MCQ Questions for Class 11 Chemistry Quiz with Answers Chapter Wise PDF Download
CBSE Multiple Choice Type Questions for 11th Class Chemistry PDF formatted study resources are available for free download. These Grade 11 Chemistry CBSE MCQ Mock Test helps you learn & practice the concepts in a fun learning way.
Class 11 Chemistry MCQs Multiple Choice Questions with Answers
Here are the chapterwise CBSE MCQ Quiz Test Questions for Class 11th Chemistry in pdf format that helps you access & download so that you can practice online/offline easily.
- Chemical Bonding And Molecular Structure Class 11 Engineering Chemistry MCQ Questions
- Classification Of Elements And Periodicity In Properties Class 11 Engineering Chemistry MCQ Questions
- Environmental Chemistry Class 11 Engineering Chemistry MCQ Questions
- Equilibrium Class 11 Engineering Chemistry MCQ Questions
- Hydrocarbons Class 11 Engineering Chemistry MCQ Questions
- Hydrogen Class 11 Engineering Chemistry MCQ Questions
- Organic Chemistry - Some Basic Principles And Techniques Class 11 Engineering Chemistry MCQ Questions
- Redox Reactions Class 11 Engineering Chemistry MCQ Questions
- Some Basic Concepts Of Chemistry Class 11 Engineering Chemistry MCQ Questions
- Some P-Block Elements Class 11 Engineering Chemistry MCQ Questions
- States Of Matter Class 11 Engineering Chemistry MCQ Questions
- Structure Of Atom Class 11 Engineering Chemistry MCQ Questions
- Thermodynamics Class 11 Engineering Chemistry MCQ Questions
- The S-Block Elements Class 11 Engineering Chemistry MCQ Questions
Chemical Bonding And Molecular Structure Class 11 Engineering Chemistry MCQ Quiz



Chemical Bonding And Molecular Structure Questions and Answers
| Chemical Bonding And Molecular Structure Quiz Question | Answer |
|---|---|
| Which of the following molecules has two sigma $$(\sigma)$$ and two pi $$(\pi)$$ bonds? | $$HCN$$ |
| Total number of lone pair of electrons in $$I_3{^-}$$ ion is : | $$9$$ |
| During the formation of a molecular orbital from atomic orbitals, probability of electron density is: | zero in the nodal plane |
| Consider the following compounds and select the incorrect statement from the following : $$NH_{3}, PH_{3}, H_{2}S, SO_{2}, SO_{3}, BF_{3}, PCl_{3}, IF_{7}, P_{4}, H_{2}$$ |
Six molecules out of above compounds are non-planar in structure |
 |
$$16$$ |
| Which of the following is/has the weakest attractive forces? | Vander Waals |
| Among the following, the molecule with the highest dipole moment is: |
$$\mathrm{C}\mathrm{H}_3 {Cl}$$ |
| Which of the following pairs of ions are isoelectronic and isostructural? | $$CO_3^{2-}, NO_3 {^-}$$ |
| Which one of the following pairs of species have this same bond order? | $$CN^{-}, CO$$ |
| Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion |
Classification Of Elements And Periodicity In Properties Class 11 Engineering Chemistry MCQ Quiz



Classification Of Elements And Periodicity In Properties Questions and Answers
| Classification Of Elements And Periodicity In Properties Quiz Question | Answer | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recently (in 2003) element with atomic number $$110$$ has been named by IUPAC as: | $$Ds$$ | ||||||||||||||||||||
| IUPAC symbol of atomic number $$119$$. | $$Uue$$ | ||||||||||||||||||||
| The correct order of electron affinity is: | Cl $$>$$ F $$>$$ O | ||||||||||||||||||||
| Within each pair of elements F & Cl, S & Se, and Li & Na respectively, the elements that release more energy upon an electron gain are: | $$Cl, S$$ and $$ Li$$ | ||||||||||||||||||||
Identify the incorrect match.
|
(d), (iv) | ||||||||||||||||||||
| Which of the following sequence contains atomic number of only representative elements? | $$3,\ 33,\ 53,\ 87$$ | ||||||||||||||||||||
| The elements $$P,Q,R,S$$ belong to group number $$14,15,16,17$$ respectively. Select the elements in increasing order of their electronegativity. | $$P< Q< R< S$$ | ||||||||||||||||||||
| Statement: Rows in the periodic table are called periods. State whether the given statement is true or false. |
True | ||||||||||||||||||||
| Fluorine has highest electron affinity in the periodic table. |
False | ||||||||||||||||||||
| Diagonal relationship is shown by : |
elements of third period |
Environmental Chemistry Class 11 Engineering Chemistry MCQ Quiz



Environmental Chemistry Questions and Answers
| Environmental Chemistry Quiz Question | Answer |
|---|---|
| Which of the following is true about acid rain? | All of the above |
| Ozone in stratosphere extends | 20-25km |
| The stratospheric ozone depletion leads to? | All of the above |
| The regions of the atmosphere, where clouds form and where we live respectively, are: | Troposphere and Troposphere |
| Which is wrong with respect to our responsibility as a human being to protect our environment? | Using plastic bags |
| Depletion of which gas in the atmosphere can lead to an increase incidence of skin cancers? | Ozone |
| World ozone day is celebrated on __________ | $$16^{th}$$ September |
| Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion. | |
| Photochemical smog formed in congested metropolitan cities mainly consists of |
Ozone, Peroxyacetyl nitrate and $$NO_x$$ |
| Nitrogen oxides produced by the emission of automobiles and power plants are the source of fine airborne particles, which lead to | Photochemical smog |
Equilibrium Class 11 Engineering Chemistry MCQ Quiz



Equilibrium Questions and Answers
| Equilibrium Quiz Question | Answer |
|---|---|
| At a temperature under high pressure $$K_w(H_2O) \, = \, 10^{10}$$, a solution of pH 5.4 is said to be: | Acidic |
| In which of the following reactions, an increase in the volume of the container will be favour the formation of products ? | $$2NO_2(g)\rightleftharpoons 2NO(g)+O_2(g)$$ |
| For the equilibrium reaction, $$H_2O(l) \rightleftharpoons H_2O(g).$$ What happens, if pressure is applied? | The boiling point of water is increased. |
| K$$_{b_1}$$ for X(OH)$$_{3}$$ (a weak base) is 10$$^{-5}$$. What is the pH of its 0.1 M solution? | 11 |
| The pH of 0.2 M solution of acid HQ =Then the value of $$K_{a}$$ for HQ will be | $$5\times10^{-6}$$ |
| The total number of different kind of buffers obtained during the titration of $$H_{3}PO_{4}$$ with NaOH are | 3 |
| In the reaction, $$2SO_{ 2 }(g)\rightleftharpoons 2SO +O_2$$, an inert gas introduced at constant temperature and volume. The dissociation of $$SO$$ is: | unaffected |
| Which statement is/are correct- | $$ 10^{-4}\ M$$ $$HCl$$ solution is less acidic than $$0.1\ M$$ $$HCN$$ $$ ( K_a HCN = 10^{-5} ) $$ |
| Assertion : - On compressing a gas to half the volume, the number of moles is halved. Reason : - Number of moles present decreases with derease is volume |
A |
| A buffer solution can be prepared from a mixture of: | $${ CH }_{ 3 }COONa$$ and $$CH_3COOH$$ in 1:1 mole ratio |
Hydrocarbons Class 11 Engineering Chemistry MCQ Quiz



Hydrocarbons Questions and Answers
| Hydrocarbons Quiz Question | Answer |
|---|---|
| Outline the reaction sequence for the conversion of ethene to ethyne (the number of steps should not be more than two). | A: Bromine water $$B: KOH$$ |
| Which statements is / are correct ? | Compound (II) in (b) shows four stereoisomers |
| Which of the following having delocalised electrons? | Benzene |
| Which of the following is highly inflammable ? |
Alkanes |
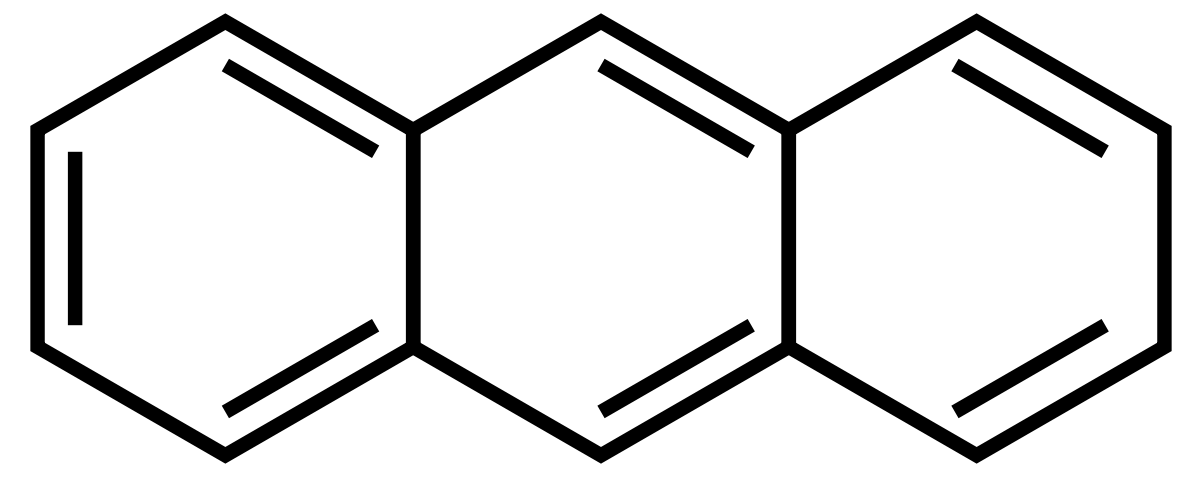
Choose the correct IUPAC name of the following compound from the given-below options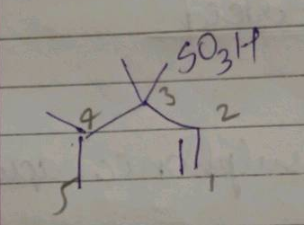 |
$$3,4-Dimethyl$$ $$Pent-1-ene$$ $$3-Sulphonic Acid$$ |
Which of the following compounds would have the same vapour pressure ?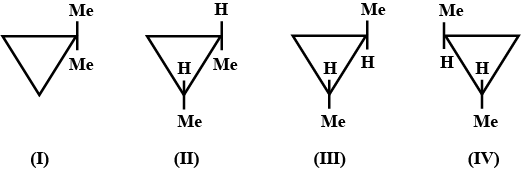 |
(III) and (IV) |
| Which one of the following has the minimum boiling point? | Isobutene |
The IUPAC name of given compound: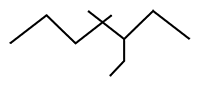 |
3-ethyl-4, 4 dimethylheptane |
| The trans- alkenes are formed by the reduction of alkynes with: | $$Na/liq.NH_3$$ |
The IUPAC name of the following compound is: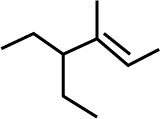 |
$$4$$-Ethyl-$$3$$-methylhex-$$2$$-ene |
Hydrogen Class 11 Engineering Chemistry MCQ Quiz



Hydrogen Questions and Answers
| Hydrogen Quiz Question | Answer |
|---|---|
| Which of the following pair behaves strong and weak base hydride : $$NH_3, PH_3, AsH_3, SbH_3, BiH_3$$ |
$$NH_3, BiH_3$$ |
| lonic hydrides is/are usually :- | Good reducing agents |
| The oxygen in hydrogen peroxide which is used in oxidation is bound by ..... bond. | covalent bound |
| Water is a ______ oxide. |
neutral |
| Hydride of which of the following element shown the highest tendency of hydrogen storage? | Al |
| $$H_2O_2$$ is stabilized by | Metals |
| $$H_2$$ gas can not be prepared by :- | $$Mg\ +\ NaOH$$ |
| Which of the following pairs will not produce dihydrogen gas ? | $$Cu + dil. HCl $$ |
| Which of the following represents saline hydrides? | $$KH,\,Ca{H_2},\,Ba{H_2}$$ |
| Which gas is evolved when potassium carbonate is treated with dilute with $$HCl$$? | Carbon dioxide $$(CO_{2})$$ |
Organic Chemistry - Some Basic Principles And Techniques Class 11 Engineering Chemistry MCQ Quiz



Organic Chemistry - Some Basic Principles And Techniques Questions and Answers
| Organic Chemistry - Some Basic Principles And Techniques Quiz Question | Answer |
|---|---|
Give $$ IUPAC $$ names of following alkyl group.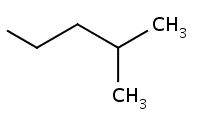 |
Isopentyl |
| $$CH_3CHO \overset{10 \, percent \,\, NaOH}{\underset{5^o C }{\longrightarrow}} \underset{\Delta}{\longrightarrow} \overset{H_2O}{\underset{Ni}{\longrightarrow}} (A) $$; Product (A) of the reaction is: |
butanol |
Then the correct structure of $$P$$ & $$Q$$ an respectively are: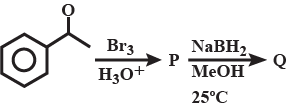 |
|
| Identity the correct sequence of increasing number of $$\pi-$$bonds in structure of the following molecules: I) $$H_{2}S_{2}O_{6}$$ II) $$H_{2}SO_{3}$$ III) $$H_{2}S_{2}O_{5}$$ |
$$II,III,I$$ |
| The functional group present in organic acid is: |
$$-COOH$$ |
| Which of these contains the carbonyl group? |
all of these |
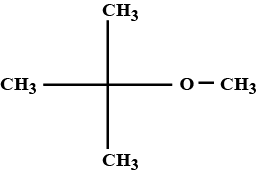
| The compound that is most difficult to protonate is: | |
The total number of $$\pi $$ electrons in the given structure are : 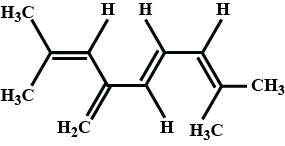 |
8 |
| Both Assertion and Reason are correct and Reason is the correct explanation for Assertion | |
 |
Both Assertion and Reason are correct and Reason is the correct explanation for Assertion |
Redox Reactions Class 11 Engineering Chemistry MCQ Quiz



Redox Reactions Questions and Answers
| Redox Reactions Quiz Question | Answer |
|---|---|
| What is the oxidation number of lithium in $$LiCl$$? | $$+1$$ |
| Which of the following arrangement represents increasing O.N. of central atom. | $${ CrO }_{ 2 }^{ - },{ ClO }_{ 3 }^{ - },{ CrO }_{ 4 }^{ 2- },{ MnO }_{ 4 }^{ - }$$ |
| Of the following transition metals, the maximum number of oxidation states is shown by | $$Mn$$ |
| What is the oxidation number of $$O$$ in a diatomic molecule $$({O}_{2})$$? | $$0$$ |
| Oxidation state of iodine in $$I Cl_{2}^{-}$$ is : |
+ 1 |
| The correct statement for the molecule $$ CsI_{3}$$ is that: | it contains $$ Cs^{+}$$ and $$ I_{3}{^-}$$ ions |
| Excess of KI reacts with $$CuSO_4$$ solution and then $$Na_2S_2O_3$$ solution is added to it. Which of the statement is incorrect for this reaction? |
$$CuI_2$$ is formed |
| The pair of compounds having metals in their highest oxidation state is: | $$[Fe(CN)_6]^{3-}$$ and $$[Cu(CN)_4]^{2-}$$ |
| $$(\mathrm{N}\mathrm{H}_{4})_{2}\mathrm{C}\mathrm{r}_{2}\mathrm{O}_{7}$$ on heating gives a gas which is also given by: |
heating $$\mathrm{N}\mathrm{H}_{4}\mathrm{N}\mathrm{O}_{2}$$ |
| For the redox reaction, $$MnO_{4}^{-} + C_{2}O_{4}^{2-} + H^{+}\rightarrow Mn^{2+} + CO_{2} + H_{2}O$$, the correct coefficients of the reactants for the balanced equation are _____________. | $$MnO_{4}^{-} = 2, C_{2}O_{4}^{2-} = 5, H^{+} = 16$$ |
Some Basic Concepts Of Chemistry Class 11 Engineering Chemistry MCQ Quiz



Some Basic Concepts Of Chemistry Questions and Answers
| Some Basic Concepts Of Chemistry Quiz Question | Answer |
|---|---|
| What is chemistry? | All of the above. |
| A balanced chemical equation is in accordance with the law of conservation of mass. | True |
| According to the Avogadro's Law, equal volumes of two different gases, under same conditions of temperature and pressure, contain equal number of: |
molecules |
| At same temperature and pressure, equal volumes of gases contain the same number of: | molecules |
| Which of the following property of an element never changes? |
Atomic weight |
| Which one of the following properties of an element is not variable? |
Atomic weight |
| Equal volume of gases contain equal number of moles. State whether the above statement is true or false. |
The statement is True |
| The value of 1 amu is equal to: |
$$1.66\times 10^{-24}g$$ |
| Avogadro's law finds an application in the determination of: |
Molecular weights of gases |
| Mass of one twelth of carbon atom is called atomic mass unit. |
True |
Some P-Block Elements Class 11 Engineering Chemistry MCQ Quiz



Some P-Block Elements Questions and Answers
| Some P-Block Elements Quiz Question | Answer |
|---|---|
| What is the general electronic configuration of $$p-block$$ elements? | $${ ns }^{ 2 }{ np }^{ 1-6 }$$ |
| What is the n factor or valancy factor of ozone during the change $$2$$O$$_3$$ $$\rightarrow$$ $$3$$O$$_2$$? | $$6$$ |
| Which is correct order of stability: | $${Tl}^{+}>{Tl}^{+3}$$ |
| Kernite is a mineral containing: | boron |
| ______ can be found in bauxite and sphalerite in traces. | Gallium |
| Lorandite is a mineral of: | thallium |
| Beryl, cryolite are minerals of: | aluminium |
| Aluminium is usually found in $$+3$$ oxidation state. In contrast, thallium exists in $$+1$$ and $$+3$$ oxidation states. This is due to : | inert pair effect |
| The element that shows greater ability to form $$p\pi -p\pi$$ multiple bonds, is: | $$C$$ |
| Correct statements among a to d regarding silicones are: (a) They are polymers with hydrophobic character. (b) They are biocompatible. (c) In general, they have high thermal stability and low dielectric strength. (d) Usually, they are resistant to oxidation and used as greases. |
(a), (b) and (d) only. |
States Of Matter Class 11 Engineering Chemistry MCQ Quiz



States Of Matter Questions and Answers
| States Of Matter Quiz Question | Answer |
|---|---|
| Which of the following law is followed by ideal gases? | All of the above |
| A spherical balloon of $$21\ cm$$ diameter is to be filled up with $$H_2$$ at $$NTP$$ from a cylinder containing the gas at $$20\ atm$$ at $$27^{\circ}C$$. The cylinder can hold $$2.82$$ litre of water. The number of balloons that can be filled up | 10 |
| Total pressure of a gaseous mix is equal to the sum of the Partial Pressures is: |
Dalton's law |
| Which of the following is not an assumption of the kinetic theory of gases? | At high pressure, gas particles are difficult to compress. |
| Which term describes the mass of $$6.022\times { 10 }^{ 23 }$$ representative particles? | Molar mass |
| Liquid $$M$$ and liquid $$N$$ form an ideal solution. The vapour pressures of pure liquids $$M$$ and $$N$$ are $$450$$ and $$700\ mm$$ $$Hg$$, respectively, at the same temperature. Then correct statement is: ($${x}_{M}=$$ Mole fraction of $$M$$ in solution; $${x}_{N}=$$ Mole fraction of $$N$$ in solution; $${y}_{M}=$$ Mole fraction of $$M$$ in vapour phase; $${y}_{N}=$$ Mole fraction of $$N$$ in vapour phase) |
$$\cfrac{{x}_{M}}{{x}_{N}}> \cfrac{{y}_{M}}{{y}_{N}}$$ |
| When an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules : |
Exert no attractive forces on each other |
| According to kinetic theory of gases : |
between collisions, the molecules move in straight lines with constant velocities |
| The correction factor $$'a'$$ to the ideal gas equation corresponds to: | forces of attraction between the gas molecules |
| Gay Lussac's law of gaseous volume is derived from : | experimental observation |
Structure Of Atom Class 11 Engineering Chemistry MCQ Quiz



Structure Of Atom Questions and Answers
| Structure Of Atom Quiz Question | Answer |
|---|---|
| The equivalent weight of a metal is 4.5 and the molecular weight of its chloride isFind the atomic weight of the metal. | 9 |
| Which letter orbital corresponds to $$l$$ = 2? | d |
| Which color of light has the highest energy? | Violet |
| The number of protons, neutrons and electrons in $$^{175}_{71}Lu$$, respectively, are | $$71, 104$$ and $$71$$ |
| If the shortest wavelength in Lyman series of hydrogen atom is A, then the longest wavelength in Paschen series of $$He^+$$ is : | $$\dfrac{36A}{7}$$ |
| Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying $$0.5\ V$$ when the radiation of $$250\ nm$$ is used. The work function of the metal is: | $$4.5\ eV$$ |
| Which of the following has the correct combination considering $$List-I$$ and $$List-II$$? |
$$(I),(T)$$ |
| For which one of the following, Bohr model is not valid? |
Singly ionised neon atom $$(Ne^+)$$ |
| Assertion: Atoms are not electrically neutral. Reason: Number of protons and electrons are different. |
Both assertion and reason statements are wrong. |
| Both Assertion and Reason are correct and Reason is the correct explanation for Assertion. |
Thermodynamics Class 11 Engineering Chemistry MCQ Quiz



Thermodynamics Questions and Answers
| Thermodynamics Quiz Question | Answer |
|---|---|
| The total heat content of a system is | Enthalpy |
| Which one of the following equations does not correctly represent the first law of thermodynamics for the given processes involving an ideal gas? (Assume non-expansion work is zero) | Adiabatic process: $$\Delta U=-w$$ |
| At $$320\ K$$, a gas $${A}_{2}$$ is $$20$$% dissociated to $$A(g)$$. The standard free energy change at $$320\ K$$ and $$1$$ atm in $$J$$ $${mol}^{-1}$$ is approximately: $$\left[ R=8.314J{ K }^{ -1 }\ { mol }^{ -1 };\ln { 2 } =0.693;\ln { 3 } =1.098 \right] $$ |
$$4281$$ |
| The INCORRECT match in the following is : | $$\Delta G^0 < 0,K< 1$$ |
| An ideal gas undergoes a cyclic process as shown in Figure. $$\Delta U_{BC}=-5$$ kJ $$mol^{-1}$$, $$q_{AB}=2$$ kJ $$mol^{-1}$$ $$W_{AB}=-5$$ kJ $$mol^{-1}$$, $$W_{CA}=3$$ kJ $$mol^{-1}$$ Heat absorbed by the system during process CA is: 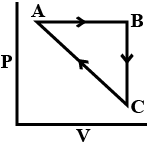 |
$$+5$$ kJ $$mol^{-1}$$ |
| The combustions of benzene (l) gives $$CO_2(g)$$ and $$H_2O(l)$$. Given that heat of combustion of benzene at constant volume is $$-3263.9 \ kJ\, mol^{-1}$$ at $$25^oC$$, heat of combustion (in $$kJ\,mol^{-1}$$) of benzene at constant pressure will be: $$(R =8.314JK^{-1}\,mol^{-1})$$ |
$$-3267.6$$ |
| A process will be spontaneous to all temperatures if: | $$\Delta H < 0$$ and $$\Delta S > 0$$ |
| A gas mixture consists of $$2$$ moles of $$O_2$$ and $$4$$ moles of Ar at temperature $$T$$. Neglecting all vibrational modes, the total internal energy of the system is |
11 RT |
| For a given reaction, $$\triangle H = 35.5\ kJmol^{-1}$$ and $$\triangle S = 83.6\ JK^{-1} mol^{-1}$$. The reaction is spontaneous at: (Assume that $$\triangle H$$ and $$\triangle S$$ do not vary with temperature) | $$T > 425\ K$$ |
| Consider the following processes: $$\Delta H\left({kJ}/{mol}\right)$$ $$\dfrac{1}{2} A \rightarrow B$$ $$+150$$ $$3B \rightarrow 2C + D$$ $$-125$$ $$E + A \rightarrow 2D$$ $$+350$$ For $$B + D \rightarrow E + 2C, \Delta H$$ will be: |
$$-175\ {kJ}/{mol}$$ |
The S-Block Elements Class 11 Engineering Chemistry MCQ Quiz



The S-Block Elements Questions and Answers
| The S-Block Elements Quiz Question | Answer |
|---|---|
| The nitride ion in lithium nitride is composed of: | 7 protons + 10 electrons |
| The correct order of reducing character of alkali metal is: | Na < K < Rb < Li |
| Which of the following is/are the correct order of mobility? | $$\mathrm { Al } ^ { 3 + } < \mathrm { Mg } ^ { 2 + } < \mathrm { Na } ^ { + }$$ |
| In aqueous solution the largest ion is: | $$Li (aq)$$ |
| Sodium metal is soft but iron is a hard metal. this difference arise due to_________ | there is one unpaired electron in sodium but four unpaired electron in iron |
| Which of the following element have maximum tendency to form complex compound_______. | Be |
| Correct order of density is:- | Mg > Ca |
| At infinite dilution whose conductivity is maximum ? | $$Cs^{ + }$$ |
| Which element among the alkali metal is more reactive towards $$O_2$$ ? | Caesium |
| Alkali metals in each period have | Lowest IE |
Chemistry MCQ Questions for Class 11 - Practice Test with Solutions
Do you want to overcome your drawbacks while attempting the quizzes or MCQ tests like time consumption, approaching questions, etc.? Take the advantage of practicing with MCQExams.com MCQ Questions for Standard 11 Chemistry Test. As it is a time-based approach and also provides answers to all questions.
One should practice the MCQs in this way for a better assessment of their preparation level. All chapters CBSE Class 11 Chemistry MCQ Quiz Questions with Solutions PDF free download links are available for easy access & quick reference.
How to Use MCQExams.com Chapterwise 11th Chemistry MCQ Interactive Quiz?
Guys do you love to share your practice hacks and tips with your friends? If yes, then our 11tth standard CBSE Chemistry MCQ interactive quiz help you do the same. Excited to know the process then jump into the below steps right away:
- Go with the respective chapter class 11 Chemistry MCQ quiz link from the above
- Now, you will find the MCQ quiz boxes for the Chemical Bonding And Molecular Structure chapter along with the interactive quiz windows.
- Click on the CBSE 11th Class Chemical Bonding And Molecular Structure MCQ Interactive Quiz and it will redirect you to another window where it displays the questions with options in stories format.
- Answer the question one after another and learn the answers right away this helps you to do a quick assessment of your knowledge.
- You can also share this cool MCQ Interactive Quiz Questions of Plus One Chemistry topicwise with your friends by just tapping on the send arrow located at the top left corner of the story.
- After clicking the button, you can opt for the copy link option and easily paste the link on your friend's chat or else in your whatsapp story too. Isn’t it cool!!
- Keep passing this interesting approach of practicing Plus One CBSE Chemistry Chemical Bonding And Molecular Structure MCQ Questions to your co-students and help them in attempting the entrance exams like JEE & NEET.