Physical And Chemical Changes - Class 7 Chemistry - Extra Questions
Write some changes happening in our body.
To walk through a waterlogged area, you usually shorten the length of your dress by folding it. Can this change be reversed?
Fill in the blanks:A new substance is formed in a _____________ change.
Give two examples of a chemical change.
Classify the following as physical or chemical change: Boiling
Churning of milk cream to get butter is a _______ change.
_____, water, and water vapour look different and display different physical properties but they are chemically the same.
Change in the color of black tea on adding lemon juice is an example of _________ changes.
Rising of hot air over a radiator is a chemical or physical change? Give reason.
Drying of a shirt in the sun is an example of __________ change.
Burning of kerosene in a lantern is an example of _______ changes.
Dissolution of salt in water is a ______ (physical/chemical) change.
Freezing of water and evaporation of water are both physical changes.
List six physical changes that matter can go through.
Iron is a solid, grey metal. Oxygen is a colourless gas. When they chemically combine, rust is made. Rust has a reddish brown colour. Why is rust different from the iron and oxygen that it is made of?
An object was originally a copper colour. After being exposed to the air, it turned a greenish colour. What kind of change happened? Explain your answer.
A .............. change is a change that does not change the identity of the substance.
Dissolution of salt in water is a _________ change
Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of Iron
(c) Mixing of iron fillings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle.
Can you change the shape of an eraser after erasing it?
Why does a blacksmith heat the metal rim to fix it on a cart wheel?
Can the rusting of iron nails occur in distilled water? Justify your answer.
What is a chemical change? Explain with example.
Give two examples of fast changes.
Why does a lump of cotton wool shrink in water? This is a physical or a chemical change?
When a candle burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
How would you show that setting of a curd is a chemical change?
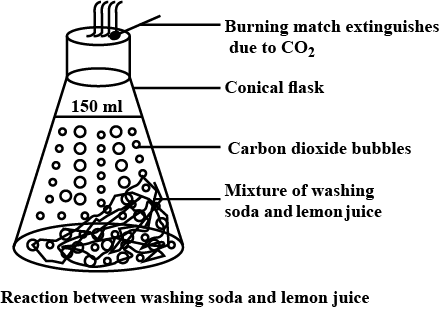
When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Give the two methods by which rusting of iron can be prevented.
Explain why rusting of iron objects are faster in coastal area than in deserts?
Explain how painting of an iron gate prevents it from rusting?
State two differences between rusting and burning.
Explain energy changes in a chemical change.
Which of the following is a physical change?
i. Burning of magnesium
ii. Dissolving of sugar in water
iii. Formation of compound from its element
iv. Rusting of iron
The burning of a piece of magnesium ribbon in air is a chemical change. Explain.
Why do we apply paints on iron goods?
What is rust?
What kind of change is shown by tearing of paper?
Classify the following process into physical or chemical changes:Digestion of food.
Match the items of Column I with the items of Column II.
Column I
Column II
a) Large crystals
i) Turns lime water milky.
b) Depositing a layer of zinc on iron
ii) Physical change
c) Souring of milk
iii) Rust
d) Carbon dioxide
iv) Sugar candy (mishri)
e) Iron oxide
v) Chemical change
f) Dissolving common salt in water.
vi) Galvanisation
Fill in the blanks:Grinding of the wheat grains is done to change their size. It is a ___________ change.
Fill in the blanks:Making sugar solution is a __________ change.
Burning of crackers is a physical or chemical change?
Melting of wax is a change where a solid changes to a liquid state. Give one more such change which you observe in your surroundings.
Give two examples for the following casePhysical changes which are reversible.
Classify the following process into physical or chemical changes:Cutting of a log of wood into pieces.
Classify the following process into physical or chemical changes:Beating of aluminium metal to make aluminium foil.
Give two examples for the following case:Chemical changes.
Define rusting? What are two necessary condition for rusting of iron. Give the chemical name of rust.
Which among the following are physical or chemical changes?
(a) Evaporation of petrol
(b) Burning of Liquefied Petroleum Gas (LPG)
(c) Heating of an iron rod to red hot
(d) Curdling of milk
What do you observe when an iron nail is kept in tap water for few days?
Give two examples for the following case:Physical changes which are not reversible.
Classify burning of kerosene in a lantern as a physical or a chemical change. Give reasons.
Classify drying of a shirt in the sun as a physical or a chemical change. Give reasons.
Classify rising of hot air over a radiator as a physical or a chemical change. Give reasons.
Classify change in the colour of black tea on adding lemon juice to it as a physical or a chemical change. Give reasons.
State whether the following is physical or chemical change: Burning of wood.
State whether the following is physical or chemical change: Water cycle in nature.
Fill in the blanks:An earthquake is a _____________ change.
A physical change is temporary. Explain.
State whether the following is physical or chemical change: Burning of sugar
Sublimation is classified as a physical change. Why?
Give reasons:Melting of solid (wax) is a physical change.
Classify the following as a physical or a chemical change:
Drying of wet clothes
Give reason :
Freezing of water to ice and evaporation of water are physical changes .
What is rusting?
Fill in the blanks :
There is no change in the __________ of the substance during a physical change.
Give reason: Cutting of a cloth piece is a physical change, though it cannot be reversed.
Classify the following as a physical or a chemical change:
Manufacture of salt from sea water
How can you say that the process of digestion is a chemical change?
Name the phenomenon which causes conversion of Ice into water.
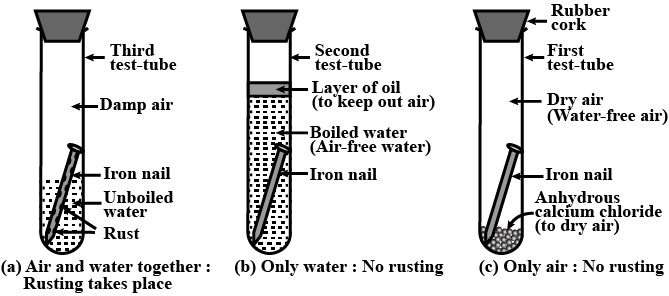
Three experiments to study the process of rusting are given below. Observe the three test tubes and answer the following questions.
A. Why the nail in the test tube 2 is not rusted?
B. Why is the nail in the test tube 1 is rusted highly?
C. Would the nail in the test tube 3 get rusted?
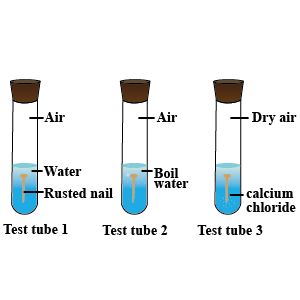
What will you take into account while identifying the following?
A physical change in a substance.
Fill in the blank:
A .......... change is a temporary change.
When hydrogen burns in oxygen, water is formed; when electricity is passed through water, hydrogen and oxygen are given out. Name the type of chemical changes involved in the two cases.
Complete the statement by filling the gap using the appropriate term.
Rusting of iron is a ..........change.
State four ways to prevent rusting.
Give a scientific explanation for following statement:
The aluminum article is used as an anode in the anodizing process.
The aluminum article is used as an anode in the anodizing process.