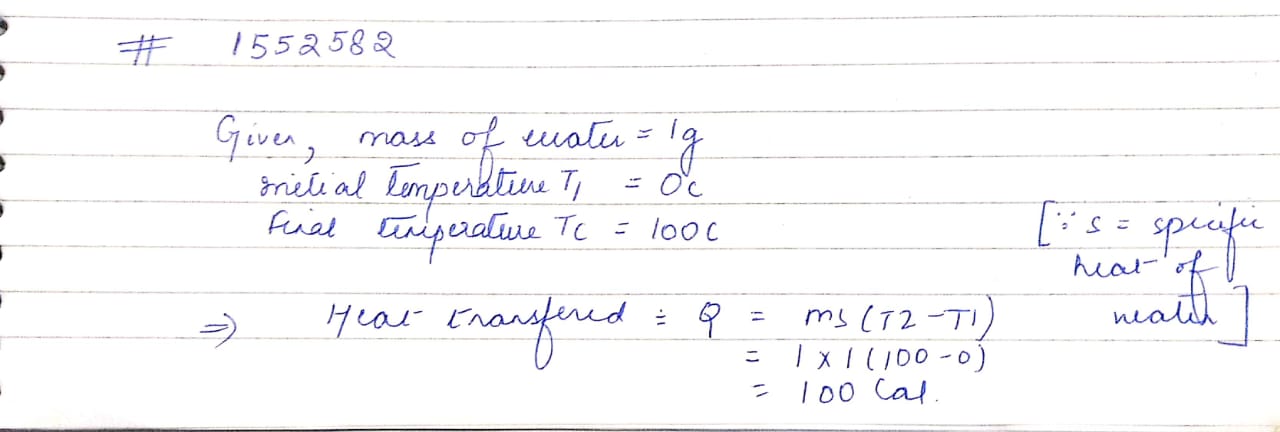Thermal Properties Of Matter - Class 11 Medical Physics - Extra Questions
Who is telling the truth? The temperature of a substance is measured in joules.
At what temperature is the Fahrenheit scale reading equal to
twice that of the Celsius scale.
At what temperature is the Fahrenheit scale reading equal to
half that of the Celsius scale?
A container with $$1 kg$$ of water in it is kept in sunlight, which causes the water to get warmer than the surroundings. The average energy per unit time per unit area received due to the sunlight is $$700 Wm^{-2}$$ and it is absorbed by the water over an effective area of $$0.05 m^2$$. Assuming that the heat loss from the water to the surroundings is governed by Newtons law of cooling, the difference (in $$^{\circ}C$$) in the temperature of water and the surroundings after a long time will be ______. (Ignore effect of the container, and take constant for Newtons law of cooling $$= 0.001 s^{-1}$$, Heat capacity of water $$= 4200 J \ kg^{-1} K^{-1}$$)
Express $$281 K$$ in $$^oC$$
Express $$0C$$ in kelvin.
The body temperature of a healthy person is $$98.6F$$. Calculate the corresponding temperature on the Kelvin scale.
Express $$45^oC$$ in kelvin.
How can the radiating power of a surface be increased?
What is the value of $$0^{\circ}$$F in celsius scale ?
The fundamental intervals of two thermometers X and Y are $$70^{\circ}$$ and $$140^{\circ}$$ respectively. Their ice points are $$10^{\circ}$$ and $$0^{\circ}$$ respectively. If Y reads $$90^{\circ}$$. What would X read?
A difference of $$1^{\circ}C$$ is _____ as the difference of $$1\ K$$.
Convert each of the following temperature in $$^{\circ}F$$ to the celsius and kelvin scale $$68^{\circ}F, 5^{\circ}F, 176^{\circ}F$$
Triple point of water is
Fill in the blanks:Heat is a form of ________.
In which mode of heat transfer , no medium is required ?
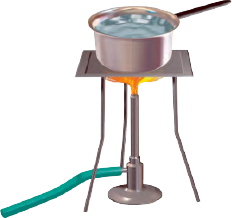
Observe the picture given in above Figure. Water is being boiled in a pan of wide base.
i) Which position $$P$$ or $$T$$ will feel warmer?
ii) Fill up the boxes $$P$$ and $$T$$ to indicate the mode of flow of heat to the hand.

The fundamental intervals of two thermometers X and Y are $$70^o$$ and $$140^o$$ respectively. Their ice points are $$10^o$$ and $$0^o$$ respectively, If Y reads $$90^o$$. What would X read?
Calculate the temperature on Celsius scale whose value is half the absolute reading?
A faulty thermometer has its fixed points marked as $$10^o$$ and $$90^o$$. The temperature of a body as measured by the faulty thermometer si $$61^o$$. Find the correct temperature of the body on celsius scale.
The temperature of a body changes from $$15^oC$$ to $$75^oC$$. What is the corresponding change in (a) Fahrenheit scale (b) kelvin scale?
At what temperature in degree Kelvin the Fahrenheit and Kelvin scales of temperature give the same reading ?
Describe an experiment to show that a blackened surface is a better emitter of heat than a polished one.
At what temperature the Fahrenheit and Celsius scale of temperature gives the same reading ?
At what temperature is the Fahrenheit scale reading equal to twice the reading of Celsius scale ?
Change each of the given temperature to the Celsius and Kelvin scales: $$68^{\circ} F, 5^{\circ} F$$ and $$176^{\circ} F.$$
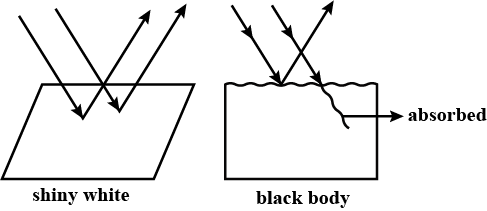
Draw a clear labelled diagram of an experiment to compare the absorbing powers of two different surfaces, a shiny white and a dull black.
Describe an experiment to show that a black (or dull) surface is a better absorber of heat radiations than a polished one.
A Faulty thermometer reads $$5^{\circ}C$$ in melting ice and $$99^{\circ}C$$ in steam. Find the correct temperature in $$^{\circ}F$$ when this faulty thermometer reads $$52^{\circ}C$$.
At what temperature do the Celsius and Fahrenheit reading have the same numerical value ?
The efficiency of an ideal engine is $$75\%$$ and it emits heat $$2 \times 103\ W$$ to sink at $$283\ K$$, then calculate :
Temperature of source.
The steam point and the ice point of a mercury thermometer are marked as $$80^{\circ}$$ and $$20^{\circ}$$. What will be the temperature in centigrade mercury scale when this thermometer reads $$32^{\circ}$$
Change each of the given temperature to the Fahrenheit and Rankine scales: $$30^{\circ} C, 5^{\circ} C$$ and $$-20^{\circ} C.$$
Derive a relation between the Celsius and Fahrenheit Scales of temperature using the fixed points in the two different scales.
The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales.
Two absolute scales A and B have triple points of water defined to be 200 A and 350 B. What is the relation between $$ \displaystyle T_{A} $$ and $$ \displaystyle T_{B} $$ ?
A faulty thermometer has its fixed points marked as $$10^{\circ}$$ and $$90^{\circ}$$. The temperature of a body as measured by the faulty thermometer is $$61^{\circ}$$. Find the correct temperature of the body on Celsius scale.
The temperature of a body changes from $$15^{\circ}C$$ to $$75^{\circ}C$$. What is the corresponding change in (a) Fahrenheit scale (b) Kelvin scale?
The boiling point of turpentine on Fahrenheit scale is double its value on Celsius scale. If the boiling point of tar on Celsius scale is $$140^{\circ}$$ more than that of turpentine on same scale, find the boiling point of tar on Kelvin scale.
Name the modes of transfer of heat in the following:(a) Solid,(b) Liquid,(c) Gas,(d) Vacuum.
Who is telling the truth? Joule is the unit of heat.
Explain conduction, convection and radiation with examples
In a scientific book that describes a temperature scale called $$Z$$, boiling and freezing point of water are referred as $${65}^{o}Z$$ and $$-{15}^{o}Z$$ respectively. (a) To what temperature on Fahrenheit scale would a temperature $$-{95}^{o}Z$$ compound? (b) What temperature change on the $$Z$$ scale that would correspond to a change of $${40}^{o}$$ on Celsius scale?
A Centigrade thermometer has its lower and upper fixed points marked $$-0.5^{\circ}C$$ and $$100.5^{\circ}$$. What is the true temperature when this thermometer reads $$30^{\circ}$$? The bore of the thermometer is uniform.
The steam point and the ice point of a mercury thermometer are marked as $$80^{\circ}$$ and $$10^{\circ}$$. At what temperature on centigrade scale the reading of this thermometer will be $$59^{\circ}$$?
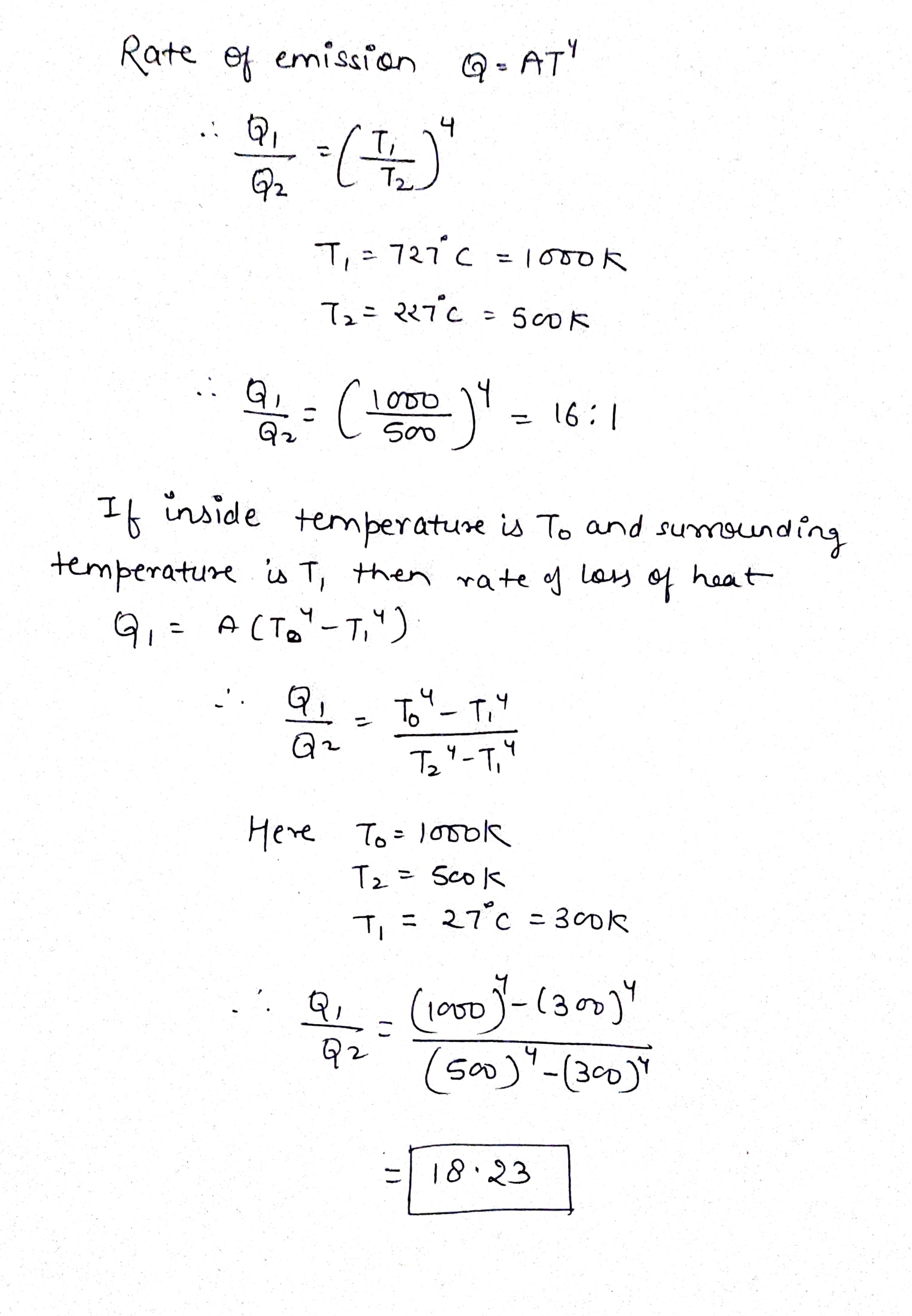
Compare the rates of emission of heat of a black body maintained at $$727^oC$$ with $$227^oC$$. If the black body is surrounded by an enclosure (black) at $$27^oC$$, what would be the ratio of their rates of loss of heat.
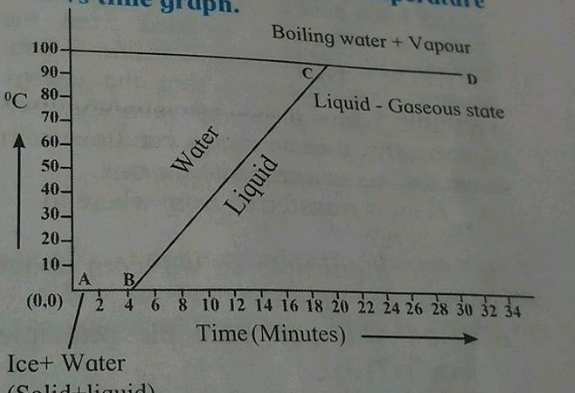
Explain the following temperature vs time graph.
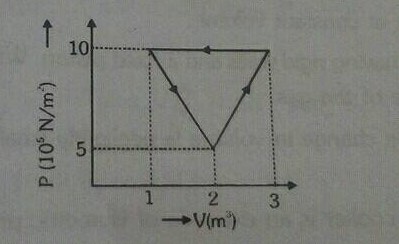
Calculate the heat supplied in figure shown for complete cycle.
How much energy is transferred when $$1 gm$$ of boiling water at $$100 ^ { \circ } C$$ cools to water at $$0^{ { \circ } }{ C }$$ ?
Two spherical bodies $$A$$ (radius $$6\ cm)$$ and $$B(radius\ 18\ cm)$$ are at temperature $$T_{1}$$ and $$T_{2}$$ respectively. The maximum intensity in the emission spectrum of $$A$$ is at $$500\ nm$$ and in that of $$B$$ is at $$1500\ nm$$. Considering them to be black bodies, what will be the ratio of the rate of total energy radiated by $$A$$ to that of $$B$$?
Look at Fig. 4.Mark where the heat is being transferred by conduction, by convection and by radiation.
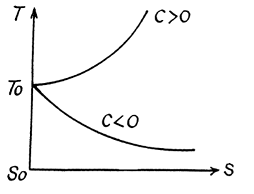
Find the temperature $$T$$ as a function of the entropy $$S$$ of a substance for a polytropic process in which the heat capacity of the substance equals $$C$$. The entropy of the substance is known to be equal to $$S_0$$ at the temperature $$T_0$$. Draw the approximate plots $$T (S)$$ for $$C > 0$$ and $$C < 0$$.
Calculate the temperature and density of carbon dioxide in critical state, assuming the gas to be a Van der Waals one.
Heat is always transfer ___ from bodies to ___ bodies.
A nurse measures the temperature of a patient to be $$41.5^0C$$. (a) What is this temperature on the Fahrenheit scale? (b) Do you think the patient is seriously ill? Explain.
Rub the palm of your hand on a metal surface for about 30 seconds. Place the palm of your other hand on an unrubbed portion of the surface and then on the rubbed portion. The rubbed portion will feel warmer.Now repeat this process on a wood surface. Why does the temperature difference between the rubbed and unrubbed portions of the wood surface seem larger than for the metal surface?
An ideal gas initially at 300 K undergoes an isobaric expansion at 2.50 kPa. If the volume increases from $$1.00 m^3$$ to $$3.00 m^3$$ and 12.5 kJ is transferred to the gas by heat, what are (a) the change in its internal energy and (b) its final temperature?
The temperature difference between the inside and the outside of a home on a cold winter day is $$57.0^0F$$. Express this difference on (a) the Celsius scale and (b) the Kelvin scale.
You need to pick up a very hot cooking pot in your kitchen. You have a pair of cotton oven mitts. To pickup the pot most comfortably, should you soak them in cold water or keep them dry?
Convert the following temperatures to their values on the Fahrenheit and Kelvin scales: (a) the sublimation point of dry ice, $$-78.5^0C$$; (b) human body temperature, $$37.0^0C$$.
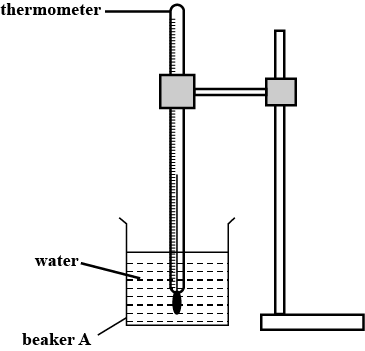
In this experiment, you will investigate the coding of water.
Carry out the following instructions referring to Figure.
Pour $$100\,cm^3$$ of hot water into beaker A. Place the thermometer in beaker A, as shown in Figure.
(i) State one precaution that you took to ensure that the temperature reading for the hot water is as reliable as possible.
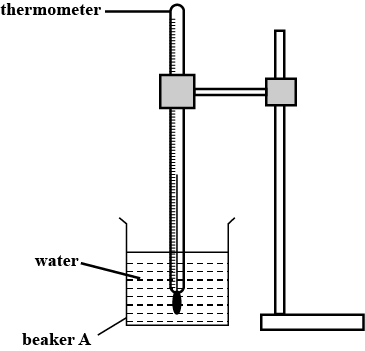
In this experiment, you will investigate the coding of water.
Carry out the following instructions referring to Figure.
Suggest two factors, other than the volume and temperature of the cold water added, that affect the decrease in temperature of the hot water.
Answer the following:(a) The triple-point of water is a standard fixed point in modern thermometry. Why? What is wrong in taking the melting point of ice and the boiling point of water as standard fixed points (as was originally done in the Celsius scale )?(b) There were two fixed points in the original Celsius scale as mentioned above which were assigned the number $$0^oC$$ and $$100^oC$$ respectively. On the absolute scale one of the fixed points is the triple-point of water, which on the kelvin absolute scale is assigned the number 273.16 K. What is the other fixed point on this (kelvin) scale?(c) The absolute temperature (Kelvin scale ) T is relate to the temperature $$t_{c}$$ on the Celsius scale by $$t_{c}=T-273.15$$. Why do we have 273.15 in this relation and not 273.16?(d) What is the temperature of the triple-point of water on an absolute scale whose unit interval size is equal to that of the Fahrenheit scale ?
You work is a material testing lab and your bess tells you to increase the temperature of a sample by $$40.0^{\circ}C$$ The only thermometer you can find at your workbench reads in $$^{\circ}F$$. If The initial temperature of the sample is $$68.2^{\circ}F$$. What is its temperature in $$^{\circ}F$$, when the desired temperature increase has been achieved ?
The fundamental interval of a thermometer $$x$$ is divided arbitrartily into $$40$$ equal and that of another thermometry into $$80$$ equal parts. If the freezing point of $$x$$ is marked $$20^{\circ}$$ and that of $$y$$ is market $$0^{\circ}$$; what is the temperature on $$x$$ when $$y$$ indicates $$70^{\circ}$$? What is the temperature in $$^{\circ}C$$? $$(55^{\circ}; 87.5^{\circ}C)$$.
What is the temperature at which we get the same reading on both the Centigrade and Fahrenheit scales?
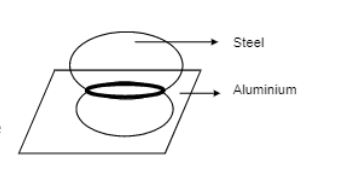
An aluminium plate fixed in a horizontal position has a hole of diameter $$2.00cm$$. A steel sphere of diameter $$2.005cm$$ rests on this hole. All the lengths refer to a temperature of $$10^oC$$. The temperature of the entire system is slowly increased. At what temperature will the ball fall down? Coefficient of linear expansion of aluminium is $$23\times 10^{-6}\ ^oC^{-1}$$ and that of steel is $$11 \times 10^{-6} \, ^oC^{-1}$$.
Name the three modes of transfer of heat.
Explain the three modes of heat transfer .
Class 11 Medical Physics Extra Questions
- Gravitation Extra Questions
- Kinetic Theory Extra Questions
- Laws Of Motion Extra Questions
- Mechanical Properties Of Fluids Extra Questions
- Mechanical Properties Of Solids Extra Questions
- Motion In A Plane Extra Questions
- Motion In A Straight Line Extra Questions
- Oscillations Extra Questions
- Systems Of Particles And Rotational Motion Extra Questions
- Thermal Properties Of Matter Extra Questions
- Units And Measurement Extra Questions
- Waves Extra Questions
- Work, Energy And Power Extra Questions