Separation Of Substances - Class 6 Chemistry - Extra Questions
Define the term handpicking.
If you are given a mix of wheat, barley, and corn and asked to segregate the three then what will you do?
What do you mean by a saturated solution?
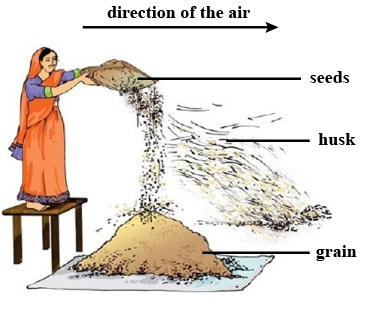
Which separation technique will you apply for the separation of wheat grains from husk?
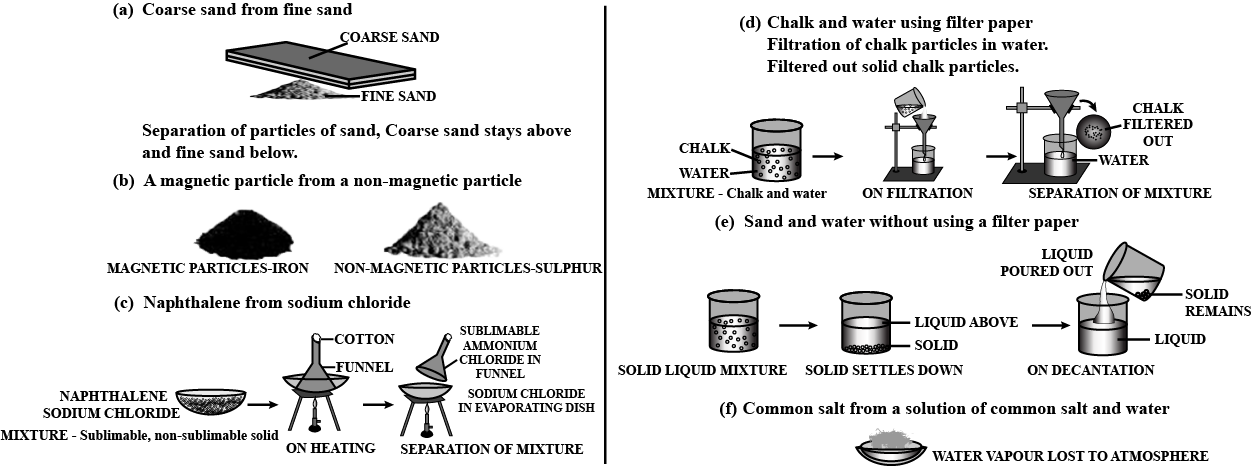
Describe an activity to separate a mixture of sand and salt.
How will you separate mangoes from a mixture of mangoes and apples?
You are given a mixture of salt and sand. Can you separate them by picking?
What is evaporation?
Name a method used to separate solid materials of different sizes.
Name the process used to separate heavier and lighter components of a mixture using air or wind.
Write various methods of separation of components from their mixture.
Name the method used to separate the pieces of stone from grain.
Can winnowing be used to separate components of a mixture having the same weight?
How can you separate grains from stalk?
Match the column.
| Separation process | The purpose for which we do the separation | What do we do with the separated components? |
| (1) Separate stones from rice. | (a) To separate two different but useful components. | (i) We throw away the solid component. |
| (2) Churning milk to obtain butter. | (b) To remove non-useful components. | (ii) We threw away the impurities. |
| (3) Separate tea leaves | (c) To remove impurities or harmful components. | (iii) We use both components. |