MCQ Questions for Class 12 Chemistry Quiz with Answers Chapter Wise PDF Download
CBSE Multiple Choice Type Questions for 12th Class Chemistry PDF formatted study resources are available for free download. These Grade 12 Chemistry CBSE MCQ Mock Test helps you learn & practice the concepts in a fun learning way.
Class 12 Chemistry MCQs Multiple Choice Questions with Answers
Here are the chapterwise CBSE MCQ Quiz Test Questions for Class 12th Chemistry in pdf format that helps you access & download so that you can practice online/offline easily.
- Alcohols,Phenols And Ethers Class 12 Engineering Chemistry MCQ Questions
- Aldehydes,Ketones And Carboxylic Acids Class 12 Engineering Chemistry MCQ Questions
- Amines Class 12 Engineering Chemistry MCQ Questions
- Biomolecules Class 12 Engineering Chemistry MCQ Questions
- Chemical Kinetics Class 12 Engineering Chemistry MCQ Questions
- Chemistry In Everyday Life Class 12 Engineering Chemistry MCQ Questions
- Coordination Compounds Class 12 Engineering Chemistry MCQ Questions
- Electrochemistry Class 12 Engineering Chemistry MCQ Questions
- General Principles And Processes Of Isolation Of Elements Class 12 Engineering Chemistry MCQ Questions
- Haloalkanes And Haloarenes Class 12 Engineering Chemistry MCQ Questions
- Polymers Class 12 Engineering Chemistry MCQ Questions
- Solutions Class 12 Engineering Chemistry MCQ Questions
- Surface Chemistry Class 12 Engineering Chemistry MCQ Questions
- The D-And F-Block Elements Class 12 Engineering Chemistry MCQ Questions
- The P-Block Elements Class 12 Engineering Chemistry MCQ Questions
- The Solid State Class 12 Engineering Chemistry MCQ Questions
Alcohols,Phenols And Ethers Class 12 Engineering Chemistry MCQ Quiz



Alcohols,Phenols And Ethers Questions and Answers
| Alcohols,Phenols And Ethers Quiz Question | Answer |
|---|---|
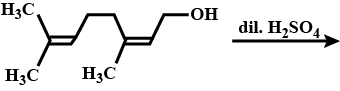 |
|
| Which of the following compounds will most readily be dehydrated to give alkene under acidic condition? | 4-Hydroxypentan-2-one |
Which of the following is not the product of dehydration of :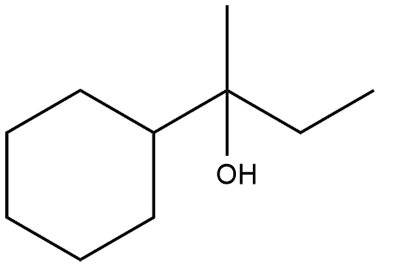 |
|
| Compound A,C8H10O, is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell. A and Y are respectively are: |
|
| The compound A on treatment with Na gives B, and with PCl5 gives C.B and C react together to give diethyl ether. A,B and C are in the order. | C2H5OH,C2H5ONa,C2H5Cl |
The reaction can be classified as: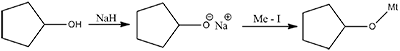 |
williamson ether synthesis reaction |
| Among the following sets of reaction which one produces anisole? | C6H5OH;NaOH,CH3I |
Degree of unsaturation of A=2 and it contains no double or triple bonds. Identify the structure of A.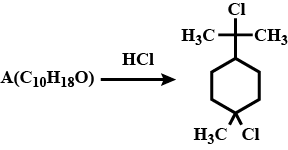 |
|
| Assertion and Reason both are incorrect. | |
| The strongest acid among the following is: | m-methoxyphenol |
Aldehydes,Ketones And Carboxylic Acids Class 12 Engineering Chemistry MCQ Quiz



Aldehydes,Ketones And Carboxylic Acids Questions and Answers
| Aldehydes,Ketones And Carboxylic Acids Quiz Question | Answer |
|---|---|
The reagents X and Y are respectively :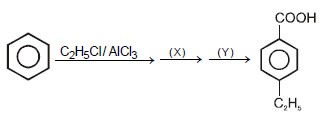 |
X=CH3COCl/AlCl3,Y=I2/NaOH, (acidification) |
| Which of the following reactions will give α− hydroxy acid as a product? | CH3−CHO(i)NaCN/HCl→(ii)H2O/H⊕/Δ |
| The IUPAC name of acetyl acetone is : | pentane-2,4-dione |
| In the given reaction, the product (B) is : CH3CHO(i)NaCN/HCl→(ii)H2O/H⊕/Δ(A)Fenton→reagent(B) |
pyruvic acid |
| Which of the following is example of 1,4- addition reaction ? | |
| When D - glucose is treated with HNO3 then the product is : | |
| Which set of reaction condition is best suited for the preparation of 5−oxohexanoic acid from 5−bromo−2−pentanone? | i) NaCN in ethanol; ii) H3O(+) & heat |
| When D-glucose is treated with Br2 water then the product is : | |
IUPAC name of the following compound is: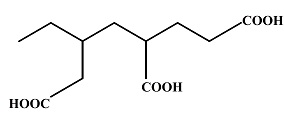 |
5-Ethyl -hexane-1, 3, 6-tricarboxylic acid |
| Which sequence of reactions would be best for the conversion of toluene into para-bromopheny lacetic acid? (NBS = N-bromosuccinimide) | i)Br2 and FeBr3;ii)NBSinCCl4;iii)NaCN in ethanol iv)H3O+ |
Amines Class 12 Engineering Chemistry MCQ Quiz



Amines Questions and Answers
| Amines Quiz Question | Answer |
|---|---|
| Which of the following is NOT a correct method of the preparation of benzylamine from cyanobenzene? | (i) HCl/H2O (ii) NaBH4 |
| Considering the basic strength of amines in aqueous solution, which one has the smallest pKb value? | (CH3)2NH |
In the following reactions, the major product W is: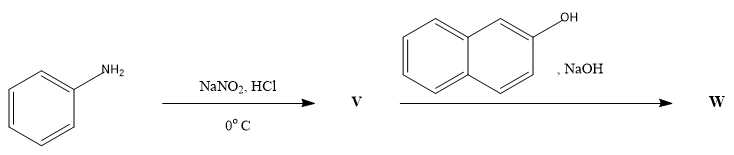 |
|
| Consider the following four compounds I, II, III, and IV. Choose the correct statement(s).  |
Steric effect makes compound IV more basic than III. |
| The correct statement regarding the basicity of arylamines is: | arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring π electron system. |
| Which of the following will be most stable diazonium salt RN+2X−? | C6H5N+2X− |
| Which of the following compounds is most basic? | |
 |
|
The major product of the following reaction is: |
|
| The correct order of the basic strength of methyl substitited amines in aqueous solution is : | (CH3)2NH>CH3NH2>(CH3)3N |
Biomolecules Class 12 Engineering Chemistry MCQ Quiz



Biomolecules Questions and Answers
| Biomolecules Quiz Question | Answer |
|---|---|
| Amylopectin is composed of: | α−D−glucose, C1−C4 and C1−C6 linkages |
| Transition state structure of the substrate formed during an enzymatic reation is | Temporary and unstable. |
| The major role of minor elements inside living organisms is to act as |
Cofactors of enzymes. |
| A non-proteinaceous enzyme is | Ribozyme |
Which of the following describes the given graph correctly?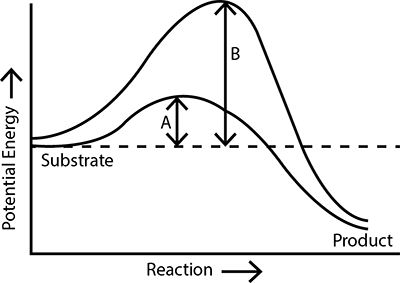 |
Exothermic reaction with energy A in presence of enzyme and B in absence of enzyme. |
| Sucrose on hydrolysis gives | α-D-Glucose +β-D-Fructose |
| Which one of the following statements is correct, with reference to enzymes? | Holoenzyme = Apoenzyme + Coenzyme |
| Starch is a mixture of two components, a water soluble component amylose(15-20%) and a water insoluble component amylopectin (80-85%). The aqueous solution of amylose gives a blue colour with iodine solution due to the formation of: | inclusion complex |
| The metal present in vitamin B12 is: | cobalt |
| Ergocalciferol is name of vitamin: |
D2 |
Chemical Kinetics Class 12 Engineering Chemistry MCQ Quiz



Chemical Kinetics Questions and Answers
| Chemical Kinetics Quiz Question | Answer |
|---|---|
| The rate of a reaction quadruples when the temperature changes from 300 to 310 K. The activation energy of this reaction is : (Assume activation energy and pre-exponential factor are independent of temperature; ln 2=0.693; R = 8.314 J mol−1K−1) |
107.2 kJ mol−1 |
| Higher order (>3) reactions are rare due to: | low probability of simultaneous collision of all the reacting species |
| Activation energy of a reaction: | is independent of temperature |
| The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the: | rate is proportional to the surface coverage |
| A first order reaction has a specific reaction rate of 10−2sec−1. How much time will it take for 20 g of the reactant to reduce to 5 g? | 138.6 sec |
| Both Assertion and Reason are incorrect | |
| For a first order gas phase reaction: A(g)→2B(g)+C(g). P0 be initial pressure of A and P, the total pressure at time 't′. Integrated rate equation is: |
2.303tlog(2P03P0−Pt) |
| The thermal decomposition of HCOOH is a first-order reaction with a rate constant of 2.4×10−3s−1 at certain temperature. How long will it take for three-fourths of the initial quantity of HCOOH to decompose? | 578 sec |
| In a homogeneous reaction A⟶B+C+D the initial pressure was P0 and after time t it was P. Expression for rate constant k in terms of P0, P and t will be: | k=2.303tlog2P03P0−P |
| What is the activation energy (KJ/mol) for a reaction if its rate constant doubles when the temperature is raised from 300K to 400K? (R=8.314 J mol−1K−1) | 6.88 |
Chemistry In Everyday Life Class 12 Engineering Chemistry MCQ Quiz



Chemistry In Everyday Life Questions and Answers
| Chemistry In Everyday Life Quiz Question | Answer | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noradrenaline is a/an: | neurotransmitter | ||||||||||||||||||||
| Which artificial sweetener contains chlorine? | Sucralose | ||||||||||||||||||||
The correct match between Item -I and Item- II is :
|
A→R;B→S;C→P;D→Q | ||||||||||||||||||||
| Which of the following compounds is not an antacid? | Phenelzine | ||||||||||||||||||||
| Artificial sweetener, which is stable under cold conditions only, is: | aspartame | ||||||||||||||||||||
| Both Assertion and Reason are correct and Reason is the correct explanation for Assertion | |||||||||||||||||||||
| Which one of the following is employed as antihistamine? | Diphenyl hydramine | ||||||||||||||||||||
| The important antioxidant used in food is: | BHT | ||||||||||||||||||||
| Veronal, a barbiturate drug, is used as a/an: | sedative | ||||||||||||||||||||
| Name two α-amino acids, which form a dipeptide, which is 100 times more sweet than cane sugar? | Phenylalanine |
Coordination Compounds Class 12 Engineering Chemistry MCQ Quiz



Coordination Compounds Questions and Answers
| Coordination Compounds Quiz Question | Answer |
|---|---|
| On treatment of 100mL of 0.1M solution of CoCl3.6H2O with excess AgNO3;1.2×1022 ions are precipitated. The complex is : | [Co(H2O)5Cl]Cl2.H2O |
| Which of the following complexes is used to be as an anticancer agent? | cis−[PtCl2NH3)2] |
| Iron carbonyl, Fe(CO)5 is __________. | mononuclear |
| The hypothetical complex chloro-diaquatriamminecobalt(III) chloride can be represented as: | [CoCl(NH3)3(H2O)2]Cl2 |
| The colour of blood is red due to the presence of: | haemoglobin |
| The properties of a compound are: | Different from the properties of its constituents |
| The brown complex obtained in the detection of nitrate radical is formulated as [Fe(H2O)5NO]SO4. What is the oxidation number of Fe in this complex? | +1 |
| Which of the following compounds will exhibit the highest magnetic moment? | (b)[Cr(NH3)6]3+ |
| The hybridization of Ag in a [Ag(NH3)2](+) is:- | sp |
| The cobalt hexammine cation has an atom of cobalt in the centre, surrounded by six molecules of ammonia that are attached to it. The shape of this ion is? | Octahedral |
Electrochemistry Class 12 Engineering Chemistry MCQ Quiz



Electrochemistry Questions and Answers
| Electrochemistry Quiz Question | Answer |
|---|---|
| Select among the following statements that characterize a galvanic cell? I: Oxidation occurs at the anode, which is negative. II: Oxidation occurs at the anode, which is positive. III. Reduction occurs at the cathode, which is positive. |
I and III |
| Assertion: The anions migrate to the cathode in an electrolytic cell. Reason: Positively charged ions are attracted to the negatively charged cathode. |
Assertion is false but Reason is true |
| In electrochemical corrosion. the metal undergoing corrosion : | acts as anode |
| Zn(s)|ZnCl2(aq)|Cl−(aq)|Cl2(g)|C(s) According to the above cell diagram the electrochemical cell described, the reaction at anode is : |
Zn → Zn2+ + 2e− |
| Zn(s)|ZnCl2(aq)||Cl−(aq)||Cl2(g)|C(s) The reaction at anode in the electrochemical cell is: |
Zn→Zn2++2e |
| What is a cathode? | Electrode where reduction takes place and electrons are gained. |
Which half-cell reactions describes what is going on at the anode, from the diagram ? 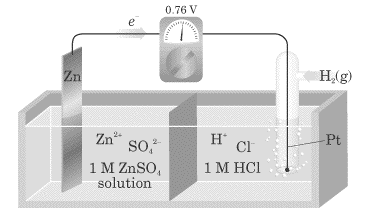 |
Zn→Zn2++2e− |
| What is the correct formula for determining the voltage of an electrochemical cell? | E0cell = E0cathode - E0anode |
| What two metals are often used in electrochemical cells? | Zinc and copper |
| At anode of an electrochemical cell, which of the following change may occur? |
Cl− to Cl2 |
General Principles And Processes Of Isolation Of Elements Class 12 Engineering Chemistry MCQ Quiz



General Principles And Processes Of Isolation Of Elements Questions and Answers
| General Principles And Processes Of Isolation Of Elements Quiz Question | Answer |
|---|---|
| The refining method used when the metal and the impurities have low and high melting temperatures, respectively, is : | liquation |
| The correct statement is : | leaching of bauxite using concentrated NaOH solution gives sodium aluminate and sodium silicate |
| Which one of the following ores is best concentrated by froth floatation method? | Galena |
| Fusion of MnO2, with KOH in presence of O2 produces a salt W. Alkaline solution of W upon electrolytic oxidation yields another salt X. The manganese containing ions present in W and X, respectively are Y and Z. Correct statement(s) is (are) |
Y is diamagnetic in nature while Z is paramagnetic |
| A tin chloride Q undergoes the following reaction (not balanced) Q+Cl−→X Q+Me3N→Y Q+CuCl2→Z+CuCl X is monoanion having pyramidal geometry. Both Y and Z are neutral compounds. Choose the correct options(s) |
There is a coordinate bond in Y |
| Bell metal is an alloy of: | Fe, Sn and Cu |
| Unwanted material with ore is called as ______ . | Gangue |
| In electrolytic refining of any metal impure metal is taken as ............... |
anode |
Complete the following statement with an appropriate word/term to be filled in the blank space. Froth floatation process is used for the concentration of _________ ores. |
Sulphide |
| Reaction takes place in aluminothermic process is also known as _______ . |
thermite reaction |
Haloalkanes And Haloarenes Class 12 Engineering Chemistry MCQ Quiz



Haloalkanes And Haloarenes Questions and Answers
| Haloalkanes And Haloarenes Quiz Question | Answer |
|---|---|
| An unknown alkyl halide [X] reacts with alcoholic KOH and produces a hydrocarbon (C4H8) as the major product. Ozonolysis of this hydrocarbon affords one mole of propanaldehyde and one mole of formaldehyde. Suggest which organic compound among the following has the correct structure of the above alkyl halide [X]? | CH3CH2CH2CH2Br |
| In alkaline hydrolysis of tertiary halide by aqueous alkali if concentration of alkali doubled, then the reaction: | will remain constant |
| An alkane C6H14 gives only two monochloro derivatives on chlorination. Its possible structure is: | CH3−CH3|CH−CH3|CH−CH3 |
| Identify X and Y in the following reaction. H2C|Br−CH2|Br+KOHalcohol→XNaNH2→Y |
X:CH2=CHBr,Y:CH≡CH |
| 2−BromopentaneistreatedwithalcoholicKOH$ solution. What will be the major product formed in this reaction and what is this type of elimination called? | Pent −2− ene, β- Elimination |
| In halogenation of aromatic hydrocarbon, a halogen carrier is used which is generally a Lewis acid. The main function of this reagent is to generate the species : | X+ |
| The correct decreasing order of following alkyl halides according to the rate of β - elimination reaction with alcoholic KOH will be: (i)CH3−H|C|CH3−CH2Br (ii) CH3−CH2−Br (iii) CH3−CH2−CH2−Br |
(i) > (iii) > (ii) |
| Which of the following compounds will react with Na to form 4,5- diethyloctane? |
CH3CH2CH2−C|BrH−CH2CH3 |
| Which of the following is a primary halide? | neo-hexyl chloride |
| SN1 reaction is fastest in? |
Polymers Class 12 Engineering Chemistry MCQ Quiz



Polymers Questions and Answers
| Polymers Quiz Question | Answer |
|---|---|
| Teflon is an example of polymer which is a/an | addition Ploymer |
| Polyester is made up of the repeating units of a chemical called: | ester |
| Plexiglass is a polymer of ____. | methyl methacrylate |
| CF2=CF2 is a monomer of: | Teflon |
| The following are synthetic polymers except : | Proteins |
| Most balloons, tyres and footballs are made-of rubber. This is because rubber is: | soft and flexible |
Ropes of nylon and polyester are commonly used in cranes and elevators. |
False |
| Synthetic fibres are synthesized from raw material called: | polymers |
| As plastics and synthetic fibers consist of repeating monomer units, they are known as ______ . | polymers |
| Which is used in making of thermocol ? | Polystyrene |
Solutions Class 12 Engineering Chemistry MCQ Quiz



Solutions Questions and Answers
| Solutions Quiz Question | Answer |
|---|---|
| When 200 g of an oleum sample labelled as 109% is mixed with 300 g of another oleum sample labelled as 118% the new labelling of resulting oleum sample becomes | 114.4% |
| A sample of oleum is labelled as 112%. In 200 g of this sample, 18 g water is added, The resulting solution will contain | 191.33 g H2SO4 and 26.67 g free SO3 |
| Vapour pressure of liquid | increases with increase in temperature |
| Which of the following behaviour is true about the ideal binary liquid solution of liquids A and B if P0A<P0B? | plot of Ptotal|vsXB is linear with +ve slope |
| Benzene and toulene from an ideal solution, the vapour pressures of the benzene and toluene are 75 mm and 25 mm respectively at 200C if the mole fractions of benzene and toluene in vapour are 0.75 and 0.25 respectively the vapour pressure of the ideal solution is | 62.5 mm |
| The vapour pressure of a soluiton of two liquids A ( P0=80mm,X=0.4) and B(P0=120mm,X=0.6) is found to be 100 mm, it shows that the solution exhibits |
negative deviation from ideal behaviour |
| Heptane and octane from ideal solution at 373 K the vapour pressure of the pure liquids are 106 kPa and 46 kpa respectively . what will be the vapour pressure, in bar of mixture of 30.0 g of heptane and 34.2 g of octane ? | 0.76 bar |
| For an ideal solution of A and B , YA is the mole fraction of A in the vapour phase at equilibrium . which of the following plot should be linear ? | 1PtotalvsYA |
| Under the condition of a similar temperature, which of the following solution will have, minimum vapour pressure? | 0.1 M Al2(SO4)3 |
| A mixture contains 1 mole of volatile liquid A(P0A=100mmHg) and 3 moles of volatile liquidB(P0B=80mmHg) if the solution behaves ideally, the total vapour pressure of the distillate is | 85 mm Hg |
Surface Chemistry Class 12 Engineering Chemistry MCQ Quiz



Surface Chemistry Questions and Answers
| Surface Chemistry Quiz Question | Answer |
|---|---|
| A particular adsorption process has the following characteristics : (i) It arises due to van der Waals forces and (ii) it is reversible. Identify the correct statement that describes the above adsorption process : | Energy of activation is low |
| The most appropriate method of making egg-albumin sol is: |
Break an egg carefully and transfer the transparent part of the content to 100 mL of 5% w/V saline solution and stir well. |
| Peptization is a : | process of converting precipitate into colloidal solution |
| The moles of HCl required to neutralise left NH3 solution after adsorption is: | 0.175 |
Mention catalyst in the following reaction: KClO3→KCl+O2 |
MnO2 |
| Which is correct in case of heterogeneous catalyst? |
The catalyst actually forms a compound with reactants. |
Substances, which are added to soaps change their properties in order to make them more useful for a particular application, are called ___. |
fillers |
| A freshly obtained precipitate of SnO2 is peptized by little of KOH to give a sol. The sol particles may be represented as : |
[SnO2]SnO2−3 |
| Milk is an example of : | Emulsion |
| Which of the following is homogeneous? |
Milk |
The D-And F-Block Elements Class 12 Engineering Chemistry MCQ Quiz



The D-And F-Block Elements Questions and Answers
| The D-And F-Block Elements Quiz Question | Answer |
|---|---|
| The ionic charges on chromate ion and dichromate ion respectively is: | −2,−2 |
| Which element is a transition metal? | melting point in oC - 651; density in g/cm3- 1.7; colour of oxide - white |
| E1, E2 and E3 are the emf values of the three galvanic cells respectively. (a) Zn|Zn+21M||Cu+20.1M|Cu (b) Zn|Zn+21M||Cu+21M|Cu (c) Zn|Zn+20.1M||Cu+21M|Cu Which one of the following is true? |
E3>E2>E1 |
| Which of the following pair has similar magnetic moment? | Fe3+,Mn2+ |
| Gd (64) has _________ unpaired electrons with sum of spin __________. | 8,4 |
| Amphoteric behaviour is shown by the oxides of: | Sn and Zn |
| The first IE of lithium is 5.4eV and first electron affinity of Cl is 3.6eV. The value of ΔH for the reaction Li(g)+Cl(g)→Li+(g)+Cl−(g), is (in eV) : |
+1.8 |
| Potassium dichromate can be prepared by: |
both A and C |
| The magnetic moment of [Cu(NH3)4]2+ was found to be 1.73 B.M. The number of unpaired electrons in the complex is: |
1 |
| AgCl and NaCl are colourless. NaBr and NaI are also colourless but AgBr and AgI are coloured. This is due to: | Ag+ polarizes Br− and I− |
The P-Block Elements Class 12 Engineering Chemistry MCQ Quiz



The P-Block Elements Questions and Answers
| The P-Block Elements Quiz Question | Answer |
|---|---|
| Which of the following elements belongs to halogen group? | [Kr],4d10,5s2,5p5 |
| In the modern periodic table, elements are arranged in order of increasing atomic number which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Aufbau principle filling of a new energy shell. In accordance with the Aufbau principle, the seven period (1 to 7) have 2,8,8,18,18,32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f - block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table. The elements with atomic number 35,53 and 85 are all ________. |
Halogens |
| The elements with atomic numbers 10, 18, 36, 54 and 86 are all: | inert gases |
| Which of the block of elements does belong to inert gases? | p-block |
| A certain element forms a solid oxide which dissolves in water to form an acidic solution. The element is: | P |
| Which of the following statements is correct? | All halogens except fluorine form oxyacids |
| A radioactive halogen is: | astatine |
| How many electrons are present in the outermost orbit of halogens? | 7 |
| Halogens show usually: | Both electrovalency and covalency |
| The outermost electronic configuration of the most electronegative element is: | ns2np5 |
The Solid State Class 12 Engineering Chemistry MCQ Quiz



The Solid State Questions and Answers
| The Solid State Quiz Question | Answer |
|---|---|
| If A is the molecular mass of an element, a is the edge length and N is the Avogadro's number, then the density for simple cubic lattice is : |
ANa3 |
Statement: The molecular orbitals in a crystalline solid are delocalised. State whether the given statement is true or false. |
True |
Statement: Imperfection in solids give rise to new properties in it. State whether the given statement is true or false |
True |
Statement: A cubic crystal possess all 23 elements of symmetry. State whether the given statement is true or false |
True |
| Identify the amorphous substance from the following. | clay |
| An element A (atomic weight =100 amu) having bcc structure has unit cell edge length of 400 pm. Calculate the density of A. |
5.188 g cm−3 |
| Which of the following is a non-crystalline solid? | Glass |
| The lattice parameters, a≠b≠c and α=γ=90∘,β≠90∘ represents a : | monoclinic system |
| Bravais lattices are of___________. | 14 types |
| Diamond belongs to which of the following crystal system? | Cubic |
Chemistry MCQ Questions for Class 12 - Practice Test with Solutions
Do you want to overcome your drawbacks while attempting the quizzes or MCQ tests like time consumption, approaching questions, etc.? Take the advantage of practicing with MCQExams.com MCQ Questions for Standard 12 Chemistry Test. As it is a time-based approach and also provides answers to all questions.
One should practice the MCQs in this way for a better assessment of their preparation level. All chapters CBSE Class 12 Chemistry MCQ Quiz Questions with Solutions PDF free download links are available for easy access & quick reference.
How to Use MCQExams.com Chapterwise 12th Chemistry MCQ Interactive Quiz?
Guys do you love to share your practice hacks and tips with your friends? If yes, then our 12tth standard CBSE Chemistry MCQ interactive quiz help you do the same. Excited to know the process then jump into the below steps right away:
- Go with the respective chapter class 12 Chemistry MCQ quiz link from the above
- Now, you will find the MCQ quiz boxes for the Chemical Kinetics chapter along with the interactive quiz windows.
- Click on the CBSE 12th Class Chemical Kinetics MCQ Interactive Quiz and it will redirect you to another window where it displays the questions with options in stories format.
- Answer the question one after another and learn the answers right away this helps you to do a quick assessment of your knowledge.
- You can also share this cool MCQ Interactive Quiz Questions of Plus Two Chemistry topicwise with your friends by just tapping on the send arrow located at the top left corner of the story.
- After clicking the button, you can opt for the copy link option and easily paste the link on your friend's chat or else in your whatsapp story too. Isn’t it cool!!
- Keep passing this interesting approach of practicing Plus Two CBSE Chemistry Chemical Kinetics MCQ Questions to your co-students and help them in attempting the entrance exams like JEE & NEET.