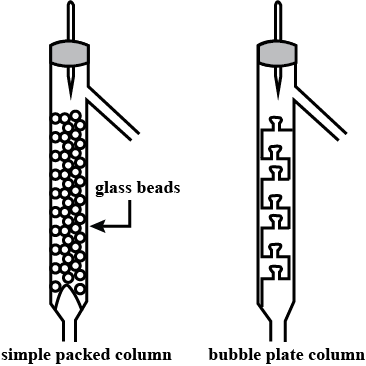
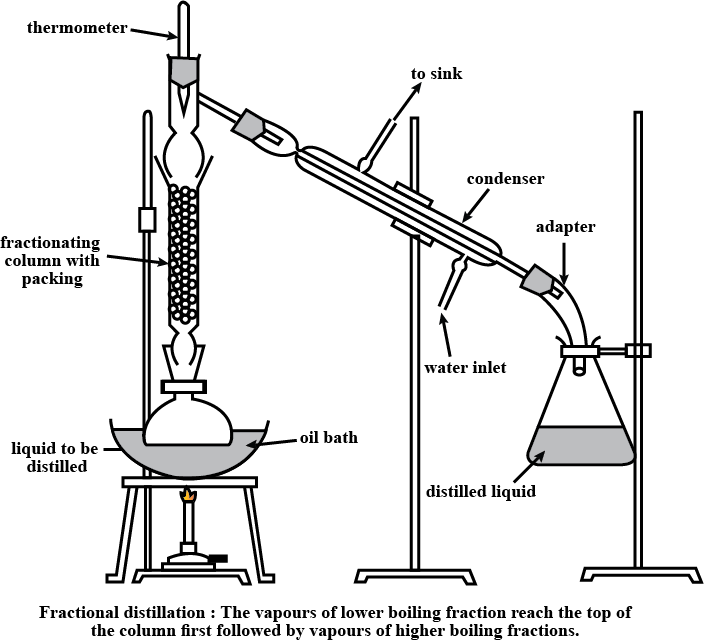
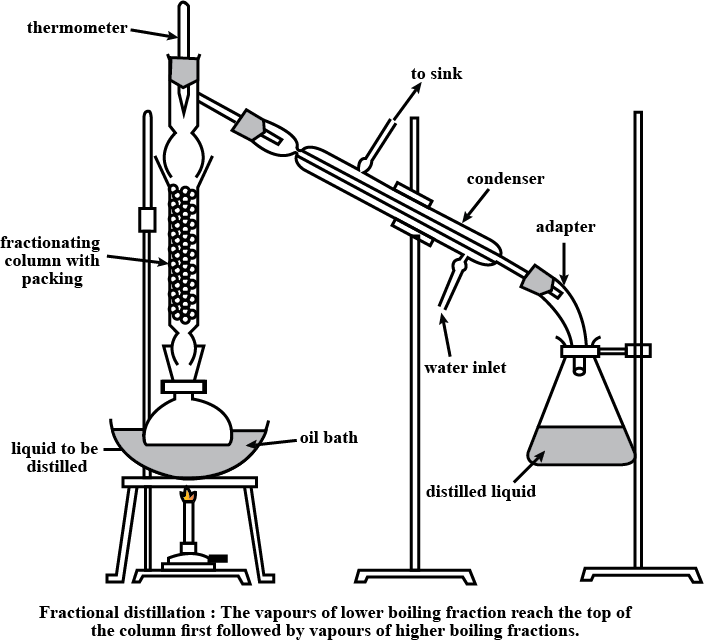
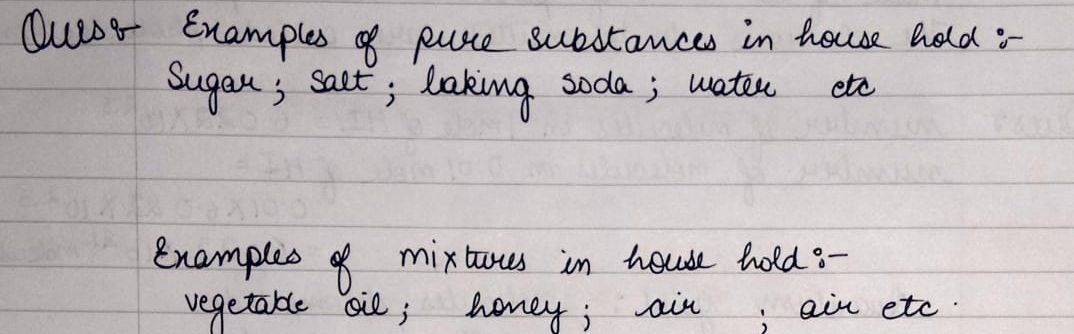Is Matter Around Us Pure - Class 9 Chemistry - Extra Questions
$$H_2O$$ contains hydrogen and oxygen but it is a pure substance. How?
What are the components of solution?
Define a compound.
Name the liquid which evaporates and then condenses during the process of distillation.
Cloud is a colloidal suspension of water droplets in air.
If true then enter 1, else enter 0.
Cloud is a colloidal suspension of water droplets in air.
If true then enter 1, else enter 0.
State two ways in which you can separate a mixture of sand and sugar.
Smoke is an aerosol and a colloidal solution of carbon in air.
If true then enter 1, else enter 0.
Two or more elements combine to form ...............
Table salt is made from sodium and chlorine which can be poisonous. Still we can eat table salt without getting poisoned. Why?
How will you obtain $$NH_4Cl$$ from a mixture of $$NH_4Cl$$ and $$KCl$$?
"The properties of the product are different from those of the constituents". State whether this statement best describes an element, a compound or a mixture.
What are pure substances? Give two example of pure substances.
How will you separate a mixture of common salt, chalk powder and powdered camphor? Explain.
What are immiscible liquids?
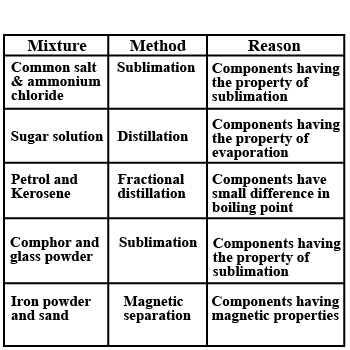
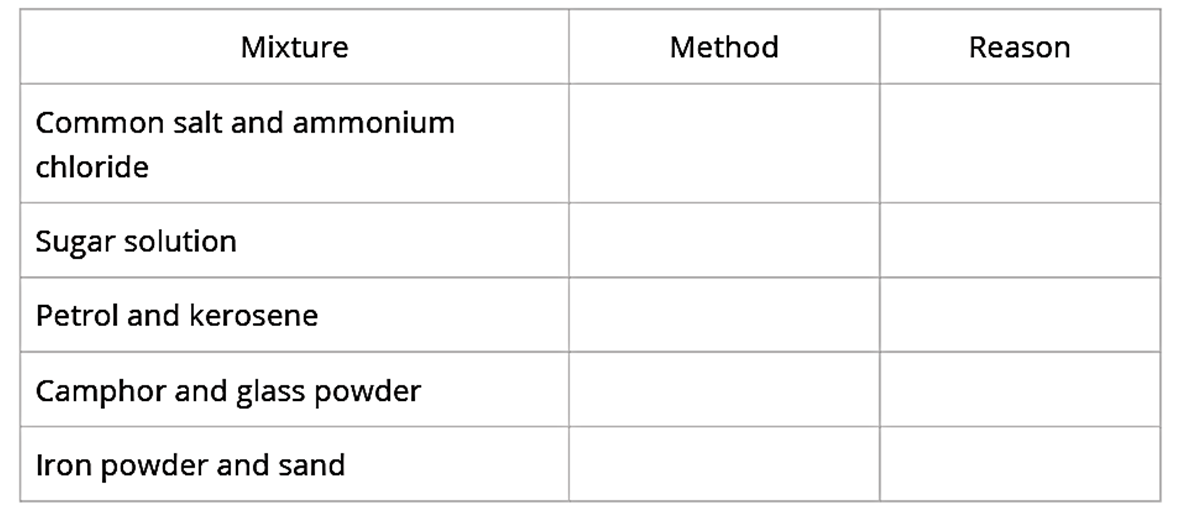
A few mixtures are given above. Tabulate the methods to separate their components and give the reasons for selecting the method.
How will you purify a liquid having non-volatile impurities?
Suggest methods for the separation of the following mixtures.
A mixture of liquid $$A\ (b.p-365\,K)$$ and liquid $$B\ (b.p-356\,K).$$
Classify the following substances into elements, compounds and mixtures:
(i) Milk (ii) 22-carat gold (iii) iodized table salt (iv) Diamond (v) Smoke (vi) Steel (vii) Brass (viii) Dry ice (ix) Mercury (x) Air (xi) Aerated drinks (xii) Glucose (xiii) Pertol/Diesel/Kerosene oil (xiv) Steam (xv) Cloud
Suggest a method to purify camphor containing traces of common salt.
A solution is prepared by dissolving certain amount of solute in 500 g of water. The percentage by mass of solute in solution is 2.38. Calculate mass of solute.
A solution that has relatively less amount of solute with respect to the solvent is called a ________ solution.
Ammonium chloride can be separated from common salt by the method of ................
If true enter $$1$$, else enter $$0$$.
A standard solution is one whose concentration is not known.
Why is air considered as a mixture?
What do you mean by:
(a) Molecule of an element
(b) Molecule of a compound, with examples
Can their be possibility of solid solutions?
The major component of steel is ________.
Define heterogeneous mixtures and write its types.
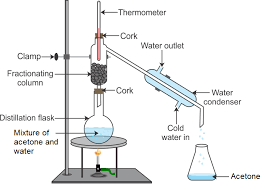
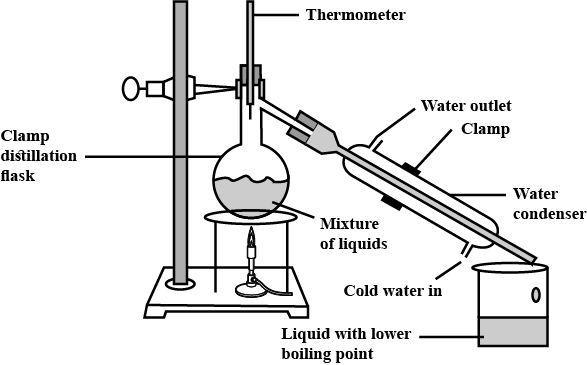
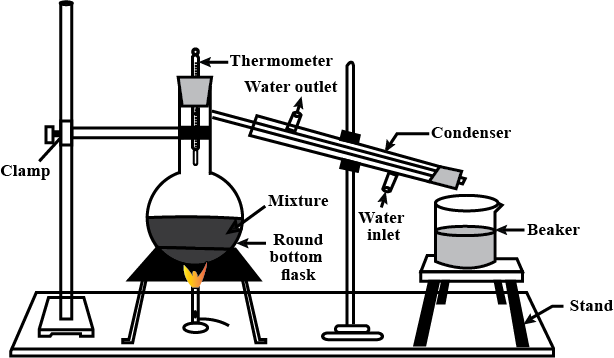
What is distillation?
Blood is an example of ________.
Water is good solvent for ionic compounds.
Give example of following type of Colloid.
Emulsion
Give example of following type of Colloid.
Solid sol
Give example of following type of Colloid.
Gel
The process of settling of sand, when a mixture of sand and water is left undisturbed for some time is known as ______________.
Suggest a suitable method to separate the components of the mixture of two volatile components with an appreciable difference in boiling points.
Under which category of mixtures will you classify alloys and why?
________ (Matter/Atom) can be classified chemically into pure substances and mixtures.
A mixture of iodine and sand are separated by method of _______ . (filtration/sublimation)
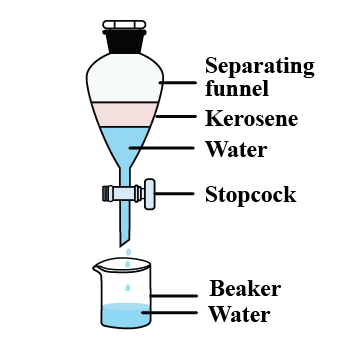
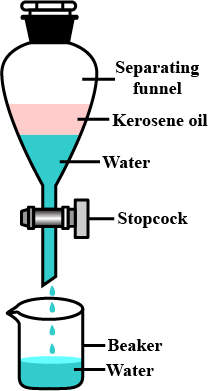
A mixture of oil and water can be separated by using ______ (separating/filtration) funnel.
How is 'chromatography' applied in the separation of coloured constituents present in a mixture of ink?
Define crystallization.
What would you observe when crystals of copper (II) sulphate and iron (II) sulphate are separately heated in two test tubes?
Explain the method of crystallization.
Blood is a _________.
true solution
colloid
suspension
(Give your answer using respective number)
Water is purified for scientific purposes by distillation. Describe the process.
Why is water called an universal solvent?
Explain the following giving examples.
$$(a)$$ Saturated solution
$$(b)$$ Pure substance
$$(c)$$ Colloid
$$(d)$$ Suspension
Classify each of the following as a homogeneous or heterogeneous mixture:
Soda water, wood, air, soil, vinegar, filtered tea.
Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Which of the following materials fall in the category of a pure substance?
$$(a)$$ Ice
$$(b)$$ Milk
$$(c)$$ Iron
$$(d)$$ Hydrochloric acid
$$(e)$$ Calcium oxide
$$(f)$$ Mercury
$$(g)$$ Brick
$$(h)$$ Wood
$$(i)$$ Air
Identify the solutions among the following mixtures.
$$(a)$$ Soil
$$(b)$$ Seawater
$$(c)$$ Air
$$(d)$$ Coal
$$(e)$$ Soda water
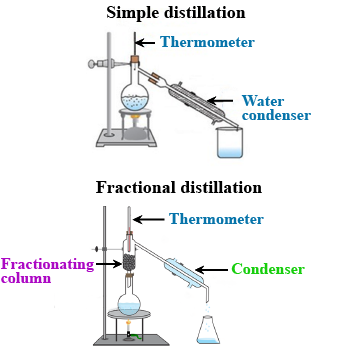
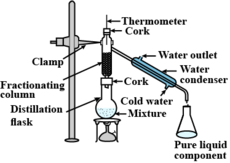
What is the difference between distillation and fractional distillation?
True or False.The properties of compounds are the same as those of the constituent elements.
Which of the following is likely to be a pure substance?
A) A colourless liquid that boils over the range 70$$^o$$C to 80$$^o$$C.
B) A green solid which starts to melt to 80$$^o$$C and is completely melted at 90$$^o$$C.
C) A white solid which produces a chromatogram consisting of only one spot.
D) A brown liquid that is completely miscible with water.
State True or False.Distilled water cannot be separated into its constituents by physical methods.
Is air considered a solution? Explain.
Match the examples with suitable separation process:
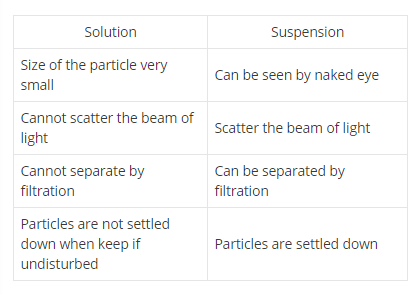
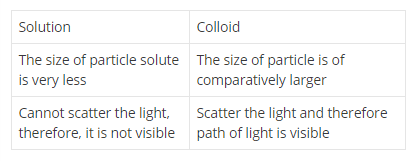
Distinguish between true solution and colloidal solution.
State differences between aerosol and foam?
Can we separate the compounds of an azeotropic mixture by fractional distillation? Explain
In centrifuge how are particles of different densities separated?
Differentiate true solution and colloidal solution.
Colloidal solution of solid in liquid is called ________.
What type of colloid is formed when a gas is dispersed in a liquid? Give an example.
Answer in one word or one sentence.
Ornamental gold-containing copper is an example of what type of solution?
Ornamental gold-containing copper is an example of what type of solution?
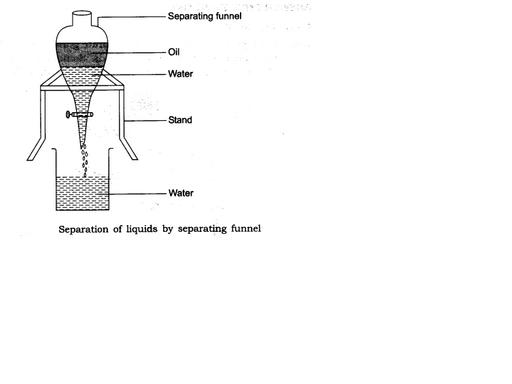
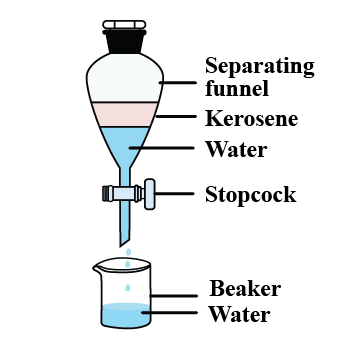
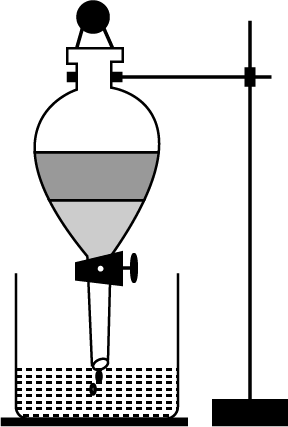
How will you separate oil and water from their mixture?
How much copper sulphate will be required to saturate 100 g of a dilute aqueous solution of $$CuSO_4$$ at $$25^oC$$ if 10 g of the dilute solution leave on evaporation and drying 1.2 g of anhydrous $$CuSO_4$$ in water at $$25^oC$$ is 25.
Define the following terms:(i) Solution(ii) Solute
What is meant by a pure substance? Give examples.
State the properties of solution.
Define elements, compounds and molecules.
Give a reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several meters away.
Name the method you will use to separate two immiscible liquids.
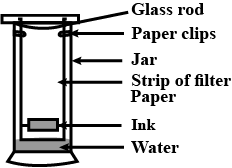
What do you observe on the filter paper on which a drop of ink is placed, and as the water rises on it?
Give two examples of homogeneous mixture.
Differentiate between homogeneous and heterogeneous mixtures with examples.
In one sample of brass, the following components were found: Copper $$(70\%)$$ and zinc $$(30\%)$$. Identify the solvent, solute, and solution from these.
Answer in one word/ a few words.
A pure substance formed when atoms or molecules of different elements combine.
A pure substance formed when atoms or molecules of different elements combine.
What kind of mixture is gunpowder?
How are sol, solution and suspension different from each other?
Define the term 'compound'?
Answer in one word/ a few words
The simplest form of a pure substance made up of only one kind of atom -_______________.
What do you mean by Solution, Solvent, Solute and Concentration.
Explain the meaning of the term matter with special reference to the term 'substance'.
Answer in one word/ a few words.
A homogeneous mixture of solvent and solute.
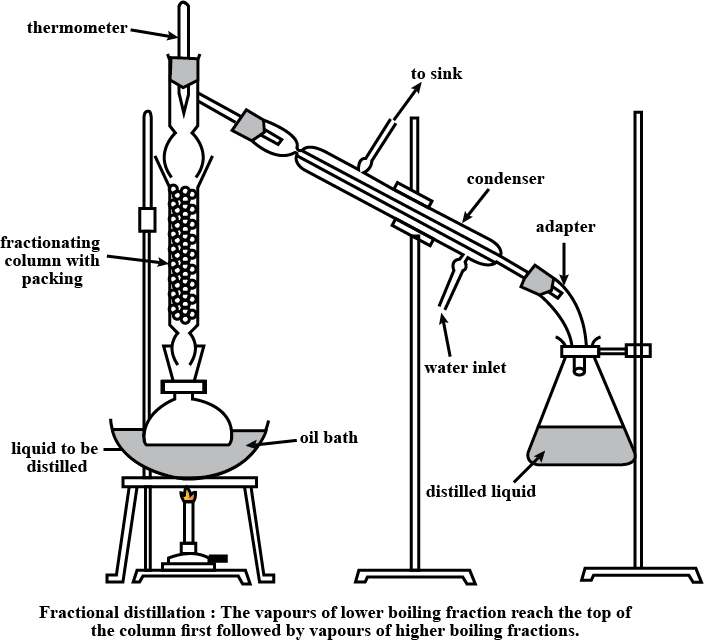
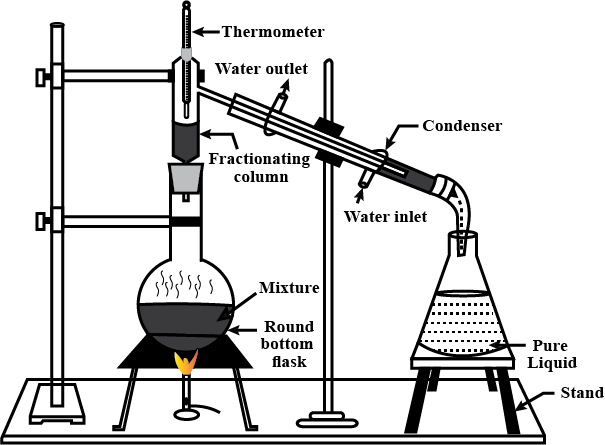
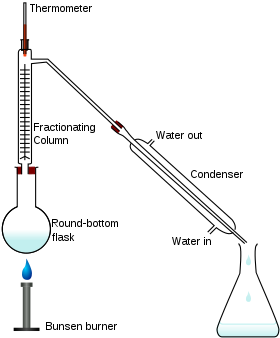
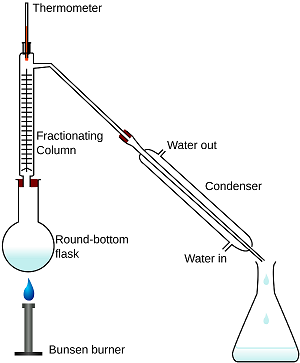
What is fractional distillation? Explain.
Name a kind of substance that is a combination of different elements.
The mass of $$10\%$$ glucose solution is $$200\ g$$. Find the masses of the solute and the solvent used to prepare the solution.
What is meant by a substance?
Give a brief description of crystallization with an illustration.
Write one example of heterogeneous mixture used in daily life situations.
Why is milk regarded as a mixture and not as a pure substance?
Write three difference between colloidal solution, solution and suspension.
What is the principle of fractional distillation?
Differenciate beetween Homogeneous and heterogeneous mixture with example :
When the stopper of a bottle containing colorless solution was removed, the bottle gave out a smell like that of vinegar. Identify the solution. What is the nature of the solution?
List any two properties of a colloid.
Which type of mixtures are separated using distillation method? If the difference in boiling points of constituents is less than $$25K$$ then which method will you adopt? Give an example where this method is used for separation.
List out the items in your house which comes under pure substances and mixtures ?
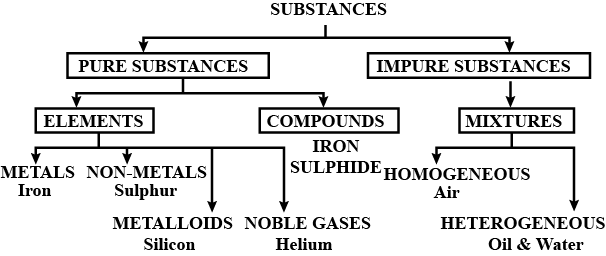
Define a pure substance. How many types of pure substance do you know?
Two volatile and miscible liquids can be separated by fractional distillation into pure component', is true under what conditions?
Write the steps you would use for making tea. Use the words, solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
What is solute?
Tyndall effect is exhibited by ________.
Write the difference between molecule and compound.
State three reasons why do you think air is a mixture and water is a compound?
What is major difference between a solution and an ordinary mixture ?
What are the two types of pure substances? Give one example of each type.
Name one element, one compound and one mixture.
Which of the following are 'Pure substances'?Ice, Milk, Iron, Hydrochloric acid, Calcium oxide, Mercury, Brick, Wood, Air
List five characteristics by which compounds can be distinguished from mixtures.
Choose the solution form among the following mixtures:Soil, Sea-water, Air, Coal, Soda-water
State two reasons for supporting that brass is a mixture and not a compound.
Which of the following are not compounds?
Chlorine gas, Potassium chloride, Iron powder, Iron Sulphide, Aluminium foil, Iodine vapour, Graphite, Carbon monoxide, Sulphur powder, Diamond
Chlorine gas, Potassium chloride, Iron powder, Iron Sulphide, Aluminium foil, Iodine vapour, Graphite, Carbon monoxide, Sulphur powder, Diamond
Give two reasons supporting that water is a compound and not a mixture.
Define a compound. Give two points of evidence to show that sodium chloride is a compound .
Draw a labelled diagram showing fractional distillation.
Give two characteristics each of:
(a) Pure substances
(b) Mixtures
Is ink a pure substance or mixture? Give reason.
Identify colloid from the following mixtures:
Muddy water, sugar in water, ink, blood, soda water, foam
Name the two components of a colloid.
Explain the following giving examples:
(a) Saturated solution
(b) Unsaturated solution
(c) Suspension.
How will you separate a mixture containing benzene and diesel (difference in their boiling points is more than $$50^oC$$), which are miscible with each other?
Define (a) solute (b) solvent.
Fractional distillation method is used for the separation of different gases from air. Why is it so?
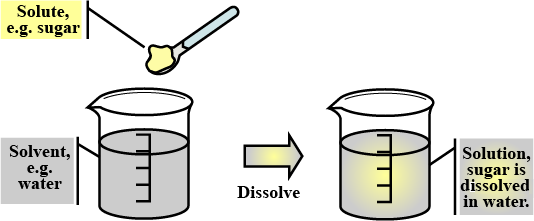
Define solution.
Show diagrammatically, how will you separate two immiscible liquids?
When blue ink is heated, what do you think has got evaporated from the watch glass ?
Give an example of solution containing a solid solute in a solid solvent.
State the basic difference between simple and fractional distillation.
Write one property of colloids.
Give one example of the following:
Aerosol
You are provided with soda water, milk, and muddy water. How can you differentiate between them in terms of:
(i) Homogeneity(ii) Filtration(iii)Tyndall effect
Give one example of the following:
Solution
Suggest separation technique(s) one would need to employ to separate Mercury and water.
A mixture of chloroform and water taken in a separating funnel is mixed and left undisturbed for some time. The upper layer in the separating funnel will be of ________ and the lower layer will be that of ________.
Which of the tubes in the figure will be more effective as a condenser in the distillation apparatus?

Suggest a suitable method to separate the components of a mixture containing two or more coloured constituents soluble in some solvent.
Suggest separation technique(s) one would need to employ to separate Kerosene oil, water and salt.
Give an example each for the mixture having the following characteristics. Suggest a suitable method to separate the components of these mixtures: A volatile and a non-volatile component.
Give an example of a mixture having two volatile components with an appreciable difference in boiling points. Suggest a suitable method to separate the components of this mixture.
Give an example of a mixture containing two immiscible liquids. Suggest a suitable method to separate the components of this mixture.
What would you observe when an aqueous sugar solution is heated to dryness?
Give two examples for each of the following :
(a) Metals
(b) Non-metals
(c) Metalloids
(d) Noble gases
State the information obtained from the formula of a compound.
Name two compounds which dissolve in water.
A solution is always a liquid. Comment.
A mixture of two or more miscible liquids, for which the difference in the boiling points is less than $$25 K$$ can be separated by the process called ________.
FIll in the blanks
A metal which is a liquid at room temperature is __________.
Fractional distillation is suitable for separation of miscible liquids with a boiling point difference of about $$25 K$$ or less. Explain how it improves the efficiency of distillation.
A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in the figure. The filter paper was removed when the water moved near the top of the filter paper. What would you expect to see, if the ink contains three different coloured components?
Define a pure substance. How many types of pure substances do you know?
State four important characteristics of compounds.
Write the molecular formulae of compounds calcium oxide, hydrogen sulphide, carbon monoxide and lead sulphide.
State two characteristics of water which prove that it is a compound.
(a) Why do sugar and water retain their individual properties in a sugar solution?
(b) Why do petrol and water form a heterogeneous mixture?
(c) Why sulphur does dissolve when carbon disulphide is added to a mixture of iron and sulphur but not when it is added to iron sulfide?
(b) Why do petrol and water form a heterogeneous mixture?
(c) Why sulphur does dissolve when carbon disulphide is added to a mixture of iron and sulphur but not when it is added to iron sulfide?
Define the following:
(a) Pure substance
(b) Impure substance
From the list given below select the correct substance which is most suitable for the statements given: (oxygen, diamond, zinc, graphite, gold)
A metal that is brittle.
Which non-metal is a good conductor of electricity?
The hardest naturally occurring substance.
The most ductile metal.
A gaseous non-metal.
Select homogeneous and heterogeneous mixtures from the following:
The salt solution, petrol and water, sand and charcoal, alcohol and water, air dissolved in water, air, seawater, fruit juices, mist, brass.
The salt solution, petrol and water, sand and charcoal, alcohol and water, air dissolved in water, air, seawater, fruit juices, mist, brass.
Which of the following options is correct for the given statement:
All pure substances have
the same physical state
the same colour
the same composition
a definite set of properties
Complete the statement - an element is a pure substance made up of (identical/different) atoms.
Classify substances into pure and impure substances in the form of a chart or tabulation.
Select the correct answer from the choice in brackets.
A compound [nitrogen / ozone/ zinc chloride]
Why do we need pure substances?
State the technique involved in separating the following:
(a) Iodine crystals and potassium chloride
(b) Iron and chalk powder
(c) Potassium chloride from an aqueous solution of potassium chloride
(d) Rice powder from soil particles
(e) Iron filings from sand
(f) Large diamonds from very small diamonds
The process of separation of liquid from solid, by removing the liquid layer at the top from the layer of settled solid below is known as ___________.
Define: Miscible liquids
Define solvent.
____________ is a process to obtain a very pure form of a solid dissolved in a liquid.
__________ and ___________ are pure substances.
Define solute.
How is distillation more advantageous than evaporation?
A pure substance has definite __________ and constant _________ .
A ________ substance has only one kind of matter.
The method used to separate miscible liquids that vary greatly in their boiling points is _______.
An _________ is a homogeneous mixture of two or more metals.
The composition and properties of a __________ mixture is uniform throughout.
Chalk powder dissolved in water is an example of a __________.
Fill in the blanks:
A _________ is a uniform mixture of a solute and a solvent.
Immiscible liquids are separated by using a _________.
Differentiate between solution and suspension.
Name the liquid or medium of dissolution which allows the solute to dissolve in it.
Give a reason why water is considered a 'universal solvent' but an alkali is not.
Define the term 'solution' with reference to addition of sodium chloride to water.
Define the term solvent with reference to addition of sodium chloride to water.
Why is iron sulphide a compound?
Define the term solute regarding the addition of sodium chloride to water.
How is distillation method different from evaporation?
Classify the following substances into compounds and mixtures: Carbon dioxide, air, water, milk, common salt, blood, fruit juice, and iron sulphide.
Give one example for each of the following types of mixtures:
(a) solid-solid homogeneous mixture
(b) heterogeneous mixture
(c) miscible liquids
(d) liquid-gas homogeneous mixture
Name the substance you will add to speed up sedimentation.
Explain the term solute.
Fill in the blank.
Chalk powder dissolved in water is an example of a ______ .
Fill in the blank.
An ____ is a homogeneous mixture of two or more metals.
Fill in the blank.
Immiscible liquids are separated by using a _________ .
Explain the term solvent.
Define a pure substance.
Explain the term solution.
Give two examples of colloid.
Give two examples of solvent other than water.
Differentiate between solution and suspension.
Explain why Fused $$CaCl_2$$ or conc.$$H_2SO_4$$ is used in a desiccator.
Differentiate between suspension and colloid.
Answer the following question.
In one sample of brass, the following ingredients were found: copper $$(70\%)$$ and zinc $$(30\%)$$ Identify the solvent, solute, and solution from these.
In one sample of brass, the following ingredients were found: copper $$(70\%)$$ and zinc $$(30\%)$$ Identify the solvent, solute, and solution from these.