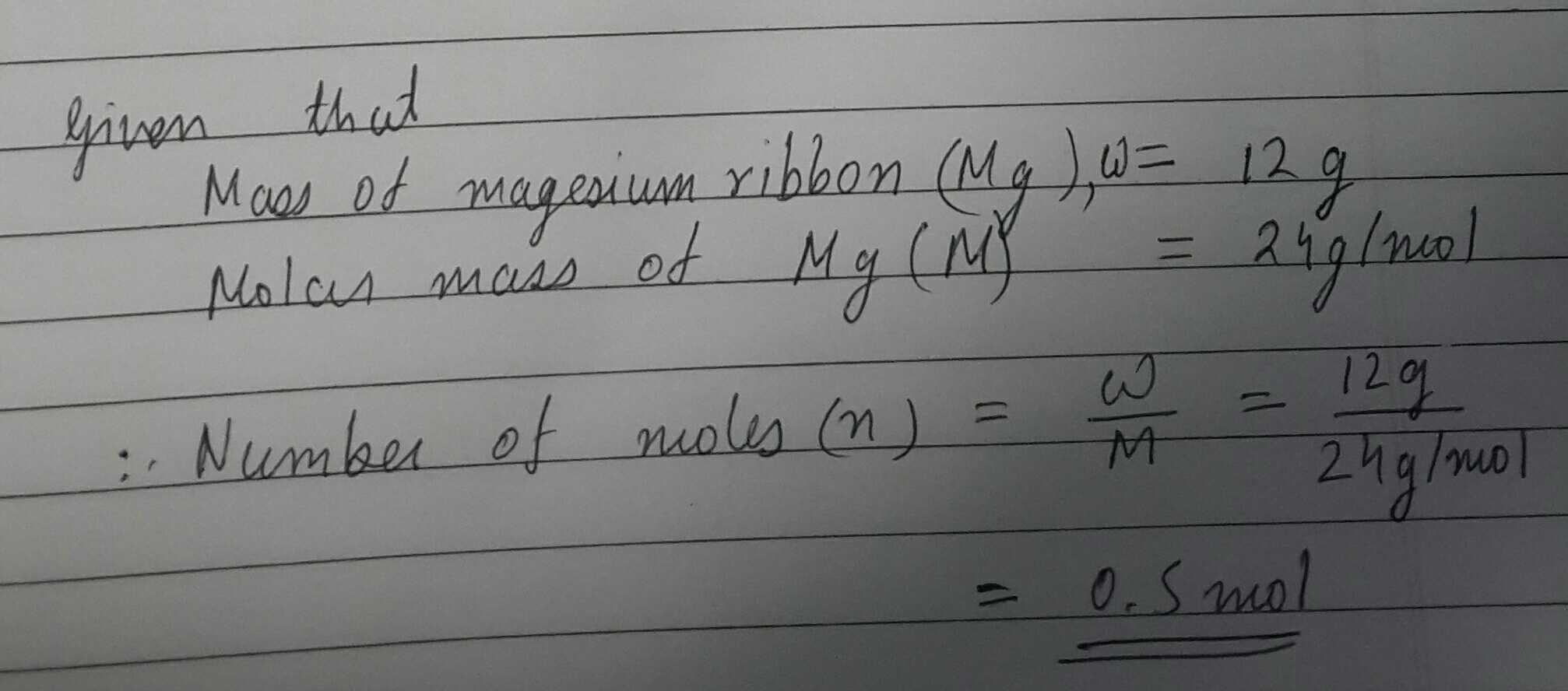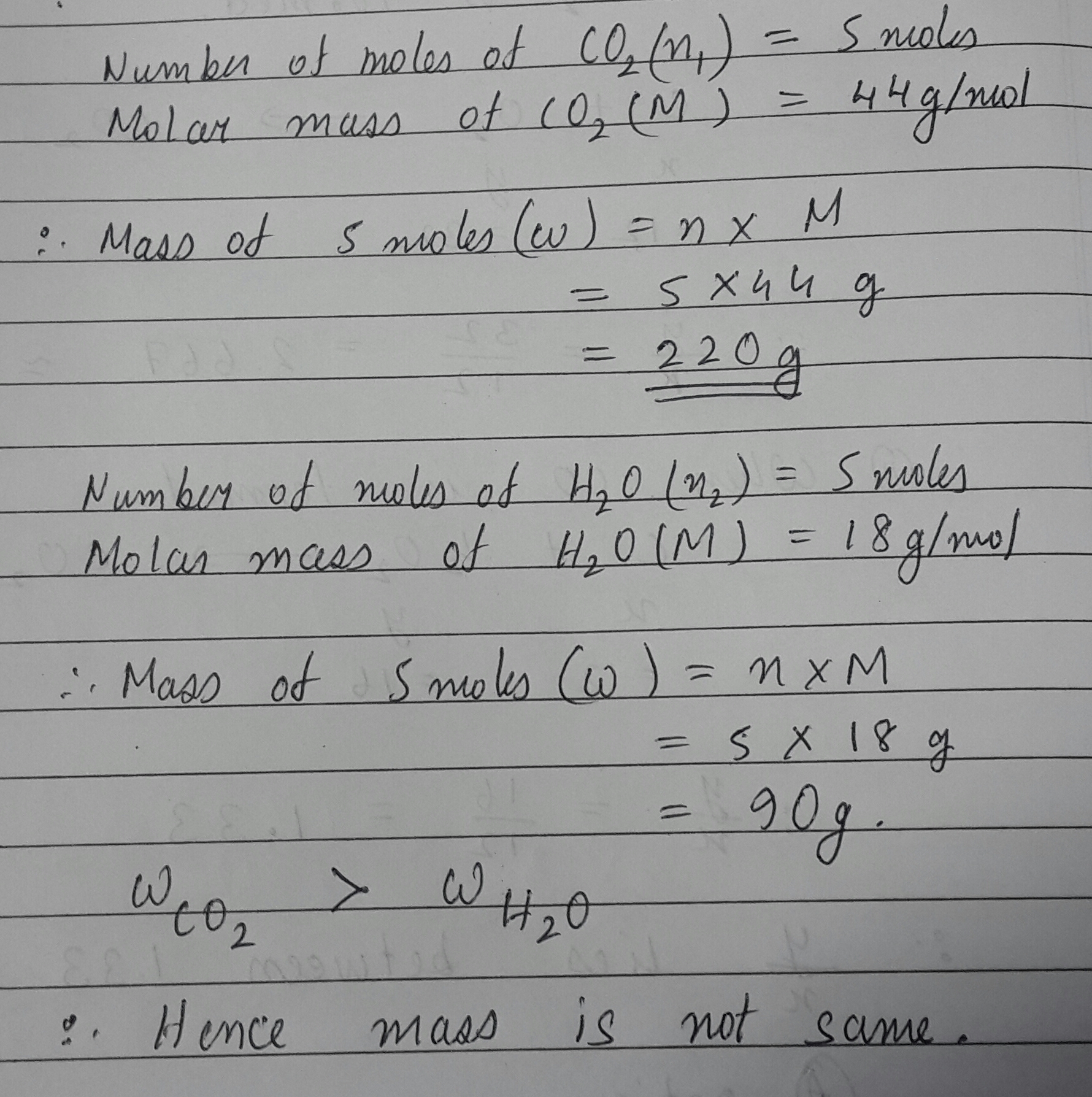Atoms And Molecules - Class 9 Chemistry - Extra Questions
Define ion.
Define Symbol.
Why does $$OH^{ - }$$ is an ion?
According to Dalton,______is the smallest indivisible particle of the matter.
Complete the statements given below by filling in the blank with the correct word/s.
The basic unit of an element is an ________.
Fill in the blanks:
The molecules are made up of _________.
__________ are made up of same kind of atoms.
An ion with positive charge is called
Complete the statements given below by filling in the blank with the correct word/s.
Atom contains ______, with positively charged _______.
Select the correct answer from the choice in brackets.
The symbol of — Mercury [Mg / Hg / Ag]
If an atom loses an electron, it acquires a ____ charge.
If an atom gains an electron, it is called _____.
State Dalton's atomic theory.
A charged particle is called an _____.
An ion with negative charge is called as_________.
What does the word 'atom' mean?
What are molecules ?
Name the smallest particle from which matter is made up.
Write the names of elements from the following symbol :
Be
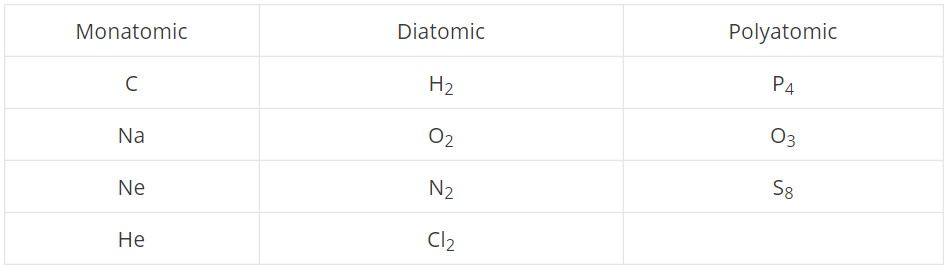
Give some more examples of molecules in the respective categories (other than the ones already mentioned).

Write the diatomic molecules from the following
$$H_2, Na, C, N_2, P_4, O_2$$
How many moles of hydrogen are present in 1 gm of hydrogen?
Calculate the molecular mass of ethanoic acid , $$ CH_3COOH $$.
(Atomic masses : $$ C = 12u ; H = 1u ; O = 16u $$)
Give the symbols for metals of group $$13$$.
The weight of $$ 1 \times 10^{22} $$ molecules of $$ \mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O} $$ is _________.
Components in a mixture are present in varying proportions and not in a fixed proportion.
Molecular mass of water is __________ u.
Molar mass of $$C_2H_6$$ (in g/mol) is:(Given atomic weight of $$C = 12 $$gm and $$H = 1$$gm)
Calculate the molar mass of $$\displaystyle { CO }_{ 2 }$$.
Give reason why an atom of an element is electrically neutral?
Calculate the molar mass of $$\displaystyle { CH }_{ 4 }$$.
Define atom.
Determine the molecular mass of water.
What is meant by a 'mole' of a substance?
Give an example of the following:
A molecule in which central atom is linked to three other atoms.
A molecule in which central atom is linked to three other atoms.
How many moles are there in $$200g$$ of $$Na$$?
Define the following terms:
Atom
Isotope
Atomic number
Atomic mass number
Write a short note on Avogadro's number .
Which qualities are required in the student to perform such an activity?
What is the difference between cation and anion?
Calculate the number of moles of magnesium present in a magnesium ribbon weighing 12 g. molar atomic mass of magnesium is $$ 24 g mole^{-1} $$
How do negative and positive ions form?
What is an atom?
Define an atom.
Calculate the number of hydrogen molecules in $$100 \,gm$$ hydrogen.
Calculate the mass (in grams) of -
(i) $$10^{23} $$molecules of $$CO_{2} $$ $$gas$$
(ii) 2 moles of nitrogen atom
(C=12 u, 0= 16 u, N = 14 u)
Fill in the blank.
Dalton said that _______ could not be divided.
$$AB_2$$ and $$A_2B_3$$ are two compounds of the elements A and B. 0.15 mole of each of these compounds weighs 9.3 and 15.9 g respectively. Find the atomic masses of A and B.
A sample of $$CaCO_3$$ has Ca (40%), C (12%), O (48%) by mass. If the law of constant proportions is true then find the mass of Ca in 5 g of $$CaCO_3$$ obtained from another source.
Calculate the number of atoms in $$120\ g\ of\ Ca$$.
Write formula of - barium sulphate
$$\text{Ravi prepared a solution of sodium chloride by mixing 5.85 g salt in 1 litre of water. Find}$$
Molar mass of sodium chloride
The mass of one steel screw is 4.11 g. Find the mass of one mole of the steel screws. Compare this value with the mass of the Earth $$(5.98 \times10^{24})$$ kg.Which one of the two is heavier and by how many times?
14 g of $$N_2$$ contains $$3.01\times 10^{23}$$ nitrogen molecules. If true then enter 1, if false enter 0.
The molar mass of $$H_2O$$ is:
The molar mass of $$CO_2$$ is :
Molar mass of $$H_2SO_4$$ is:(Given: Atomic mass of $$H=1, S=32, O=16$$)
If the atomic mass of carbon were to set at $$100$$ amu, the Avogrado's number will be equal to $$5.01\times {10}^x$$ atoms. Find the value of $$x$$.
Select the correct answer from the given choices for the statement given below.
A positively charged ion is called [cation / anion]________.
Cations are formed by .............. (loss/ gain) of electrons and anions are formed by ................. (loss/ gain) of electrons.
Identify the odd one out:Mercury, Gold, Brass, Nitrogen
The weight of $$1\times 10^{22}$$ molecules of $$CuSO_4\cdot 5H_2O$$ is:(nearest integer)
What is denoted by the chemical formula $$S_8$$.
What does the symbol $$H_2$$ convey?
Calculate the formula mass of compound given below: NaCl (common salt)Given: Atomic masses of Na = 23, Cl = 35.5.
Calculate the molar mass of the following:
(i) $$\displaystyle { H }_{ 2 }O$$
(ii) $$\displaystyle { CO }_{ 2 }$$
(iii) $$\displaystyle CH_{ 4 }$$
(i) $$\displaystyle { H }_{ 2 }O$$
(ii) $$\displaystyle { CO }_{ 2 }$$
(iii) $$\displaystyle CH_{ 4 }$$
What will be the mass of one $$\displaystyle ^{ 12 }{ C }$$ atom in g ?
Calculate the mass and charge of one mole of electrons.
Given: Mass of one electron = $$9.10 \times 10^{-31}$$ kgCharge of one electron = $$1.602 \times 10^{-19}$$ coulomb
What are the difference between atoms and molecules?
In an experiment 0.2430 gm of magnesium on burning with oxygen yielded 0.4030 gm of magnesium oxide. In another experiment 0.1820 gm of magnesium on burning with oxygen yielded 0.3020 gm of magnesium oxide. Show that the data explain the law of definite proportions.
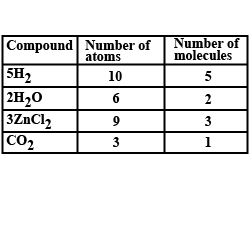
Mention the difference between $$N_2$$ and $$2N$$?
Convert into mole.
$$(a)$$ $$12$$ g of oxygen gas
$$(b)$$ $$20$$ g of water
$$(c)$$ $$22$$ g of carbon dioxide.
The symbol for element lead is derived from the latin name ---------------.
A symbol of an element is always represented by the first alphabet of an atom of the element.
Match the mass of elements given in column I with the no. of moles given in column II and mark the appropriate choice.
The sodium salt of methyl orange has 7% sodium. What is the minimum molecular weight of the compound?
Which of the following contains the largest number of oxygen atoms? 1.0 g of $$O$$ atoms, 1.0 g of $$O_2$$, 1.0 g of ozone $$O_3$$?
The order of increasing relative molecular mass of the following gases is, hydrogen, oxygen, carbondioxide, sulphurdioxide, chlorine. Given 8 gram of each gas at STP, which will contain the least number of molecules and which the most?
In two different experiments, the copper oxide was obtained from copper. The ratio of the mass of copper and the mass of oxygen found in the two experiments are the same. They are in the ratio of $$4: 1$$. What of you infer about the experiments?
Find the molecular mass of sulphuric acid.
Find out the molecular mass of sulphuric acid.
Give two reasons to prove that water is a compound and not an element.
A molecule formed by combination or association of three molecules of the same substance is known as _______ .
Aluminium reacts with sulphuric acid to form Aluminium sulphate. Answer the following:
What is the charge carried by sulphate ion?
Formation of ions from neutral atoms or molecules known as :
Calculate the total number of electrons present in one mole of methane.
Match the List of scientists and philosophers(List 1) with the list of statements(List 2).
(a) State the postulates of Dalton's atomic theory.(b) Calculate the concentration in terms of mol/kg when 6.35g of KCl is dissolved in 300g of water. [At mass of K=39u, At mass of Cl=35.5u]
How many water molecules will be there in $$3\times 10^{-23}$$ g sample of water?
Find the molecular mass (in kilogram) of an ammonia molecule $$NH_3$$.
Write the molecular mass of
$$HgCO_3,CaCO_3,NaHCO_3,CH_3CH_2OH, Al_2,(SO_4)_2$$
Number of molecules present in $$4.2$$ g $$N_2$$ is $$X\times10^{22}$$. What is the value of $$X$$(round off to 1 digit)?
Calculate the number of molecules in a drop of water weighing $$0.05$$ gm.
In an experiment 3g of hydrogen was obtained. If hydrogen and oxygen combine in the ratio of 1:Prove the law of constant proportion.
What is a mole?
Cost of sugar ($${C}_{12}{H}_{22}{O}_{11}$$) is Rs. $$40$$ per kg. Calculate its cost per mole.
What does $$^{12}C$$ mean in amu?
A compound contains 1 carbon atom and 2 oxygen atoms. The molecular mass of compound (in gm) is __________.
In an experiment, 1.288 g of copper oxide was obtained from 1.03 g of copper. In another experiment, 3.672 g of copper oxide gave, on reduction, 2.938 g of copper. Show that these figures verify the law of constant proportions.
Name of the ion with symbol $$S^{2-}$$_______
Give the answers of the following:(a) What is the mass of one atom of S? (b) What is the atomic mass of S? (c) what is the gram atomic mass of S?
What are anions?
Write the complete symbol for the atoms with given atomic and mass no, $$Z=18\ A=40\ $$ and $$A=9$$
($$Z=$$Atomic no and $$A=$$mass)
Write the limitations of Dalton's atomic theory.
Calculate the molecular masses of $$H_2, O_2, Cl_2, CO_2, CH_4, C_2H_6, C_3H_6, NH_3, CH_3OH$$
$$2.8\ g$$ of calcium oxide prepared by heating limestone were found to contain $$0.8\ g$$ of oxygen. When one gram of oxygen was treated with calcium, $$3.5\ g$$ of calcium oxide was obtained. Show that the results illustrate the law of definite proportions.
Complete the following table of radicals.
| Name of Radical | Molecular representation |
| Sulphate | |
| $$N^{3-}$$ | |
| Nitrate | |
| Bisulphite | |
| $$CO_{3}^{2-}$$ | |
| $$OH^{-}$$ | |
| Phosphate |
State the difference between cations and anions.
Explain Dalton's atomic theory.
Name the Indian sage who first propounded the idea of atom (amu).
State law of constant properties. Give one example to illustrate this law.
Calculate the molar mass of the following substances (a) Ethyne $$C_2H_2$$ (b) Sulphur molecule $$S_8$$(c) Phophorous molecule $$P_4$$ (d) Nitric acid $$HNO_3$$ (e) Hydrochloride acid $$HCl$$.
Find the molecular mass of: (a) $$H_2S$$ (b) $$HCl$$ (c)$$NH_3$$ (d) $$Cl_2$$ (e) $$CH_3COOH$$ (f) $$CH_3CHO$$
The molecular weight of hydrogen peroxide is $$34$$. What is the unit of molecular weight?
Why is it not possible to see an atom with naked eyes?
State the various postulates of Dalton's atomic theory of matter.
Write symbol of silver.
Write the symbol of gold.
Calculate the formula unit mass of $$CaSO_{4.}2H_{2}O$$.
Write symbol of copper.
0.607 g of the silver salt of a tribasic acid on combustion deposited 0.37 g of pure silver Calculate the molecular weight of the acid.
What is the symbol of the species with number of electrons equal to $$36$$ protons equal to $$35$$ and neutrons equal to $$45$$?
Which postulate of Dalton's atomic theory can explain law of definite proportions?
Complete the following table.
| Atomic Number | Mass Number | Number of Nutrons | Number of Protons | Number of Electrons | Name of the Atomic Species |
| 9 | - | 10 | - | - | - |
| 16 | 32 | - | - | - | Sulphur |
| - | 24 | - | 12 | - | - |
| - | 2 | - | 1 | - | - |
| - | 1 | 0 | 1 | 0 | - |
Give postulates of Dalton's atomic theory.
Write the names of the following compounds and deduce their molar masses.
$${ N }a_{ 2 }{ SO }_{ 4 },\quad { K }_{ 2 }{ CO }_{ 3 },\quad { CO }_{ 2 },\quad { MgCl }_{ 2 },\quad NaOH,\quad { AlPO }_{ 4 },\quad { NaHCO }_{ 3 }$$
How does the Modern atomic theory contradict and correlate with Dalton's atomic theory?
Name the reaction in which one of the reacting species of loses electrons, while the other gains electrons.
An atom of an element is denoted by a "symbol"- Explain the meaning of the term 'symbol'. State a reason for representing the following elements by their symbols.
(a) Hydrogen by 'H'
(b) Helium by 'He'
(c) Copper by 'Cu'
Calculate the molecular masses of $$H_{2},O_{2},Cl_{2},CO_{2},CH_{4}, C_{2}H_{6},C_{2}H_{4},NH_{3},CH_{3}OH $$.
What are the ions present in this compound?$$Al_2(SO_4)_3$$
Apply the law of constant proportions to calculate the mass of oxygen that will be used up for combustion of 5 g of $${H_2}$$ gas.
How many atoms of element 35 can combine with ab atom of element 20?
State the law of definite proportions. Explain it with the help of an example
Calculate the number of moles of the following:
(I) $$52g$$ of $$He$$
What are ions? Give some examples.
Is it possible to see atoms these days? Explain your answer.
What are the main postulates of Dalton's atomic theory? What were it's limitations.
Water $$(H_2O$$) obtain from any source contains hydrogen and oxygen in the ratio 1:8 by mass. Which Law follows it? Define the Law?
(a) State six postulates of Daltons atomic theory.
(b) A 0.24g Sample of compound of boron and oxygen on analysis was found to contain 0.096g of boron and 0.144g of oxygen. Find the percentage composition of the compound by wight.
Verify by calculation that 5 moles of $$CO_2$$ and 5 moles of $$H_2O$$ not have same mass.
Explain the mole concept. What is the importance of mole concept.
State law of definite proportions.
Differentiate between $${\text{2H}}$$ and $${{\text{H}}_{\text{2}}}$$.
Mass of 1.5 mole of $${ CO }_{ 2\ }$$ molecule
Calculate the mass of the following
(i) $$0.5$$ mole of $$N_{2}$$ gas (mass from mole of molecule) (ii) $$0.5$$ mole of $$N$$ atoms (mass from
mole of atom) (iii) $$3.011\times 10^{23}$$number of N atoms (mass from number) (iv) $$6.022\times 10^{23}$$number of $$N_2$$ atoms (mass from number)
Which postulate of Dalton's atomic theory is the result of the law of conservation of mass?
How to calculate the molar mass?
What is the weight of $$3.01 \times 10^{23}$$ molecules of ammonia?
Which postulate of Dalton's atomic theory is the result of law of conservation of mass given by Lavoisier?
Which part of Dalton's atomic theory came from the law of constant proportions given by Proust?
What is the scientific name of particles which make up matter?
Calculate the molecular masses of the following compounds:
(a) Methanol $$ CH_3OH $$
(b) Ethanol, $$ C_2H_5OH $$
Define 'molecular mass' of a substance.
What is meant by saying that ' the molecular mass of oxygen is $$ 32 $$ '?
Calculate the molecular masses of the following compounds :
(a) Methane , $$ CH_4 $$
(b) Ethane , $$ C_2H_6 $$
(c) Ethene , $$ C_2H_4 $$
(d) Ethyne , $$ C_2H_2 $$
(Atomic masses : $$ C =12u , H = 1u $$ )
The molecular formula of glucose is $$ C_6H_{12} O_6 . $$ Calculate its molecular mass ( Atomic mass : $$ C = 12 u : H = 1u ; O= 16u $$ )
Calculate the molecular mass of chloroform $$ (CHCl_3) $$.
(Atomic masses: $$ C = 12u ; H = 1u ; Cl = 35.5u $$ )
(a) Name the element used as a standard for atomic mass scale.
(b) Which particular atom of the above element is used for this purpose?
(c) What value has been given to the mass of this reference atom?
Calculate the molecular masses of the following :
(a) Hydrogen , $$ H_2 $$
(b) Oxygen , $$ O_2 $$
(c) Chlorine , $$ Cl_2 $$
(d) Ammonia , $$ NH_3 $$
(e) Carbon dioxide , $$ CO_2 $$
(Atomic masses : $$ C = 12 u ; H = 1u ; O = 16 u ; Cl = 35.5 u ; N = 14u $$ )
(a) What is an atom? How do atoms usually exist?
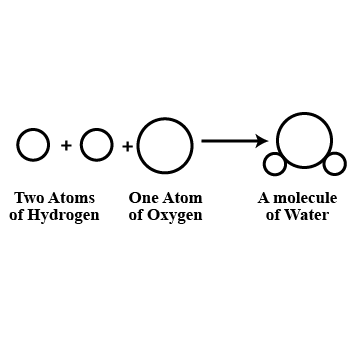
(b) What is a molecule? Explain with an example.
(c) What is the difference between the molecule of an element and the molecule of a compound? Give one example of each.
Give reasons for the following:
Atoms of most elements cannot exist independently, then how do these atoms form the matter that we can feel, see, or touch?
What is meant by molecular mass?
Give reasons for the following:
Give the difference between an atom and an ion.
Give the difference between an atom and an ion.
Calculate the molecular masses of the following compounds:
(a) Hydrogen sulphide, $$ H_2S $$
(b) Carbon disulphide, $$ CS_2 $$
(Atomic masses: H = 1u , S = 32u, C = 12u)
(a) Hydrogen sulphide, $$ H_2S $$
(b) Carbon disulphide, $$ CS_2 $$
(Atomic masses: H = 1u , S = 32u, C = 12u)
Calculate the molecular mass of hydrogen bromide $$ (HBr) $$ .
(Atomic masses: $$ H = 1u ; Br = 80u $$ )
State the law of constant proportions . Give one example to illustrate this law.
Why is atom considered as a neutral particle?
Explain giving a suitable example: Law of constant proportion.
Give the main postulates of Dalton's atomic theory.
Define the following term:
Avogadro's number
The chlorine atom is electrically neutral but chloride ion is charged . Explain .
Give the symbols for:
Beryllium, Barium, Radium, Sodium
Give the symbols for:
Two non-metals of group $$16$$
Name the smallest particle of an element that can retain all the chemical properties.
Size of the nucleus of an atom is _______ as compared to the size of the atom .
Find the ratio by mass of the combining elements in the following compound:
$$H_2SO_4$$
Find the ratio by mass of the combining elements in the following compound:
(I) $$Ca(OH)_2$$
Find the ratio by mass of the combining elements in the following compound:
(I) $$NH_3$$
Find the ratio by mass of the combining elements in the following compound:
$$C_2H_5OH$$
Find the ratio by mass of the combining elements in the following compound:
$$MgCl_2$$
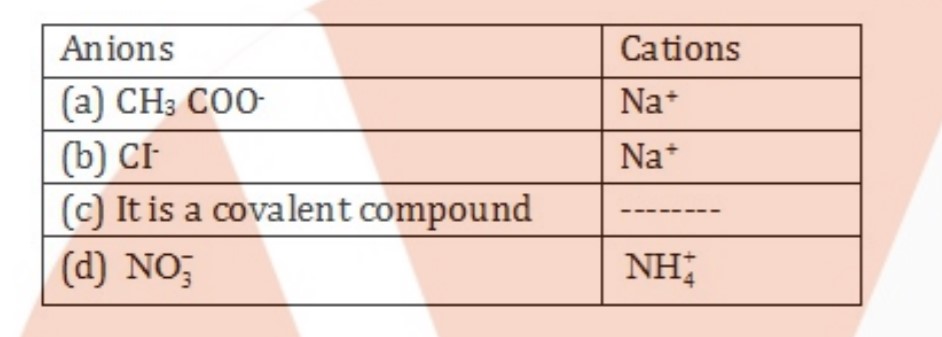
Write the cations and anions present(if any) in the following compounds:
(a) $$CH_3COONa$$
(b) $$NaCl$$
(c) $$H_2$$
(d) $$NH_4NO_3$$
Verify by calculating that $$240$$ g of calcium and $$240$$ g of magnesium have a mole ratio of $$3:5$$.
Find the ratio by mass of the combining elements in the following compound:
$$CaCO_3$$
Write any two observations which support the fact that atoms are divisible.
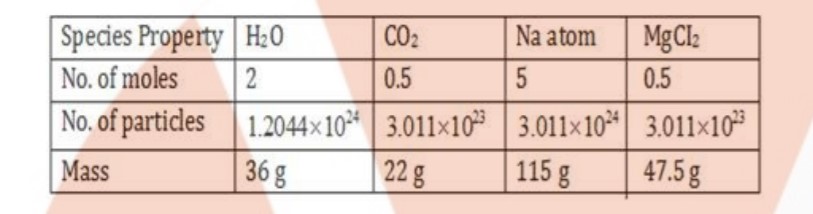
Fill in the missing data in Table.
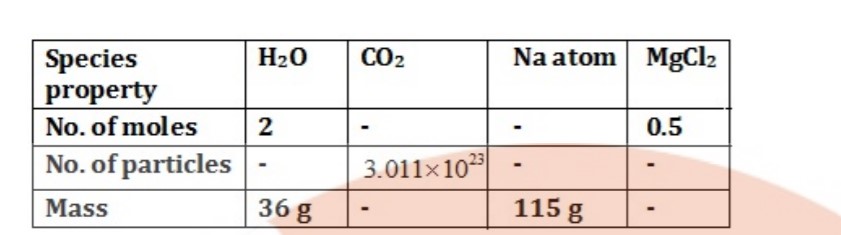
What is the symbol for SI unit of mole? How is the mole defined?
Name the scientists who discovered the following:
Atoms
The visible universe is estimated to contain $$10^{22}$$ stars. How many moles of stars are present in the visible universe?
What will be the mass of one atom of $$C-12$$ in grams?
State three characteristics of molecules of matter.
Write the chemical names of the following and also give their molecular formulae:
(a) Baking soda (b) Vinegar
(c) Marble (d) Sand
(c) Marble