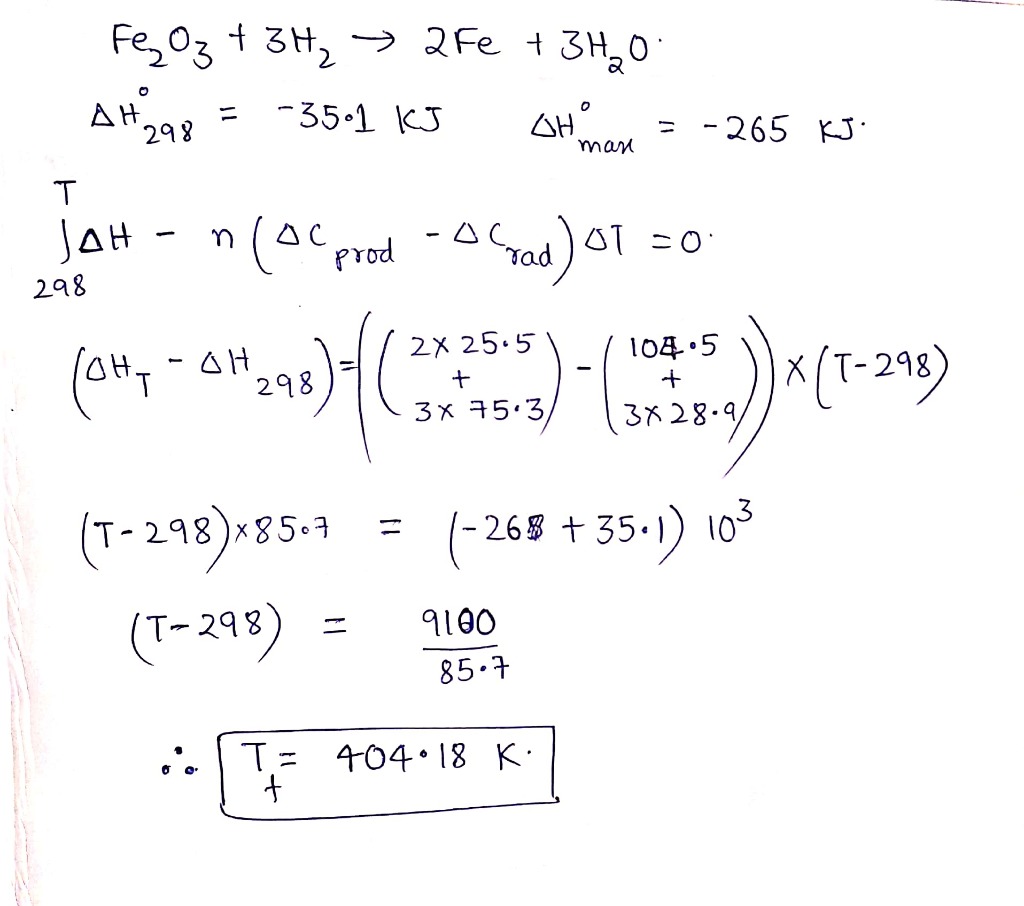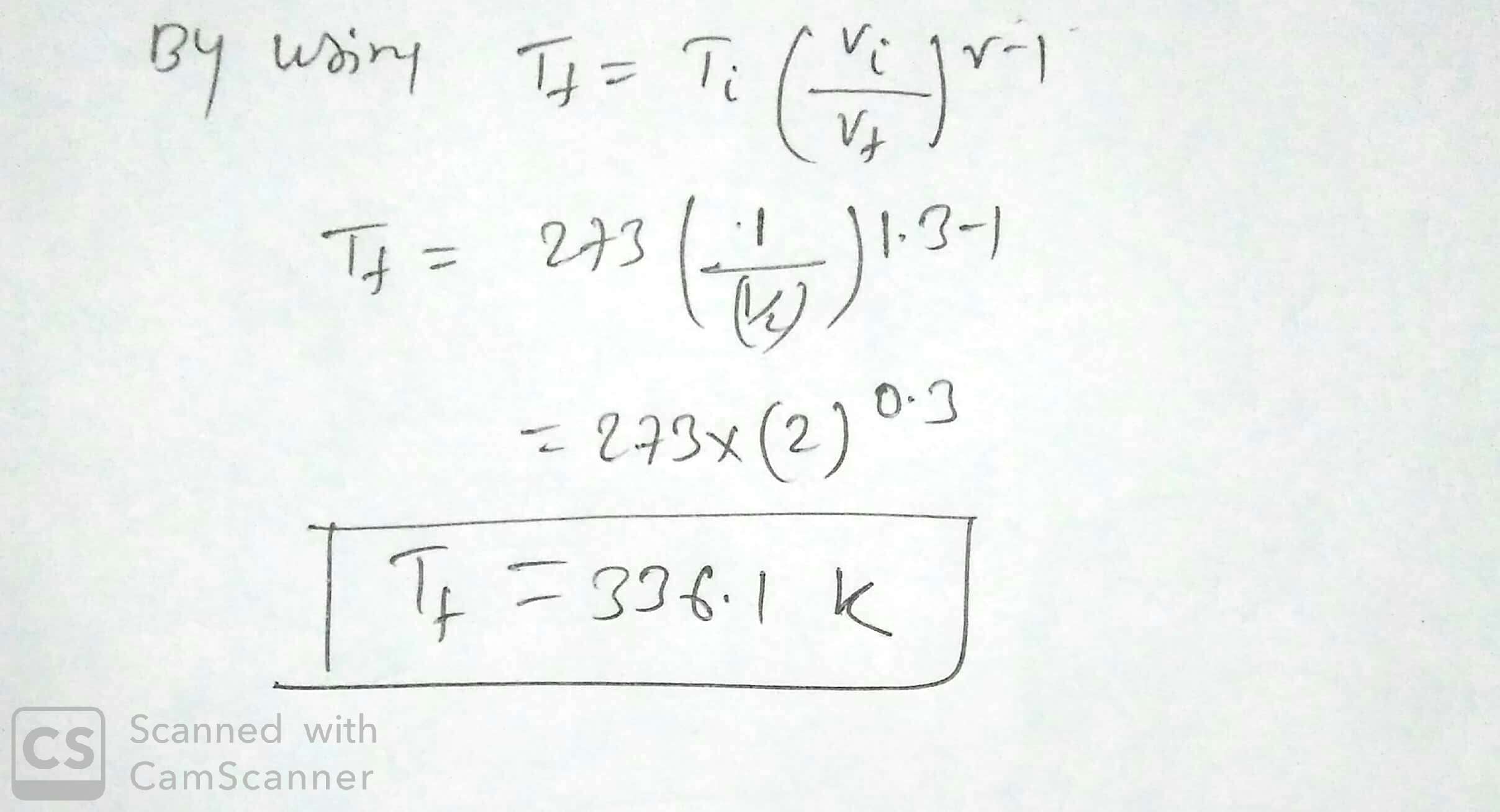Kinetic Theory - Class 11 Engineering Physics - Extra Questions
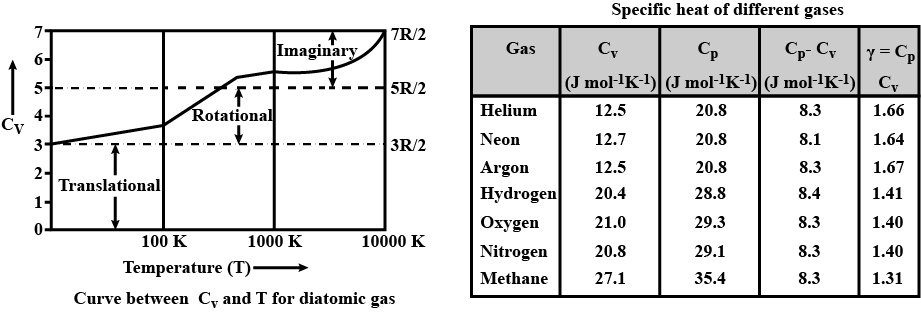
The heat capacity of a diatomic gas is higher than that of a mono-atomic gas.If true enter 1 else 0
Molecules of a gas has -------------- intermolecular spaces. (maximum / minimum)
In the graph shown, U is the internal energy of gas and $$\rho$$ the density. Corresponding to given graph match the following two columns
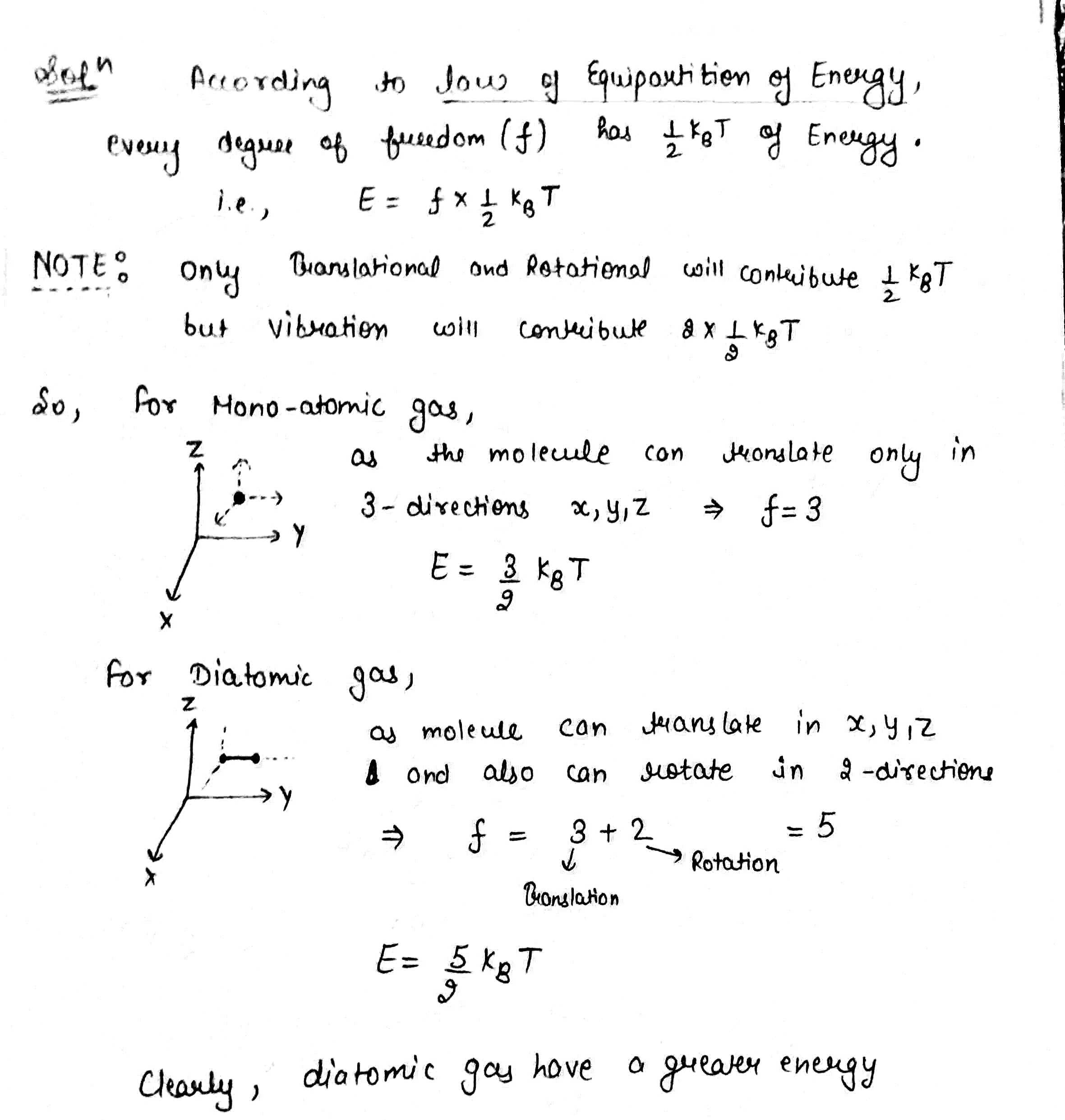
State the law of equipartition of energy.
When the hydrogen atom in going from $$n=5$$ to $$n=1$$ state, its recoil speed is nearly
$$C_p - C_v$$ for an ideal gas is ______.
What is the name of h & what is the value?
The value of rotational K.E. at temperature T of one gram molecules of a diatomic gas will be-
Calculate the average molecular kinetic energy:
Per kilogram, of oxygen at $$27^{o}C$$
($$R=8320J/ Kmol K$$, Avogadro's number $$=6.03\times 10^{26}m$$ molecules /$$K mole$$)
What is the kinetic energy of an object?
What is the average translational kinetic energy of a molecule of an ideal gas at temperature of $$27^{\circ}C$$
The average speed of all the molecules in a gas at a given instant is zero, whereas the average velocity of all the molecules is zero. Explain why?
What is the average kinetic energy per atom of each gas ?
If $$x\, \times\, 10^{-2}\,$$ moles of helium at temperature 300 K and 1.00 atm pressure are needed to make the internal energy of the gas 100 J. Find $$x$$ (Round off to the nearest integer)
A sample of helium gas is at a temperature of 300 K and a pressure of 0.5 atm. What is the average kinetic energy of a molecule of a gas?
For any distribution of speeds $$V_{rms}\, \geq\, V_{av}$$. Is this statement true or false?
A $$2.00\ mL$$ volume container contains $$50\ mg$$ of gas at a pressure of $$100\ kPa$$. The mass of each gas particle is $$8.0\, \times\, 10^{-26}\ kg$$. Find the average translational kinetic energy of each particle.
Match the followung two columns for 2 moles of an ideal diatomic gas at room temperature T.
Match following
Match following
Heat capacity of a diatomic gas is higher than that of monoatomic gas.
If true enter 1, else enter 0.
The ratio of average translational kinetic energy to rotational kinetic energy of a diatomic molecule at temperature T is x/y.
then value of x+y is...........
The ratio of a specific heats of a gas is 9/7, then the number of degrees of freedom of the gas molecules for translation motion is:
Calculate the kinetic energy of $$10$$ gram of Argon molecules at $$127^\circ C$$.
[Universal gas constant $$R=8320{ J }/{ }\ mol\ K$$. Atomic weight of Argon $$=40kg/ mol$$]
Calculate the average molecular kinetic energy :
(a) per kilomole, (b) per kilogram, of oxygen at $$27^\circ C$$.
($$R=8320{ J }/{ K }moleK$$, Avogadro's number $$=6.03\times { 10 }^{ 26 }{ molecules }/{ K }mole$$)
The specific heat at constant volume for a gas is $$0.075\ cal/g$$ and at constant pressure it is $$0.125\ cal/g$$. Calculate:
(i) the molecular weight of gas,
(ii) atomicity of gas
Specific heat of a monoatomic gas at constant volume is $$315\ J\ kg^{-1} K^{-1}$$ and at a constant pressure is $$525\ J\ kg^{-1} K^{-1}$$. Calculate the molar mass of the gas.
If the average distance between collisions of nitrogen molecules at $$NTP$$ is $$9.44 \times {10}^{-6}\ cm$$, what is the time between collisions?
Given $$R=8.31 \times {10}^{3}\ J/k\ mol\ K$$
Explain the inter-conversion of the three states of matter in terms of force attraction and K.E of the molecules.
State the applications of compressibility of gases in our day-today life.
Find the average kinetic energy per molecule at temperature $$T$$ for an equimolar mixture of two ideal gases $$A$$ and $$B$$ where $$A$$ is monoatomic and $$B$$ is diatomic.
The average translational kinetic energy of air molecules is $$0.040eV$$ ($$1eV=1.6\times {10}^{-19}J$$). Calculate the temperature of the air. Boltzman's constant $$k=1.38\times {10}^{-23}J{K}^{-1}$$
Find the number of degrees of freedom of molecules in a gas if its heat capacities are $$ C_v = 650 \ J Kg^{-1} K^{-1} $$ and $$C_p = 910 \ J Kg^{-1} K^{-1}$$.
$$0.040$$ $$g$$ of He is kept in a closed container initially at $$100.0C$$. The container is now heated. Neglecting the expansion of the container, calculate the temperature at which the internal energy is increased by $$12$$ $$J$$. [ R = $$\dfrac {25}{3}$$ j - $$moi^{-1}$$ - $$k^{-1}$$]
Calculate the max. work done by system in an irreversible (single step) adiabatic expression of 1 mole of a polyatomic gas from 300K pressure 10 atm to 1 atm.($$\gamma$$ = 1.33)
For a gas, $$\gamma=9/7$$. What is the number of degrees of freedom of the molecules of the gas?
The kinetic energy of $$1\ kg$$ of oxygen at $$300\ K$$ is $$1.356 \times 10^{6}\ J$$. Find the kinetic energy of $$4\ kg$$ of oxygen at $$400\ K$$.
Volume of vessel A is twice the volume of vessel B. Both are filled with the same gas. If the gas in A is at twice the temperature and twice the pressure to the gas in B, what is the ratio of gas molecules in A and B?
State law of equipartition of energy. Use this law of equipqrtition to calculate specific heats of monoatomic , diatomic and triatomic gases.
A gas of molecular weight $$40$$ has a specific heat $$0.075\ cal/g/deg$$ at a constant volume. What is the $$C_v$$ value for it and what is the atomicity of the gas?
The molar heat capacities of water , $$ H_{2 (g)} $$ and $$ O_{2(g)} $$ are $$ 18 , 6.76 $$ and $$ 6.62 \,cal $$ respectively . the heat of formation of water at $$ 25^{\circ} \,C $$ is $$ -68.4\,kcal $$ . Calculate its heat of fomation at $$ 90^{\circ} \,C $$ .
An ideal gas undergoes isothermal compression from an initial volume of $$4.00\ m^3$$ to a final volume of $$3.00\ m^3$$. There is $$3.50$$ mol of the gas, and its temperature is $$10.0^oC$$.
How much energy is transferred as heat between the gas and its environment?
One way to keep the contents of a garage from becoming too cold on a night when a severe subfreezing temperature is forecast is to put a tub of water in the garage. If the mass of the water is $$125\ kg$$ and its initial temperature is $$20^oC$$,
how much energy must the water transfer to its surroundings in order to freeze completely.
Specific heat of $$Li(s), \ Na(s), \ K(s), \ Rb(s)$$ and $$Cs(s)$$ at $$398\ K$$ are $$3.57,\ 1.23,\ 0.756, \ 0.363$$ and $$0.243\ Jg^{-1}K^{-1}$$ respectively. Compute the molar heat capacity of these elements and identify any periodic trend. If there is trend, use it to predict the molar heat capacity of $$Fr$$.
For the reduction of ferric oxide by hydrogen,
$$Fe_{2}O_{3(s)} + 3H_{2(g)} \rightarrow 2Fe_{(s)} + 3H_{2}O_{(l)}$$; $$\Delta H^o_{298}= -35.1\ kJ$$.
The reaction was found to be too exothermic to be convenient. It is desirable that $$\Delta H^o$$ should be at most $$-26\ kJ$$. At what temperature it is possible.
$$C_{P\ Fe_{2} O_{3}}=104.5$$, $$C_{p\ Fe(s)} = 25.5$$, $$C_{p\ H_{2}O_{(|)}} = 75.3$$,
$$C_{p \ H_{2(g)}} =28.9$$ (All in $$J/mol^{-1}$$ )
1 g of arsenic dissolved in 86 g of benzene brings down the freezing point to $$ 5.31 ^0C $$ from $$ 5.50 ^0 C $$. If $$ K_f $$ of benzene is $$ 4.9 \frac { ^0C}{m} $$, the atomicity of the molecule is : ( Atomic mass of As = 75 )
The heat of reaction for, $$C_{10}H_{8(s)} + 12O_{2(g)} \rightarrow 10CO_{2(g)} + 4H_{2}O_{(1)} $$ at constant volume is $$- 1228.2\ kcal$$ at $$25^oC$$. Calculate the heat of reaction at constant pressure at $$25^oC.$$
Suppose $$12.0\ g$$ of oxygen $$(O_2)$$ gas is heated at constant atmospheric pressure from $$25.0^o$$ to $$125^oC$$.
How much energy is transferred to the oxygen as heat? (The molecules rotate but do not oscillate).
Under constant pressure, temperature of $$2.00\ mol$$ of an ideal monatomic gas is raised $$15.0\ K$$. What are
the change $$\Delta E_{int}$$ in the internal energy of the gas.
Calculate the number of degrees of freedom of molecules of hydrogen in $$1cc$$ of hydrogen gas at NTP.
We give $$70\ J$$ as heat to a diatomic gas, which then expands at constant pressure. he gas molecules rotate but do not oscillate. By how much does the internal energy of the gas increase?
The difference between $$C_{p}$$
and $$C_{v}$$ can be derived using the empirical relation $$H=U +\mathbf{p}
\mathbf{V}$$. Calculate the difference between $$\mathbf{C}_{\mathbf{p}}$$ and $$\mathbf{C}_{\mathbf{v}}$$
for 10 moles of an ideal gas.
Suppose $$4.00$$ mol of an ideal diamotic gas, with molecular reaction rotation but not oscillation experienced a temperature increase of $$60.0\ K$$ under constant-pressure conditions. What are the change $$\Delta E_{int}$$ in internal energy of the gas?
When $$20.9\ J$$ was added as heat to a particular ideal gas, the volume of the gas changed from $$50.0\ cm^{3}$$ to $$100\ cm^{3}$$ while the pressure remained at $$1.00\ atm$$
By how much did the internal energy of the gas change ? If the quantity of gas present was $$2.00\times 10^{-3}\ mol$$.
Suppose $$1.00\ L$$ of a gas with $$\gamma =1.30$$, initially at $$273\ K$$ and $$1.00\ atm$$, is suddenly compressed adiabatically to half its volume. Find its final temperature.
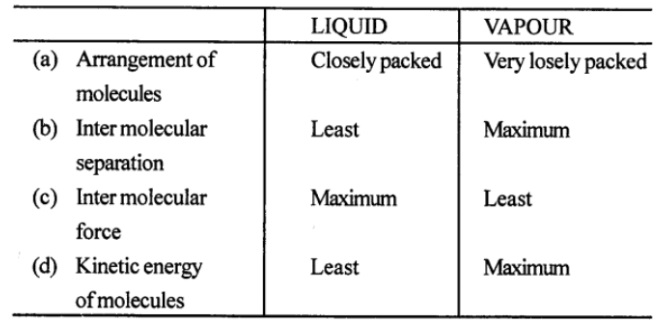
Distinguish between liquid and vapour (or gas) states of matter on the basis of following factors
(a) Arrangement of molecules
(b) Inter-molecular separation
(c) Inter-molecular force, and
(d) Kinetic energy of molecules
What is boiling ? Explain it on the basis of molecular motion ?
What is the degree of freedom for an airplane flying in the sky ?
Why does bubbles appear when a liquid is heated ?
Give the relation between degree of freedom (f) and $$\gamma.$$
State the main postulates of kinetic theory of matter.
Give the value of Cp for a diatomic gas.
Which energy gained due to the moment of molecule? Potential energy / Kinetic, energy.
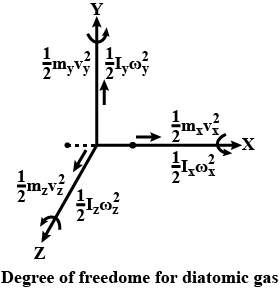
What do you understand by degree of freedom ?
A gas contained in a cylinder surrounded by a thick layer of insulating material is quickly compressed has there been a transfer of heat ?
On what factors do the degrees of freedom depend?
Find the kinetic energy of $$5$$ liters of a gas at STP, given the standard pressure, is $$1.013 \times 10^5\, N/m^2$$.
Mention the conditions under which a real gas obeys the ideal gas equation.
A gas contained in a cylinder surrounded by a thick layer of insulating material is quickly compressed has work been done ?
How many degrees of freedom have the gas molecules, if under standard conditions the gas density is $$\rho= 1.3 \ mg/cm^3$$ and the velocity of sound propagation in it is $$v = 330 \ m/s$$.
Find the mass and the number of degrees of freedom of molecules of freedom of molecules in a gas if its heat capacities are known: $$c_v = 0.65 \ J/(g.K)$$ and $$c_p = 0.91 \ J/(g.K)$$.
A solar cooker and a pressure cooker both are used to cook food. Treating them as thermodynamic systems, discuss the similarities and differences between them.
When a gas is heated, its temperature increases. Explain this phenomenon on the basis of the kinetic theory of gases.
Using the Maxwell distribution function, calculate the mean velocity projection ($$v_x$$) and the mean value of the modulus of this projection ($$| v_x |$$) if the mass of each molecule is equal to $$m$$ and the gas temperature is $$T$$.
How long will a $$2\ kW$$ heater take to boil $$2\ L$$ of water taken at $$25^oC$$? Given that the average specific heat of water in the range $$25^o C-100^oC$$ is $$4.184\ JK^{-1}g^{-1}$$.
Find the statistical weight of the most probable distribution of $$N = 10$$ identical molecules over two halves of the cylinder's volume. Find also the probability of such a distribution.
Using the Maxwell distribution function, determine the pressure exerted by gas on a wall, if the gas temperature is $$T$$ and the concentration of molecules is $$n$$.
Making use of the Maxwell distribution function, calculate the number $$v$$ of gas molecules reaching a unit area of a wall per unit time, if the concentration of molecules is equal to $$n$$, the temperature to $$T$$, and the mass of each molecule is $$m$$.
From the Maxwell distribution function find ($$v_x^2$$), the mean value of the squared $$v_x$$ projection of the molecular velocity in a gas at a temperature $$T$$. The mass of each molecule is equal to $$m$$.
Why does a diatomic gas have a greater energy content per mole than a monatomic gas at the same temperature?
Making the use of the Maxwell distribution function, find $$(1/v)$$, the mean value of a reciprocal of the velocity of molecules in an ideal gas at temperature T, if the mass of each molecule is equal to $$m$$. Compare the value obtained with the reciprocal of the mean velocity.
A light container having a diatomic gas enclosed with in is moving with velocity $$v$$. Mass of the gas is $$M$$ and number of moles is $$n$$.
- What is the kinetic energy of gas with respect to centre of mass of the system?
- What is the kinetic energy of gas with respect to ground?
A diatomic is expanded according to $${ PV }^{ 3 }= $$ constant under very high temperature (Assume vibration mode active). Calculate the molar heat capacity of gas (in cal/mol K) in this process:
Calculate
- the average kinetic energy of translation of an oxygen molecule at $$27^\circ C$$,
- the total kinetic energy of an oxygen molecule at $$27^\circ C$$,
- the total kinetic energy in joule of one mole of oxygen at $$27^\circ C$$.
Given Avogadro's number$$=6.02\times 10^{23}$$ and Boltzmann's constant$$=1.38\times 10^{-23}\:J/(mol-K)$$.
The pressure of a gas in a 100 mL container is 200 kPa and the average translation kinetic energy of each gas particle is $$6\, \times\, 10^{-21}$$. Find the number of gas particles in the container. How many moles are there in the container ?
One mole of a non-linear triatomic gas is heated in a closed rigid container from $$500^{\circ}C$$ to $$1500^{\circ}C$$. The amount of energy required is $$500xR$$ if the vibrational degree of freedom become effective only above $$1000^{\circ}C$$. Find the value of $$x$$.
What is the total random translational energy of the molecules in one mole of this gas ?
If K is the average kinetic energy per atom, find $$x$$, such that $$x=K\times{10}^{23}$$
An ideal gas occupies $$2$$ $$cubic meters$$ at a pressure of $$2$$ $$atm$$. Taking $$1$$ atm. $$= 10^5$$ $$Pa$$, find the energy density of the gas.
A sheet of $$15.0 g$$ of gold at $$25.0C$$ is placed on a $$30.0 g$$ sheet of copper at $$45.0C$$. What is the final temperature of the two metals assuming that no heat is lost to the surroundings. The specific heats of gold and copper are $$0.129 { J }/{ gC }$$ and $$0.385 { J }/{ gC }$$ respectively.
Nitrogen gas is filled in an insulated container. If $$\alpha$$ fraction of moles dissociates without exchange of any energy, then the fractional change in its temperature is
Oxygen is filled in a closed metal jar of volume $$1.0\times 10^{-3}\ m^{3}$$ at a pressure of $$1.5\times 10^{5}$$ Pa and temperature $$400\ K$$. The jar has a small leak in it. The atmospheric pressure is $$1.0\times 10^{5}$$ Pa and the atmospheric temperature is $$300\ K$$. Find the mass of the gas that leaks out by the time the pressure and the temperature inside the jar equalize with the surrounding.
Two moles of an ideal monoatomic gas at $$27^{o}$$C occupies a volume of $$V$$. If the gas is expanded adiabatically to the volume $$2\ V$$, then the work?
Hydrogen gas is contained in a closed vessel at 1 atm (100 kPa) and 300 K. (a) Calculate the mean speed of the molecules (b) Suppose the molecules strike the wall with this speed making an average angle of $$45^{\circ}$$ with it. How many molecules strikes each square meter of the wall per second?
Consider a classroom that is roughly $$8.21m\times 10m\times 3m$$. Initially $$T=290K$$ and $$P=1$$ atm. There are $$50$$ people in the class, each losing energy to the room at the average rate of $$166W$$. Assume that the walls, ceiling, floor, and furniture are perfectly insulated and do not absorb any heat. Also assume that all the doors and windows are tightly closed to prevent any exchange of air from the surrounding. How long (in sec) will the physical chemistry examination last if the professor (Mr. Neeraj Kumar) has foolishly agreed to dismiss the class when the air temperature in the room reaches body temperature, $$310K$$?
For air, $${ C }_{ P,m }=7R/2, \left[ R=0.0821L-atm/K-mol=8.3J/K-mol \right] $$
A molecule of a gas, filled in a discharge tube, gets ionized when an electron is detached from it. An electric field of $$5.0 kV m^{-1}$$ exists in the vicinity of the event. (a) Find the distance travelled by the free electron in $$1 \mu s$$ assuming no collision. (b) If the mean free path of the electron is $$1.0 mm$$, estimate. the time of transit of the free electron between successive collisions.
When $$229 \,J$$ of energy is supplied as heat at constant pressure to $$3.0$$ mole of argon gas, the temperature of the sample increases by $$2.55 \,K$$. Calculate the molar heat capacities at constant volume and constant pressure of the gas.
The specific heat at constant volume for a gas $$0.075\ cal/g$$ and at constant pressure is $$0.125\ cal/g$$. Calculate:
(i) The molecular weight of gas,
(ii) Atomicity of gas,
(iii) No. of atoms of gas in its $$1$$ mole.
(a) For two particles with speeds $$ v_{1} $$ and $$ v_{2} $$ , show that $$ v_{rms} \geq v_{av.} $$ , regardless of the numerical values of $$ v_{1} $$ and $$ v_{2} $$ . then show that $$ v_{rms} > v_{av.} $$ if $$ v_{1} \neq v_{2} . $$ (b) Suppose that for a collection of $$ N $$ particles you know . Another particle , with speed $$ u $$ , is added to the collection of particles . If the new rms and average speeds are denoted a $$ {v}'_{rms}$$ and $$ {v}'_{av.} $$ , show that
$$\displaystyle {v}'\,_{rms} = \sqrt{\dfrac{Nv^{2}_{rms} + u^{2}}{N + 1}} $$
and $$ {v}'_{rms} = \dfrac{Nv_{av.} + u}{N + 1} $$
(c) Use the expressions in part (b) to show that $$ {v}'_{rms} > {v}'_{av.} $$ , regardless of the numerical value of $$ u $$. (d) Explain why your results for (a) and (c) together show that $$ v_{rms} > v_{av.}$$ for any collection of particles if the particles do not all have the same speed .
The volume of an ideal gas is adiabatically reduced from $$200\ L$$ to $$74.3\ L$$. The initial pressure and temperature are $$1.00\ atm$$ and $$300\ K$$. The final pressure is $$4.00\ atm$$. Is the gas monotonic, diatomic, or polyatomic?
An adiabatic vessel contains $$ n_{1}=3 $$ mole of diatomic gas. Moment of inertia of each molecule is $$ I=2.76 \times 10^{-46} \mathrm{kg} $$ $$ \mathrm{m}^{2} $$ and root-mean-square angular velocity is $$ \omega_{0}=5 \times $$ $$ 10^{12} \mathrm{rad} / \mathrm{s} . $$ Another adiabatic vessel contains $$ n_{2}=5 \mathrm{mole} $$ of a monatomic gas at a temperature $$ 470 \mathrm{K} $$. Assume gases to be ideal, calculate root-mean-square angular velocity of diatomic molecules when the two vessels are connected by a thin tube of negligible volume. Boltzmann constant $$ k=1.38 \times 10^{-23} \mathrm{J} / $$ molecule.
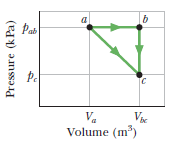
One mole of an ideal diatomic gas goes from $$a$$ to $$c$$ along the diagonal path in Fig . The scale of the vertical axis is set by $$p_{ab}=5.0\ kPa$$ and $$p_{c}=2.0\ kPa$$, and the scale of the horizontal axis is set by $$V_{bc}=4.0\ m^{3}$$ and $$V_{a}=2.0\ m^{3}$$. During the transition,
What is the change in internal energy of the gas.
Show that the number of collisions a molecule makes per second , called the collision frequency , $$ f $$ , is given by $$ f = \bar{v}/l_{m} $$ , and thus $$ f = 4\sqrt{2} \pi r^{2} \bar{v} N / V $$
Calculate the average molecular kinetic energy (i) per kmol (ii) per kg (iii) per molecule of oxygen at $$127^\circ C$$, given that the molecular weight of oxygen is $$32$$, R is $$8.31 J\,mol^{-1}K^{-1}$$ and Avogadros number $$N_A$$ is $$6.02 \times 10^{23}$$ molecules $$mol^{-1}$$.
What is meant by degree of freedom. Explain the specific heats for monoatomic, diatomic and polyatomic gases.
A gas is to be expanded from initial state $$i$$ to final state $$f$$ along either path $$1$$ or path $$2$$ on a $$P-V$$ diagram. Path $$1$$ consists of three steps: an isothermal expansion ( work is $$40\ J$$ in magnitude), an adiabatic expansion ( work is $$20\ J$$ in magnitude), and another isothermal expansion ( work is $$30\ J$$ in magnitude ). Path $$2$$ consists of two steps: a pressure reduction at constant volume and an expansion at constant pressure. What is the change in the internal energy of the gas along the path $$2$$?
State the law of equipartition of energy and hence calculate the molar specific heat of mono- and diatomic gases at constant volume and constant pressure.
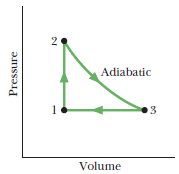
Figure shows a cycle undergone by $$1.00\ mol$$ of an ideal monatomic gas. The temperatures are $$T_1=300\ K, T_2=600\ K$$, and $$T_3=445\ K$$. For $$3\to 1$$, what is $$\Delta E_{int}$$?
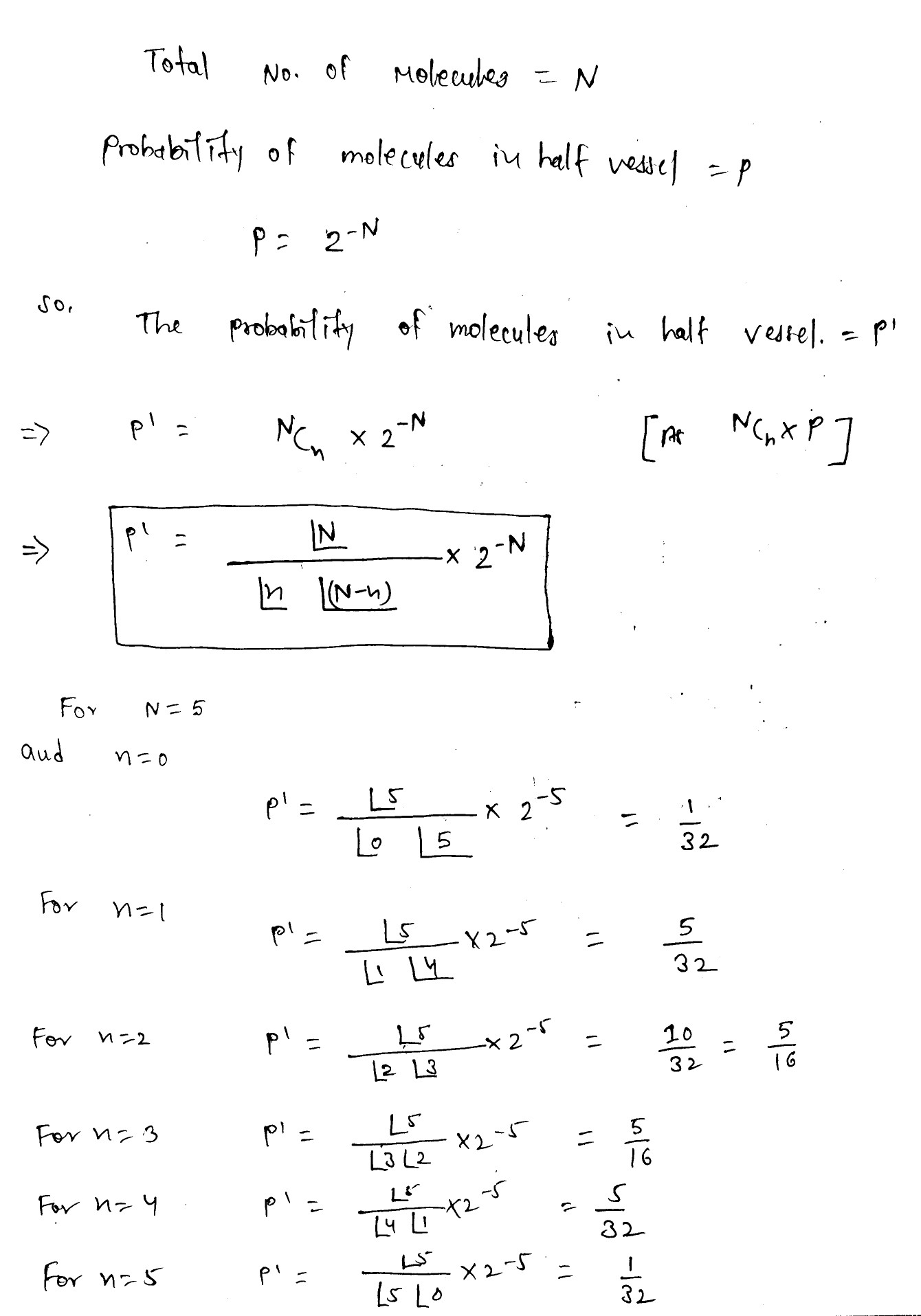
A vessel contains $$N$$ molecules of an ideal gas. Dividing mentally the vessel into two halves $$A$$ and $$B$$, find the probability that the half $$A$$ contains $$n$$ molecules. Consider the cases when $$N = 5$$ and $$n = 0, 1, 2, 3, 4, 5$$.
Show that the average value of $$c^3$$ of a Maxwellian gas is $$4\pi^{-1/2}(2kT/m)^{3/2}$$.
In 1801, Humphry Davy rubbed together pieces of ice inside an ice house. He made sure that nothing in the environment was at a higher temperature than the rubbed pieces. He observed the production of drops of liquid water. Make a table listing this and other experiments or processes to illustrate each of the following situations. (a) A system can absorb energy by heat, increase in internal energy, and increase in temperature. (b) A system can absorb energy by heat and increase in internal energy without an increase in temperature. (c) A system can absorb energy by heat without increasing in temperature or in internal energy. (d) A system can increase in internal
energy and in temperature without absorbing energy by heat. (e) A system can increase in internal energy without absorbing energy by heat or increasing in temperature.
A spherical rubber balloon inflated with air is held stationary, with its opening, on the west side, pinched shut. (a) Describe the forces exerted by the air inside and outside the balloon on sections of the rubber. (b) After the balloon is released, it takes off toward the east, gaining speed rapidly. Explain this motion in terms of the forces now acting on the rubber. (c) Account for the motion of a skyrocket taking off from its launch pad.
Class 11 Engineering Physics Extra Questions
- Gravitation Extra Questions
- Kinetic Theory Extra Questions
- Laws Of Motion Extra Questions
- Mechanical Properties Of Fluids Extra Questions
- Mechanical Properties Of Solids Extra Questions
- Motion In A Plane Extra Questions
- Motion In A Straight Line Extra Questions
- Oscillations Extra Questions
- Physical World Extra Questions
- Systems Of Particles And Rotational Motion Extra Questions
- Thermal Properties Of Matter Extra Questions
- Units And Measurement Extra Questions
- Waves Extra Questions
- Work,Energy And Power Extra Questions