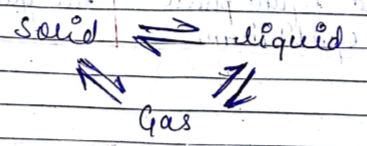Matter In Our Surroundings - Class 9 Chemistry - Extra Questions
Explain why evaporation leads to cooling of the liquid?
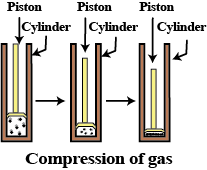
What happens when we apply pressure to the particles of the matter?
Water kept in sunlight gets heat from the sun and is evaporated. But how does water kept under the shade of a tree also get evaporated? Explain.
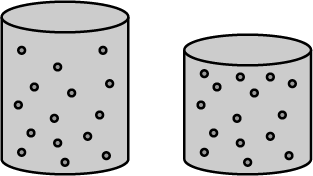
Compare the three states of matter i.e. solids, liquids, and gases with reference to inter-particle space.
State some physical properties of matter.
Name the phenomenon which causes the following change:
Formation of water vapour from water.
Fill in the blanks:Form of matter which has a definite volume but no definite shape is called a ___________.
State if the following is a physical property of a substance.
Hydrogen sulphide gas has a strong rotten egg-like odour.
Fill in the blank:
Liquids have a definite _______ .
Give scientific reasons.
A wet cloth is wrapped around water storage container in summer.
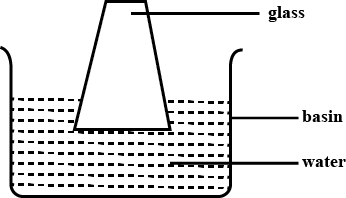
How can we describe the movement of the molecules in the figure?
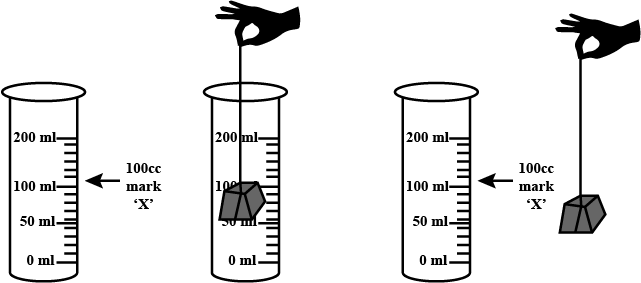
What produces more severe burns, boiling water or steam?
Give reason:
Icebergs float on ocean water.
Icebergs float on ocean water.
When a crystal of copper sulphate is placed at the bottom of a beaker containing water, the water slowly turns blue. Why?
What is the evaporation what are the factors effecting it.
Give reason for the following.
We need to classify things.
Explain what you understand from the diagram.

Why steam is used to run machines?
The molecules in solids and liquids are in contact with each other. Explain.
Define evaporation.
Why does a desert cooler cools better on a hot dry day?
Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
What type of clothes should we wear in summer?
For any substance, why does the temperature remain constant during the change of state?
How does the water kept in an earthen pot (matka) become cool during summer?
Arrange the following substances in increasing order of forces of attraction between the particles: water, sugar, oxygen.
What is the physical state of water at:
a. 250oC
b. 100oC
A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
What is the physical state of water at:
(a) 25∘C
(b) 0∘C
(c) 100∘C
Boiling water and steam both have the same temperature (1000C), but steam causes many severe burns than boiling water. Explain
(a) Tabulate the differences in the characteristics of states of matter.(b) Comment upon the following:
Rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Why is ice at 273 K more effective in cooling than water at the same temperature?
When water is boiled to 100oC, it converts into steam which on condensation gives back-water. Here, the heat given out is called ___________________ which is equal to the latent heat of vaporization.
How do you appreciate sweating mechanism of human body to control the temperature of the body?
A swimmer coming out of a pool is covered with a film of water weighing about 80g. How much heat must be supplied to evaporate this water?
The inter particle spaces are __________ in gaseous and ___________ in solids.
Explain why dogs pant during hot summer days using the concept of evaporation?
What is meant by intermolecular interaction ? What is its role in the states of matter ?
State the appropriate scientific reasons for the following statement:
The rate of evaporation decreases with increase in humidity.
What is evaporation? Explain four factors which affect the rate of evaporation.
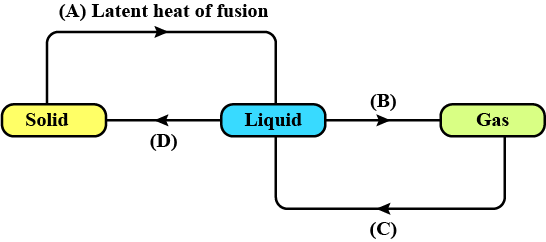
Define the latent heat of fusion.
Can matter change its state?
List down the characteristics of particles of matter.
Write the characteristic property of matter responsible for:(a) Smell of perfume spreads in the room.(b) Water takes the shape of the container in which it is kept
For any substance, why does the temperature remain constant during the change of state?
What are the factors affecting evaporation?
Name two gases which are supplied in compressed form in homes and hospitals.
A manometer is connected to a gas containing bulb. The open arm reads 43.7 cm whereas the arm connected to the bulb reads 15.6 cm. If the barometric pressure is 743 mm mercury. What is the pressure of gas in bar?
Even two or three crystals of potassium permanganate can impart colour to a very large volume of water. Which characteristic of particles of matter is illustrated by this observation?
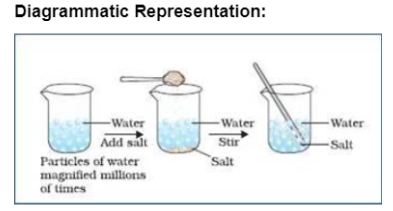
Describe in your own words, what happens to the particles when salt dissolves in water.
A piece of chalk can be broken into small particles by hammering but a piece of iron cannot be broken into small particles by hammering. Which characteristic of the particles of matter is illustrated by these observations?
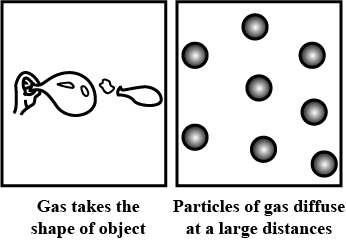
Why do gases have neither fixed shape nor a fixed volume?
Why can we easily move our hands in the air but do the same through a plank of wood, we need a karate expert? Explain.
What do you think matter is made up of small particles or not ?
What are the factors which affect the rate of evaporation ? Explain.
How is a melting point related to intermolecular forces of attraction?
Why water kept in an earthen pot during summers cools on its own?
Why do people in villages use earthen pots in summer to cool water?
Conversion of ice into water and water into ice is an example of change that can be reversed. Give four more examples where you can say that the changes can be reversed.
Paheli kept some water in a beaker for heating. She observed that tiny bubbles appeared before the water started to boil. She boiled the water for about 5 minutes and filled it in a bottle up to the brim and kept the bottle air tight till it cooled down to room temperature. Why did the tiny bubbles appear?
Water can be made to boil even at a temperature below its normal boiling point (100∘C). Explain.
Why do wet clothes placed on a clothes line get dry after some time? Explain.
Give one example in each case.
Change which occurs on heating but can be reversed.
A potter working on his wheel shaped a lump of clay into a pot. He then baked the pot in an oven. Do these two acts lead to the same kind of changes or not? Give your opinion and justify your answer.
Show experimentally that matter is made up of small particles.
Define Heat of Vaporization.
State the three effects of heat on matter.
The behavior of matter in different states is governed by various physical laws. According to you, what are the factors that determine the state of matter?
Give reason:
Liquids have definite volume but no definite shape.
Give reason:
When a teaspoon of sugar is added to half a glass of water and stirred, the water level in the glass remains unchanged.
Give reason:
When an empty gas jar is inverted over a gas jar containing a coloured gas, the gas also spreads into the empty jar.
At room temperature, the forces of attraction between the particles of solid substances are_________ than those which exist in the gaseous state.
Give reason:
A gas fills up the space available to it.
Conversion of solid state to liquid state is called fusion. What is meant by latent heat of fusion?
Give reason:
The odour of scent spreads in a room.
Paheli kept some water in a beaker for heating. She observed that tiny bubbles appeared before the water started to boil. She boiled the water for about 5 minutes and filled it in a bottle up to the brim and kept the bottle airtight till it cooled down to room temperature.
Do you think tiny bubbles will appear on heating the water taken out from the bottle? Justify your answer.
Fill in the blanks with the correct word/s from the bracket.Matter can change from one state to another by change in ___________ [temperature or pressure, temperature only].
[Enter 1 if answer is temperature or pressure else 0 if temperature only]
[Enter 1 if answer is temperature or pressure else 0 if temperature only]
Give a reason for the following.
Gases can be compressed easily.
Explain the term vaporization.
Fill in the blank:
Liquids have no definite _______ .
Fill in the blanks with the correct word/s from the bracket.
The space between atoms in molecules of solids is ___________ [minimum / maximum].
The space between atoms in molecules of solids is ___________ [minimum / maximum].
Fill in the blank:
The temperature at which a liquid starts to boil is called the _______ point of that liquid.
What was the ancient belief regarding the nature of matter?
Write your observation and conclusion for the following.
Ice is kept at room temperature.
What do you observe when a spoon of sugar is heated in a pan?
Name a chemical change which takes place in presence of heat.
Fill in the blanks:
The temperature at which a liquid starts changing into its vapour state is ______.
A certain quantity of water is heated from 20∘C to 100∘C. Its temperature is recorded after each 1 minute. The observations are:
| Time (in minute) | Temperature (∘C) |
| 0 | 20 |
| 1 | 30 |
| 2 | 40 |
| 3 | 50 |
| 4 | 60 |
| 5 | 70 |
| 6 | 80 |
| 7 | 90 |
| 8 | 100 |
| 9 | 100 |
| 10 | 100 |
| 11 | 100 |
| 12 | 100 |
What conclusion do you draw from the above table about the boiling point of water? Explain.
Fill in the blanks .
The temperature at which a solid converts into a liquid is called its ________.
State three properties of molecules of a matter .
Why does evaporation produce cooling?
Why are volatile liquids such as alcohol and spirit stored in tightly closed bottles ?
Wet clothes dry more quickly on a warm day than on a cold humid day. Explain
Water in a dish evaporates faster than in a bottle. Give reason.
Give reasons for the following.
Gases can be compressed easily.
Liquids can flow easily.
We need to classify things.
Pure substances have fixed melting or boiling points.
Electricity is not considered a matter.
Fill in the blanks:
When a gas is cooled, its molecules _____ energy.
Fill in the blanks:
The temperature at which a liquid boils is called the ______ point of that liquid.
Fill in the blank:
Any matter which has a definite ____ but no definite shape is called a ____.
A liquid can change into a vapor state
(a) at a fixed temperature, and
(b) at all temperature
Name the process involved in two cases.
Some ice is taken in a beaker and its temperature is recorded after each one minute . The observation are listed below
from the above observation what conlusion do you draw about the melting point of ice ?
Doctors advise putting strips of wet cloth on the forehead of a person having a high fever. Why?
A certain quantity of water is heated from 20∘C to 100∘C. Its temperature is recorded after each minute. The observations are: Time in minutes Temperature (∘C) 0 20 1 30 2 40 3 50 4 60 5 70 6 80 7 90 8 100 9 100 10 100 11 100
What conclusion do you draw from the above table about the boiling point of water? Explain.
| Time in minutes | Temperature (∘C) |
| 0 | 20 |
| 1 | 30 |
| 2 | 40 |
| 3 | 50 |
| 4 | 60 |
| 5 | 70 |
| 6 | 80 |
| 7 | 90 |
| 8 | 100 |
| 9 | 100 |
| 10 | 100 |
| 11 | 100 |