Acids Bases And Salts - Class 7 Chemistry - Extra Questions
Acids contain replaceable __________.
Why would it be useful for a gardener or a vegetable farmer to use litmus paper to test soil samples?
A restaurant owner has a few bottles of soft drinks in his restaurant. But, unfortunately, these are not labeled. He has to serve the drinks on the demand of customers. One customer wants an acidic drink, another wants a basic and the third wants a neutral drink. How will the restaurant owner decide which drink is to be served to whom?
A yellow curry stain on a white shirt turns red when it is washed with soap. Explain why?
How do you prepare your own indicator using beetroot? Explain.
Name a synthetic indicator.
Give two important uses of washing soda and baking soda.
Explain why:
(a) An antacid tablet is taken when you suffer from acidity.
(b) Calamine solution is applied on the skin when an ant bites.
(c) Factory waste is neutralized before disposing it into the water bodies.
Dry ammonia has no action on litmus paper but a solution of ammonia in water turns red litmus paper blue. Why is it so?
Litmus paper is a__________ indicator.
Cutting onions taken from fridge is more comfortable than cutting onions at room temperature. Explain why?
China rose indicator is turned _______by a basic solution.
Write the scientific reason.
Lemon sherbat has sweet, sour and salty taste and it can be poured in a glass.
Blue litmus solution is added to two test tubes A and B containing dilute hydrochloric acid and sodium hydroxide solutions, respectively. In which test tube a colour change will be observed? State the colour change and give its reason.
What is an acid? Write some of the properties of acids.
Why does dry HCl gas not change the colour of the dry litmus paper?
Define the following terms:
(a) Standard solution (b) Normal solution (c) Indicator
If someone is suffering from the problem of acidity after overeating, which of the following would you suggest as a remedy?
Lemon juice, vinegar, baking soda solution. Give reason for your choice.
Give reason for your choice.
(a) Define an acid and a base. Give two examples of each.
(b) Give the names and formulae of two strong bases and two weak bases.
(c) What type of ions are formed:
(i) when an acid is dissolved in water?
(ii) when a base (or alkali) is dissolved in water?
(d) Write the neutralisation reaction between acids and bases in terms of the ions involved.
(e) Write any two important uses of bases.
What would be the color of litmus in a solution of sodium carbonate?
Give any two practical applications of neutralisation reaction.
A knife that is used to cut fruit was immediately dipped into water containing drops of blue litmus solution. The color of the solution changes to red. What is the nature of the fruit?
What type of reaction is represented by the digestion of food in our body?
(i) Name one antacid. How does it help to relieve indigestion in the stomach?
(ii) A farmer treats the soil with quicklime or calcium carbonate. What is the nature of soil? Why does the farmer treat the soil with quicklime?
Give reason why antacids are required when there is pain or irritation in the stomach ?
Hritik tested rainwater and found a pH of 5-6 due to presence of H+ ions formed by reaction of rainwater with carbon dioxide. When pH of the rainwater drops below 5-6, then it is called acid rain. Oxides of nitrogen and sulphur are acidic in nature. Burning of fossil fuels such as coal and oil in power stations and furnace produce oxides of nitrogen and sulphur. He told all of his classmates living nearby to use bicycle instead of school bus so as to reduce air pollution and prevent acid rain.
(a) Which acid is formed by sulphur dioxide in the presence of air?
(b) Which acid is formed by nitrogen dioxide in the presence of air?
(c) Why is acid rain harmful to agriculture, trees and plants?
(d) How can we prevent from acid rain?
(e) What values are possessed by Hrithik?
(a) Which acid is formed by sulphur dioxide in the presence of air?
(b) Which acid is formed by nitrogen dioxide in the presence of air?
(c) Why is acid rain harmful to agriculture, trees and plants?
(d) How can we prevent from acid rain?
(e) What values are possessed by Hrithik?
Match the substances in Column I with those in Column II
| Column I | Column II |
| a) Tartaric acid | i) Soap |
| b) Calcium hydroxide | ii) curd |
| c) Formic acid | iii) Unripe mangoes |
| d) Sodium hydroxide | iv) Ant's sting |
| e) Lactic acid | v) Lime water |
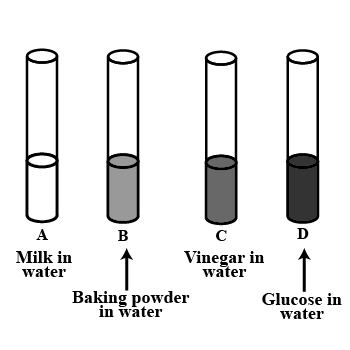
Look at Figure which shows solutions taken in test tubes A,B,C and D. What colour is expected when a piece of red litmus paper is dropped in each test tube?
Nature of the solutions is given in the table for your help.
| Test | Nature of solution | Change in colour of red litmus |
| A | Neutral | |
| B | Basic | |
| C | Acidic | |
| D | Neutral |
Look at the given reaction.
Hydrochloric acid + Sodium hydroxide (base) → Sodium Chloride(salt) + Water
Solium chloride formed in this reaction remains in solution form. Can we get solid sodium chloride from this solution? Suggest a method (if any).
Form a sentence using the following words - baking soda, ant bite, moist, effect, neutralised, and rubbing.
While playing in a park, a child was stung by a wasp. Some elders suggested applying paste of baking soda and others lemon juice as remedy. Which remedy do you think is appropriate and why?
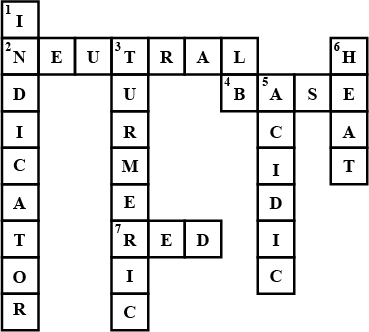
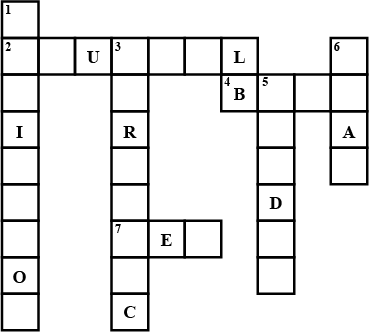
Fill in the cross word given as Figure with the help of the clues provided.
Across
(2) The solution which does not change the colour of either red or blue litmus.
(4) Phenolphthalein gives pink colour in this type of solution.
(7) Colour of blue litmus in lemon juice.
Down
(1) It is used to test whether a substance is acidic or basic.
(3) It is a natural indicator and gives pink colour in basic solution.
(5) Nature of ant's sting.
(6) It is responsible for increase in temperature during a neutralisation reaction.
Explain two neutralisation reactions related to daily life situation.
Fill the blanks in the following sentences
Phenolphthalein gives ________ colour with lime water.
You are provided with four test tubes containing sugar solution, baking soda solution, tamarind solution, salt solution. Write down an activity to find the nature (acidic/basic/neutral) of each solution.
A farmer was unhappy because of his low crop yield. He discussed the problem with an agricultural scientist and realised that the soil of his field was either too acidic or too basic. What remedy would you suggest the farmer to neutralise the soil?
Fill in the blanks with correct options given with question:Turmeric and litmus are ________ acid-base indicators. (natural/synthetic)
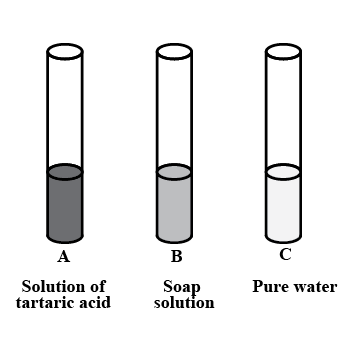
You are provided with three test tubes A, B and C as shown in figure with different liquids. What will you observe when you put:
a) A piece of blue litmus paper in each test tube.
b) A piece of red litmus paper in each test tube.
c) A few drops of phenolphthalein solution to each test tube.
Fill the blanks in the following sentences.
Lemon juice and vinegar taste _______ because they contain ________.
Paheli observed that most of the fish in the pond of her village were gradually dying. She also observed that the waste of a factory in their village is flowing into the pond which probably caused the fish to die.
i) Explain why the fish were dying.
ii) If the factory waste is acidic in nature, how can it be neutralised?
What will be the action of the following substances on litmus paper? Dry HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
Name the acid present in ant sting and give its chemical formula. Also, give the common method to get relief from the discomfort caused by the ant sting.
What do you observe when the following substance is burned and then tested with moist blue and red litmus paper?Phosphorous
Fill the blanks in the following sentencesWhen an acidic solution is mixed with a basic solution, they ________ each other forming ________ and water.
Paheli is suffering from indigestion due to acidity. Is it advisable to give her orange juice in this situation and why?
What do you observe when the following substance is heated and then tested with moist blue and red litmus paper?Magnesium
Give reason:
A wasp sting is treated with vinegar.
What do you observe when blue litmus paper is dipped in dilute hydrochloric acid?
Write down the acids present in vinegar, grapes and lemon.
Give reason:
A person suffering from acidity is advised to take an antacid.
What do you understand by the terms acid and base.
You have been provided with three tubes. One of them contains distilled water and the other two have an acidic solution and a basic solution respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Write the ion that turns red litmus blue.
What do you observe when blue litmus solution is added to water?
Give reason:
Acidic soil is treated with quick lime.
Write the names of any two natural indicators.
Write down the changes that will be seen in the instance and explain the reason behind it.
A litmus paper was dropped into 2ml dilute HCI. Then 2ml concentrated NaOH was added to it and stirred.
Dry HCl gas does not change the colour of dry litmus paper. Why ?
Fill in the blanks using appropriant words.
Main constituent of acid is _________
Bases turn red litmus to ....................
Match the columns
Fill in the blank.
If M is a non-metal, then the blue litmus will turn _______.
If a solution changes the colour of litmus from red to blue, what can you say about its pH?
Write down only the word which will correctly complete each of the following sentences.
If M is a metal, then the litmus will turn__________.
Write three differences between acids and bases.
Write the name of two artificial indicators.
What are indicators? Name any two indicators. Explain the effect of these indicators on acid and bases.
What happens if waste materials of industries is drained off without treatment?
What are acids? Write the name of two food items which contains acids.
What are antacids?
When a blue litmus is dipped in a solution it becomes red. Write the nature of the solution. Explain it
The substances which have no effect on litmus paper are called ...................... substances.
Acid turns blue indicator to ________.
Acid is derived from word .....................
Give examples of natural indicators.
Write the conjugate acid of the following:
S2−,NH3,H2PO−4,CH3NH2
What is the role of bases in disposal of wastes from factories?
What will you take to get relief from hyperacidity?
Write some properties of acids.
What is an acid?
Find the characteristics of the substances given in the table below using litmus papers.
| Substance | Change in the color of litmus | Characteristics |
| Vinegar | ||
| Lime water | ||
| Soap solution | ||
| Hydrochloric acid |
What can be inferred about the nature of the following substance from the litmus test?Distilled water
Organic acids are present in a number substances we use in our daily life.
(eg. Tomato, orange, apple, grapes, curd, etc.) Identify the organic acids in each of them and tabulate.
From the characteristics given below, find out those that are suitable for acids and put (✓) Mark.
Have a bitter taste.
Turn blue litmus red.
React with carbonates to form carbon dioxide gas
Soapy to touch
Liberate hydrogen on reaction with highly reactive metals like Mg and Zn
Have a sour taste
Turns red litmus blue.
Take equal quantities of dilute hydrochloric acid, sodium hydroxide solution and distilled water in three test tubes. Use red litmus paper and blue litmus paper to find out the nature of the solutions. Also, add two or three drops of phenolphthalein solution to the three test tubes. Record the observation and find out the nature of the substances.
Do you notice any colour change in distilled water? What property of water is revealed here?
Blue litmus paper is dipped in a solution. It remains blue. What is the nature of the solution? Explain
Dip a red litmus paper in dilute hydrochloric acid. What change do you observe?
Solution of sodium carbonate, potassium chloride, and ammonium sulfate are taken in separate beakers. Dip a litmus paper (red, blue) in each beaker.
| Salt | Colour of litmus paper | Nature of the substance |
| A | ||
| B | ||
| C |
Wet blue and red litmus papers are placed over the watch glass containing soap solution. Which litmus paper shows a color change?
Give a method that is used to identify acids and bases.
Fill in the blanks:
China rose indicator turns acidic solutions to dark __________ and basic solutions to green.
When an ant bites, it injects _________ into the skin.
Litmus is extracted from ______.
Lichens are a source of which natural indicator?
The color of a blue litmus paper when dipped in a solution remains blue. The solution is .......... or ..........
You are provided with three test tubes A, B and C which contain distilled water, acidic solution and basic solution respectively. If you are given blue litmus paper only, how will you identify the contents of each test tube.
(a) What is litmus? How is litmus paper obtained?
(b) Write the method of preparation of turmeric paper.
Match the List I(Indicator) with List II(Types of titration)
The pH values of three solutions A, B and C are given in the table. Answer the following questions:
| Solution | pH value |
| A B C | 12 2 7 |
(ii) Which solution will liberate CO2 when reacted with sodium carbonate?
(iii) Which solution will turn red litmus solution blue?