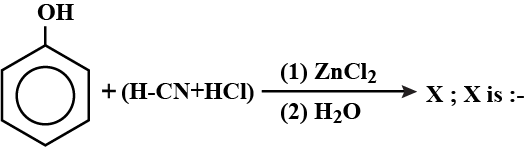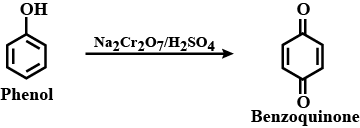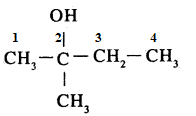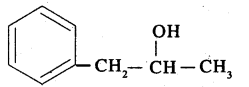Alcohols, Phenols And Ethers - Class 12 Medical Chemistry - Extra Questions
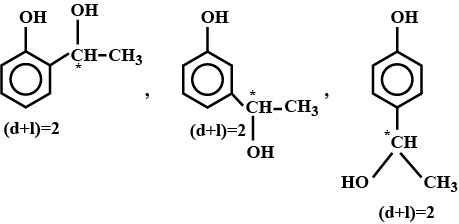
An organic compound $$(C_8 H_{10} O_2)$$ rotates plane-polarized light. It produces pink color with neutral $$FeCl_3$$ solution. What is the total number of all the possible isomers for this compound?
Solve:
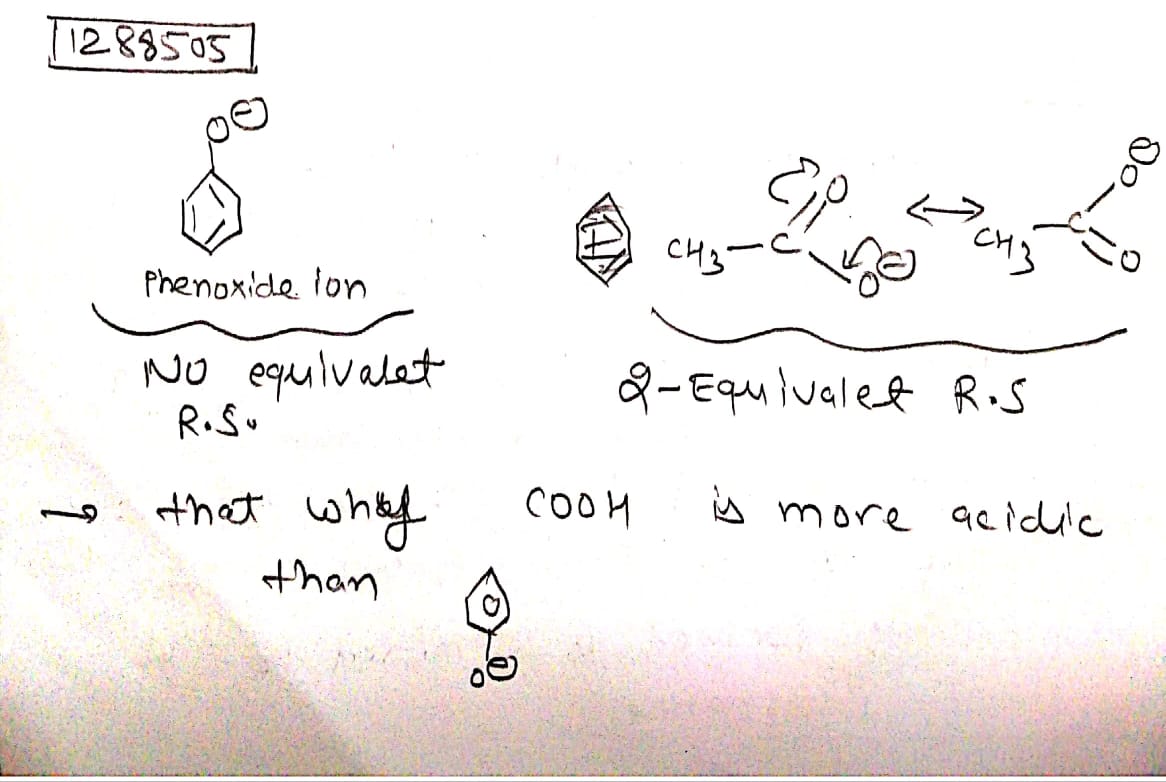
Explain why hydroxyl group attached to an aromatic ring is more acidic than the one in which hydroxyl group is attached to an alkyl group?
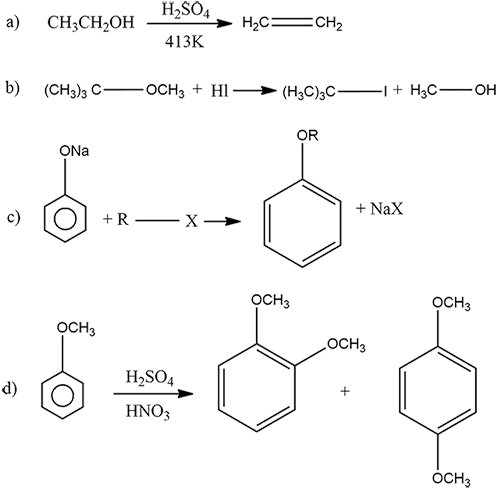
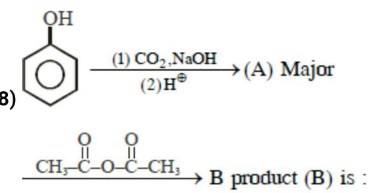
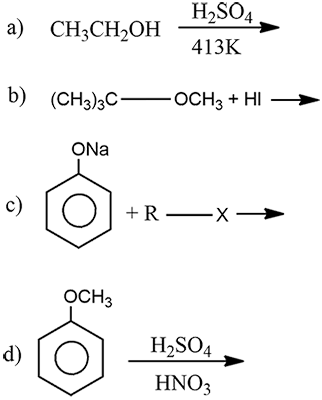
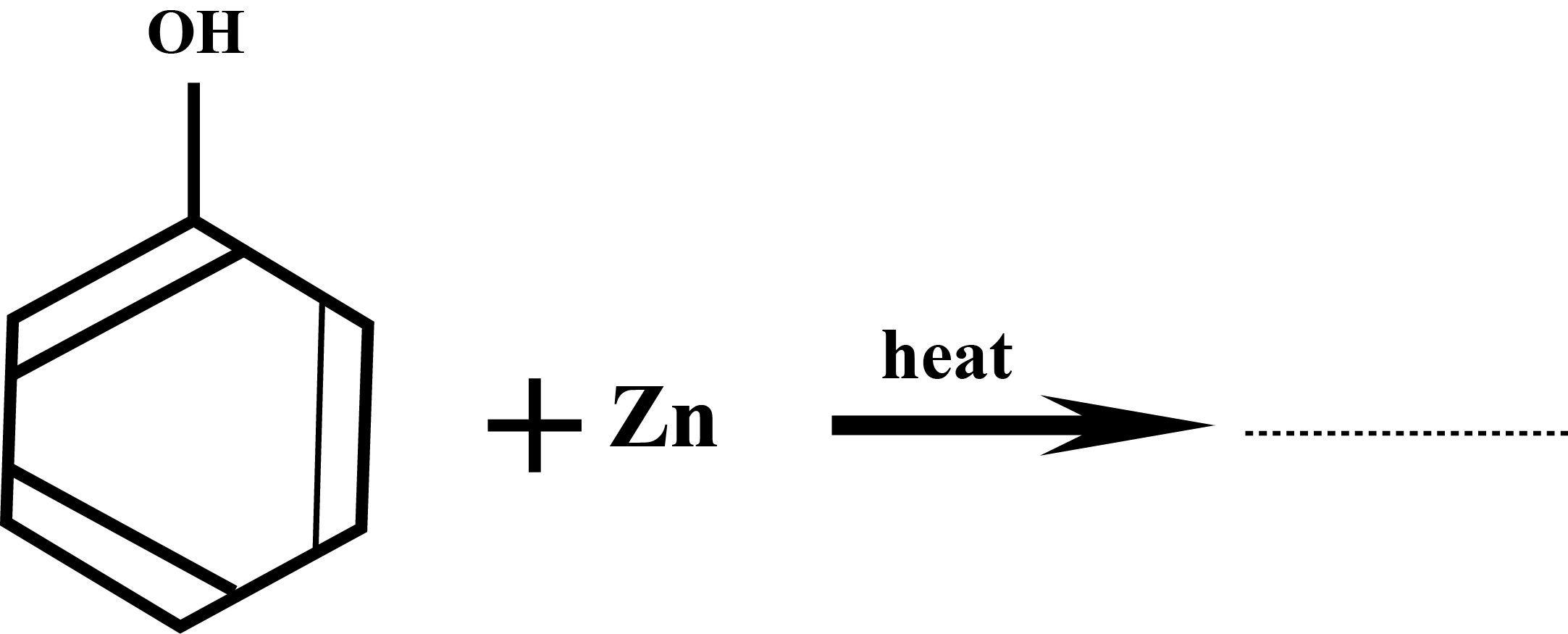
Complete the following chemical reactions:
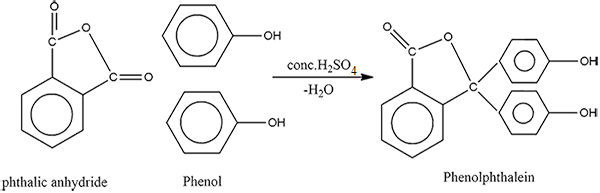
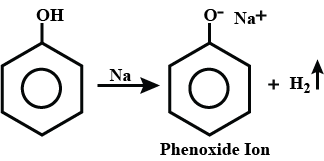
How will you convert phenol to phenolpthalein?
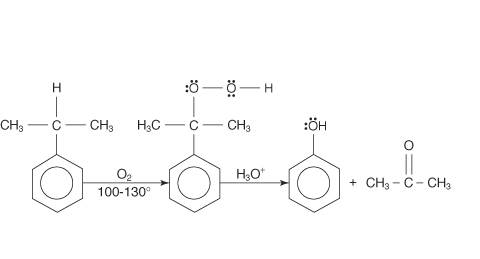
a) i) Explain the preparation of phenol from cumene.
ii) Complete the reaction (please find above image).
b) Explain Williamson's ether synthesis.
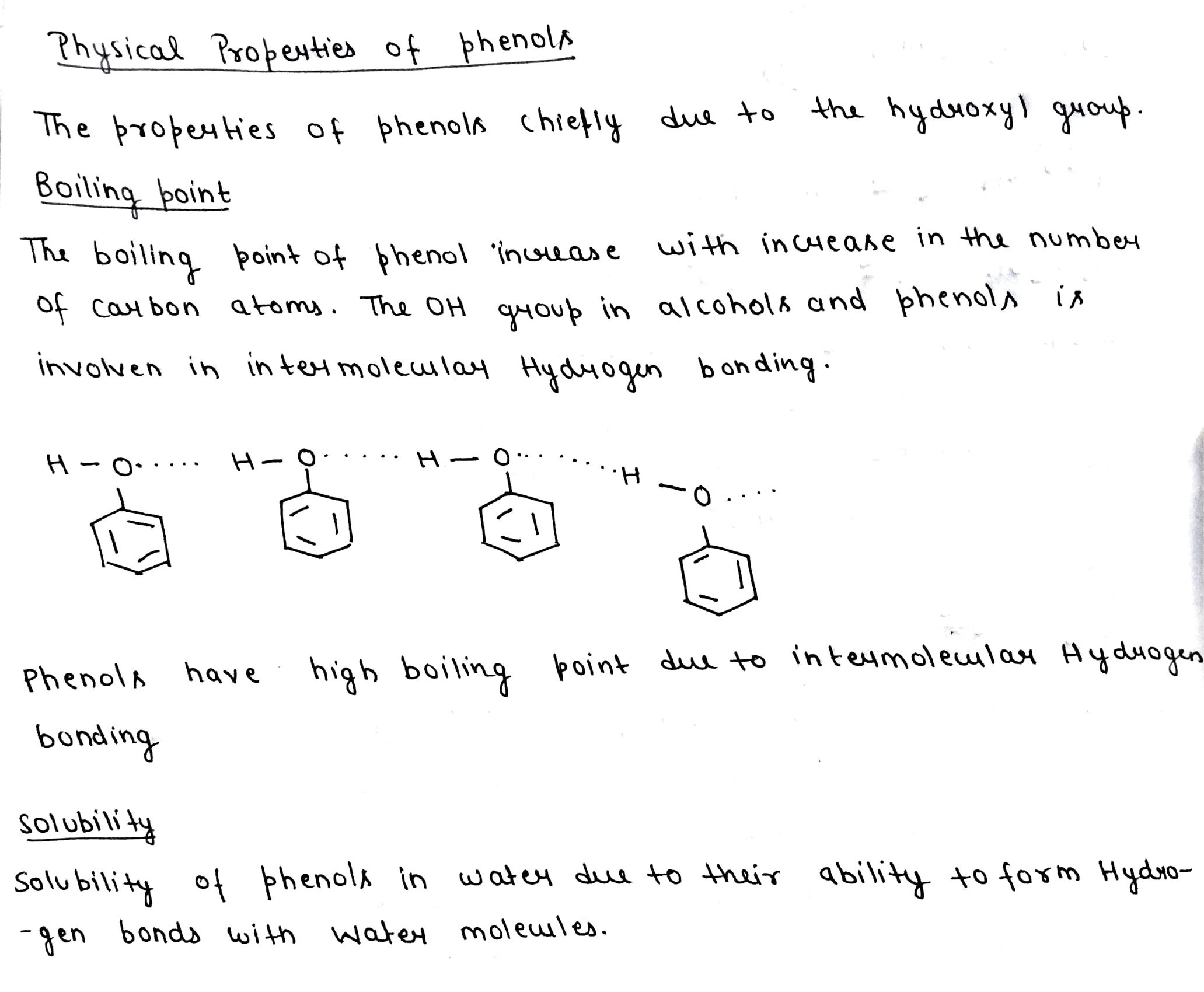
Why do alcohols possess higher boiling points as compared to those of corresponding alkanes?
Find the output for the given reaction.
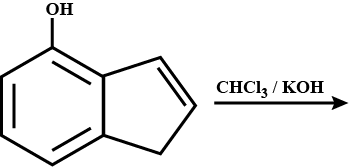
Double bond equivalent of $$D$$ is:
Distinguish between phenol and benzoic acid with a suitable chemical equation.
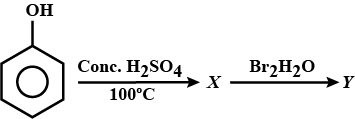
Identify the end product (Y) of the following sequence of reaction.
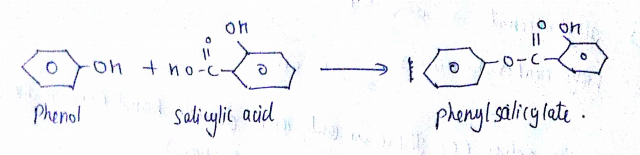
Can we react Salicylic acid with $$ph-OH$$?
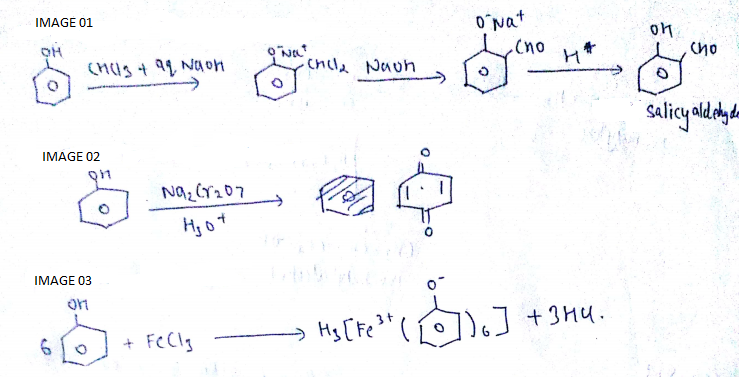
Write the reactions for the conversion of phenol to:
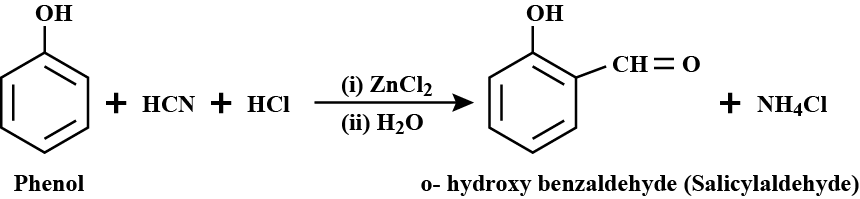
a) Salicylaldehyde
b) Benzoquinone
c) Ferric phenoxide
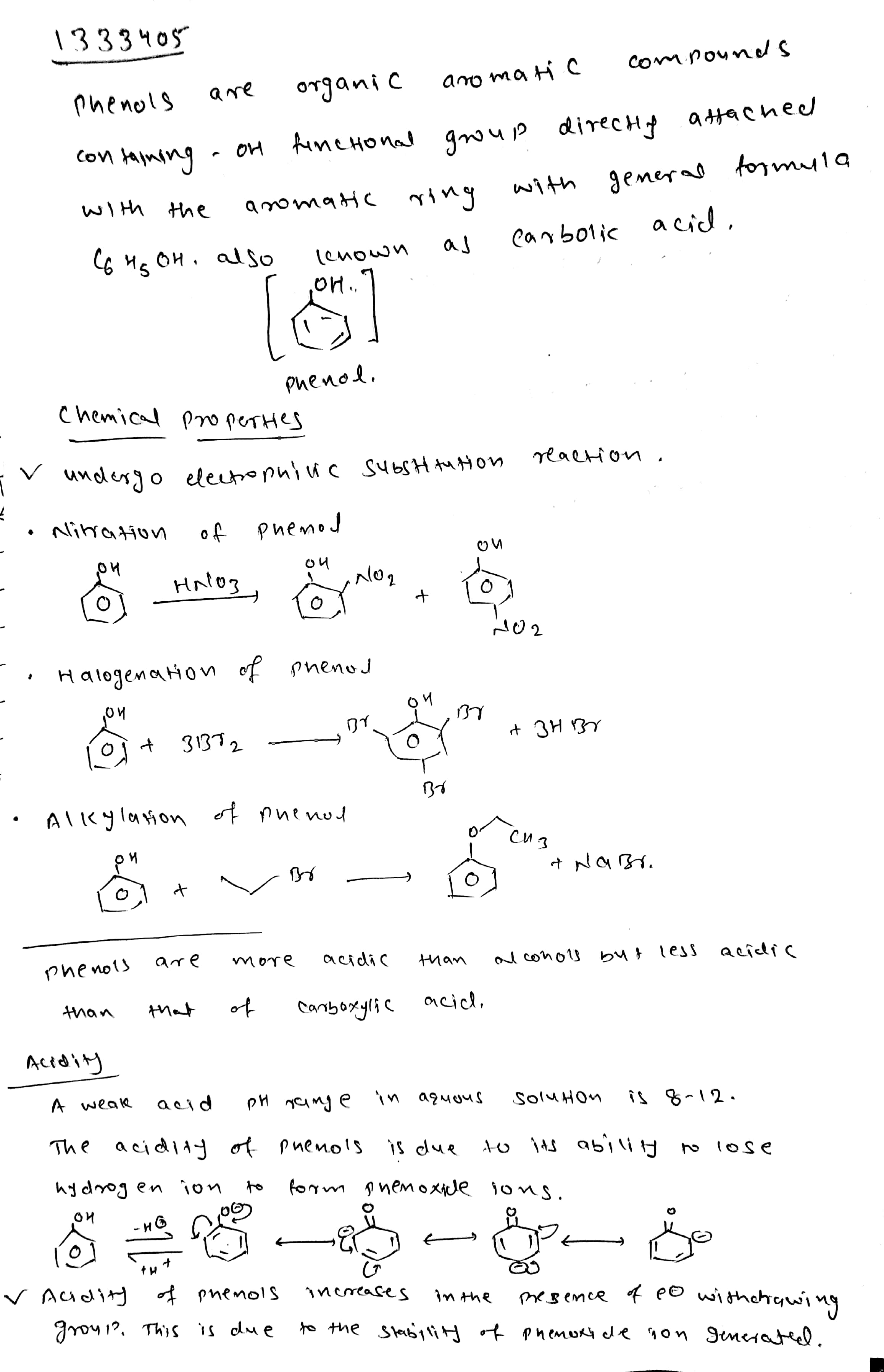
Explain why phenol is more acidic than ethanol?
What will be product T?
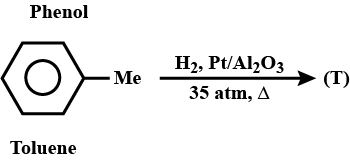
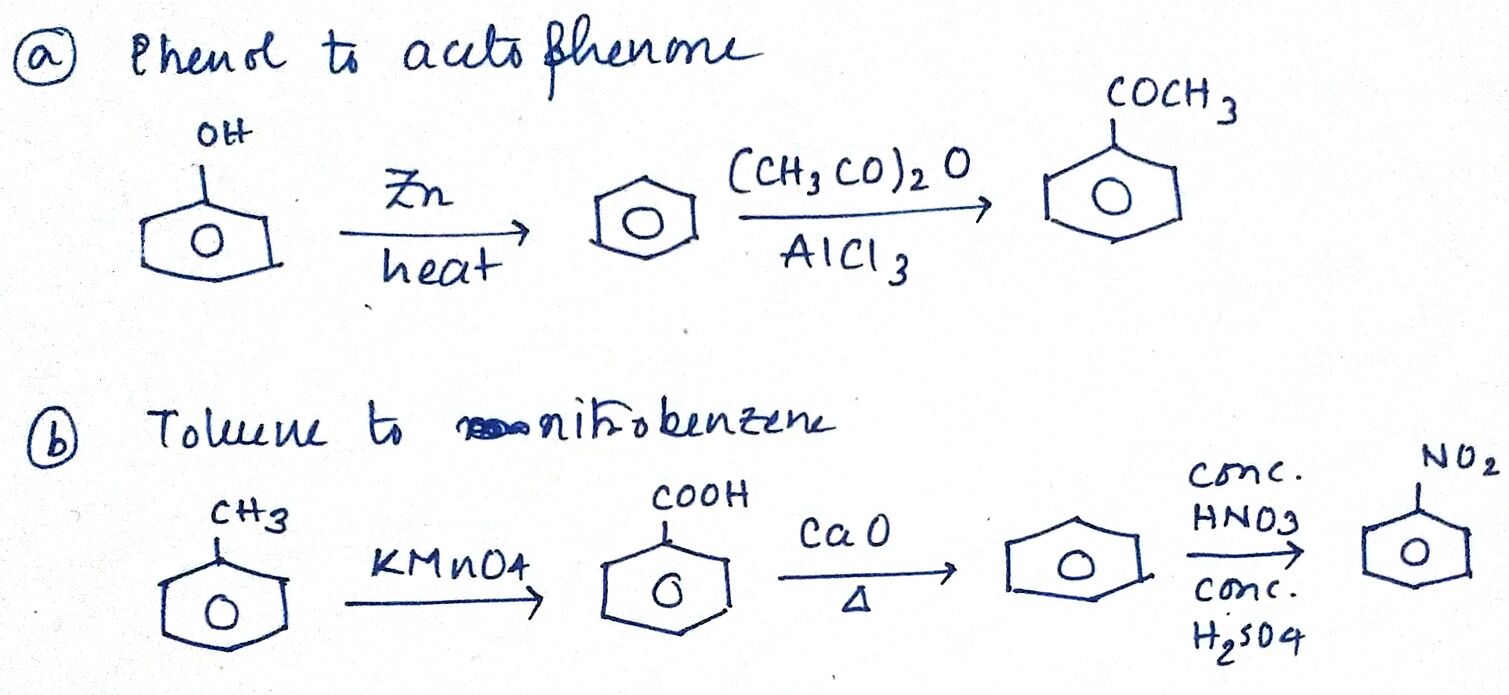
Convert
(a) phenol to acetophenone.
(b) toluene to m-nitrobenzene.
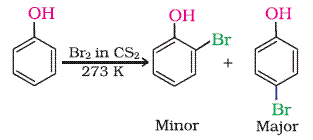
Explain why phenol in water or polarprotic give $$O-P$$ tribromo product but in nonpolar medium like $$C{ S }_{ 2 }\ C{ Cl }_{ 2 }$$ image ortho or p bromo product?
Find the major Product of the reaction
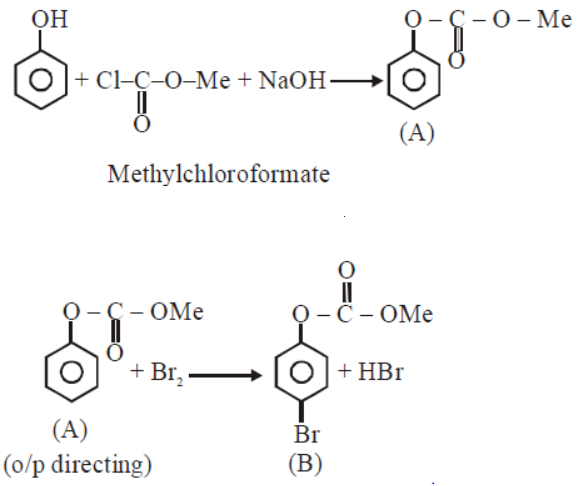
Phenol reacts with methly chloroformate in the presence of $$NaOH$$ to from product $$A$$. $$A$$ reacts with $${Br}_{2}$$ to from product $$B$$. $$A$$ and $$B$$ are respectively:
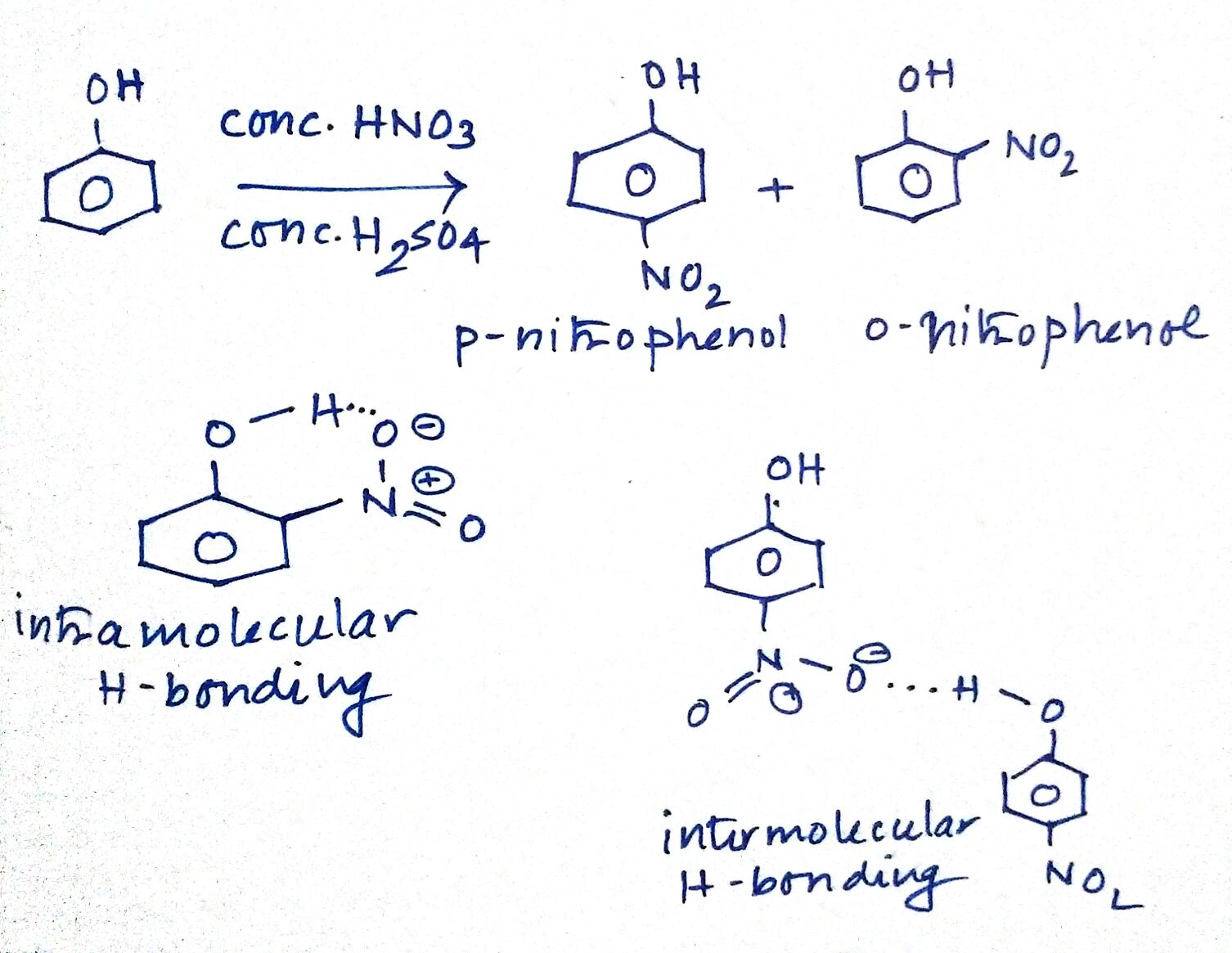
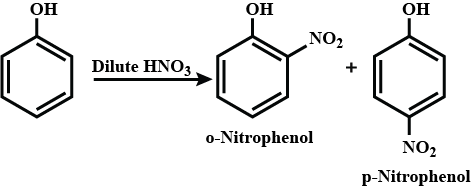
What is the action of the nitrating mixture on phenol?
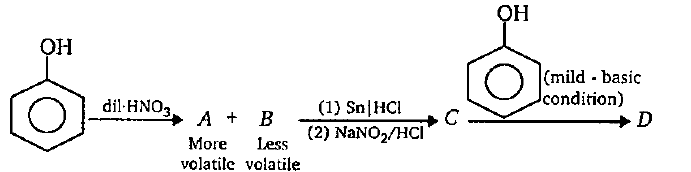
While separating O- and P- nitrophenol by steam distillation, Name the isomer which is steam volatile. Give reason.
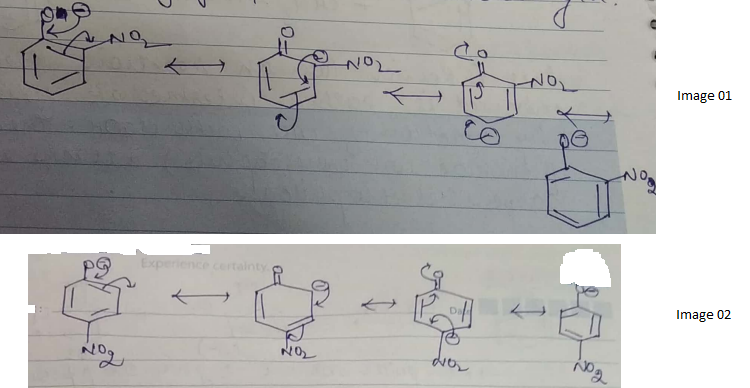
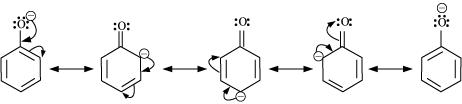
Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide lons.
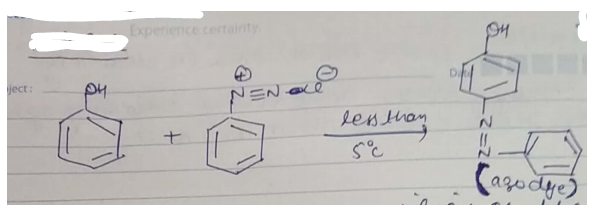
(a) What happens when Phenol is treated with benzene diazonium chloride? Give balance chemical equation.
Why phenol is more acidic than ethyl alcohol?
How the following Conversion can be carried out-
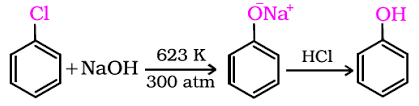
Chloro benzene to Phenol
Phenols are more acidic than alcohols. Explain why?
$$+{ Br }_{ 2 }\xrightarrow [ { CS }_{ 2 } ]{ } $$
Although phenoxide ion has more number of resonating structures than carboxylate ion, calculate acid is a stronger acid than phenol. Why?
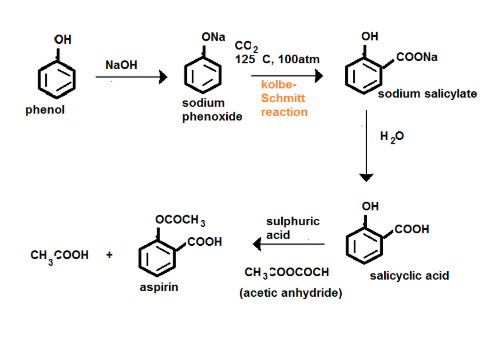
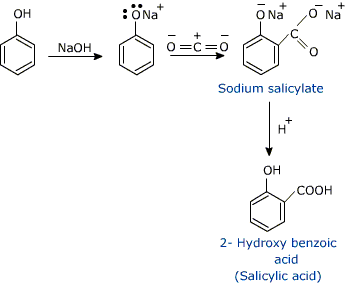
How will you prepare salicylic acid from phenol?
Why phenols are acidic in nature ?
Fill in the blanks:
Phenol is ______ while $$C_{2}H_{5}OH$$ is ______.
Fill in the blanks:
Phenol is acidic because of resonance stabilization of its conjugate base namely _________.
Account for the following:
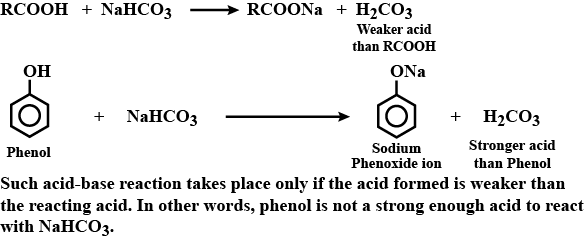
Phenol does not react with $$NaHCO_3$$, whereas carboxylic acids react.
Arrange: water, ethanol, and phenol in increasing order of acidity and give reason for your answer.
Phenol is an acid but does not react with sodium bicarbonate solution. Why?
Carry out the following conversions:
Phenol to benzoquinone
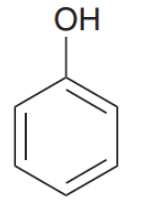
Write IUPAC name of the above compound:
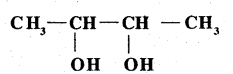
Write IUPAC name of the above compound:
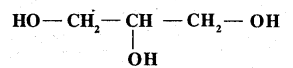
Write the structure of the compound whose IUPAC name is as follows:
$$2-$$Methyl butan$$-2-$$ol
Write structure of the compound whose IUPAC name is as follows:
phenyl propan-$$2$$-ol
Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
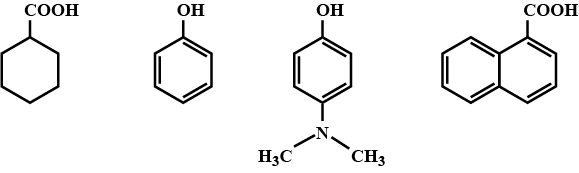
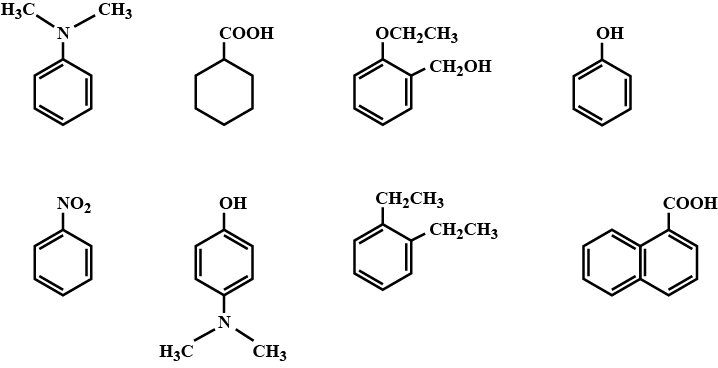
Among the following, the total number of compounds soluble in aqueous $$NaOH$$ is:
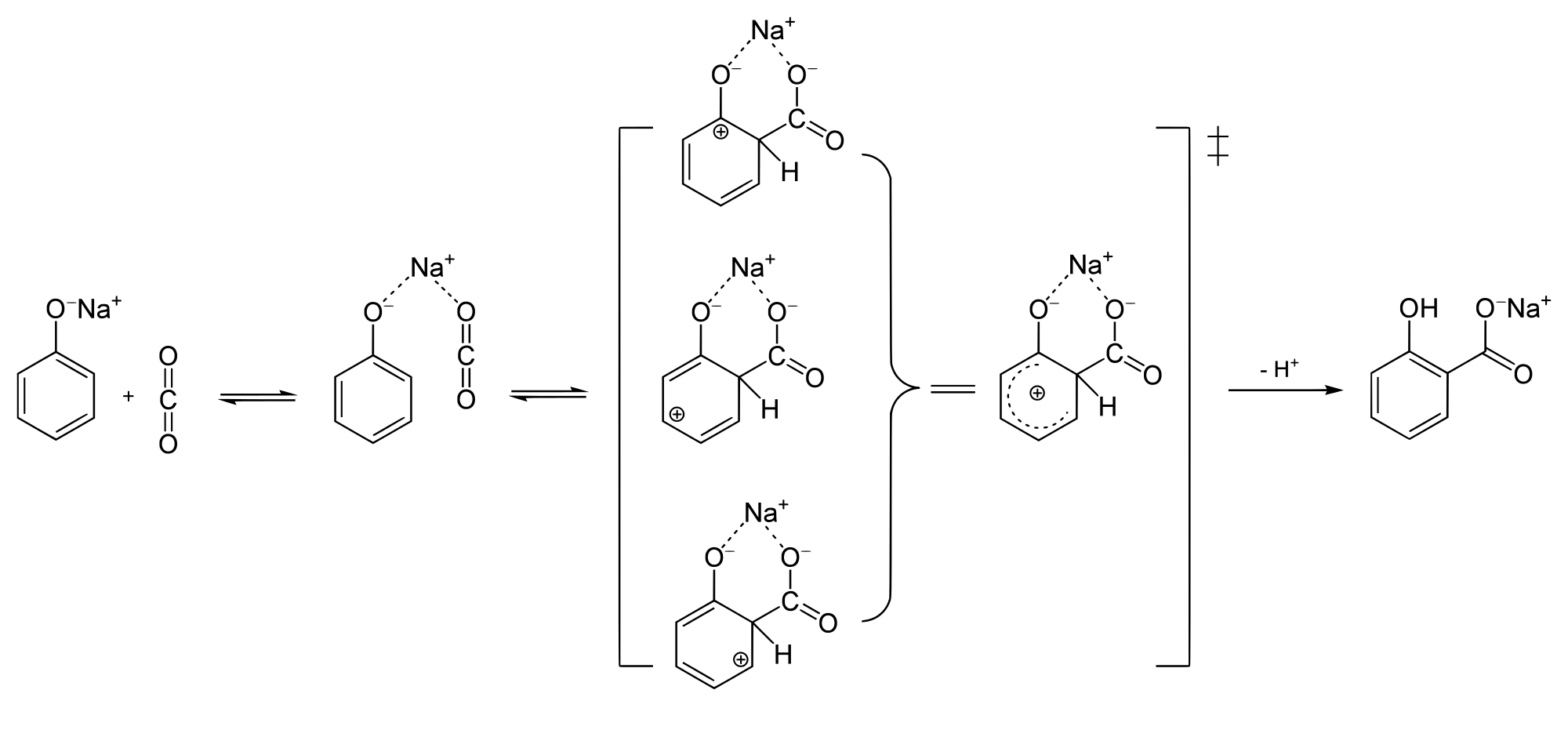
Phenol reacts with sodium hydroxide to give sodium phenoxide. Phenoxide ion undergoes electrophilic substitution with carbon dioxide ( a weak electrophile) because phenoxide ion is more reactive than phenol. Salicylic acid is formed as a major product. At lower temperature ortho isomer predominant, whereas para isomer is obtained at a higher temperature. Give the reason behind it.
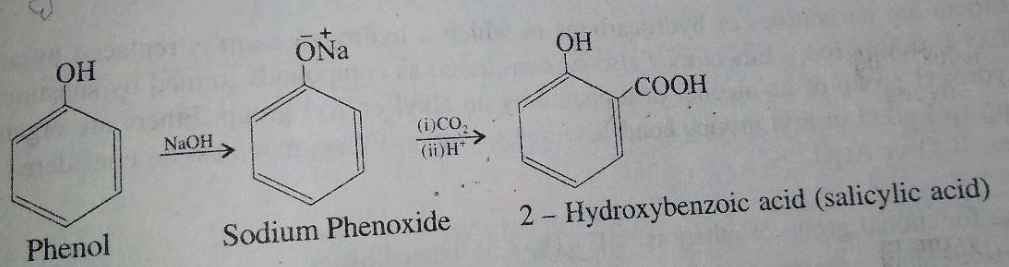
Salicylic acid does not obtained by the reaction of phenol and $${ CO }_{ 2 }$$ directly?
Correct acidic order of acidity is
Explain acidic nature of phenol with the help of reactions.
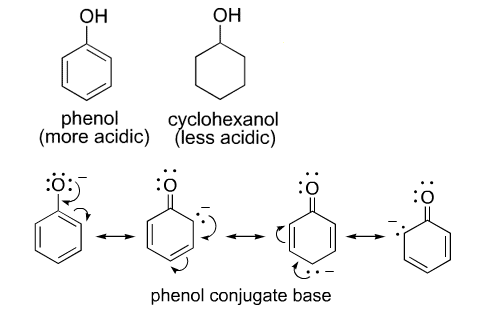
Phenol is more acidic than cyclohexanol. How?
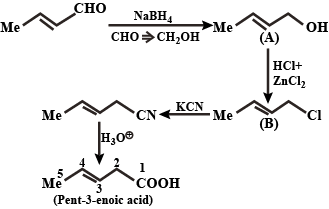
In the following, identify the compounds/reaction conditions represented by the alphabets $$(A),(B)$$ and $$(C)$$
$${H}_{3}C-CH=CH-CHO\xrightarrow [ ]{ Na{ BH }_{ 4 } } (A)\xrightarrow [ Zn{ Cl }_{ 2 } ]{ HCl } (B)\xrightarrow [ { H }^{ + } ]{ KCN } (C)$$
Draw the structure of major monohalo product in the following reaction:
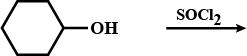
Explain the following in one or two sentences only :
'Phenol is an acid, but it does not react with sodium bicarbonate'.
The acidity of phenol is due to the _______ of its anions.
Out of o-nitrophenol and p-nitrophenol, which is more volatile. Explain.
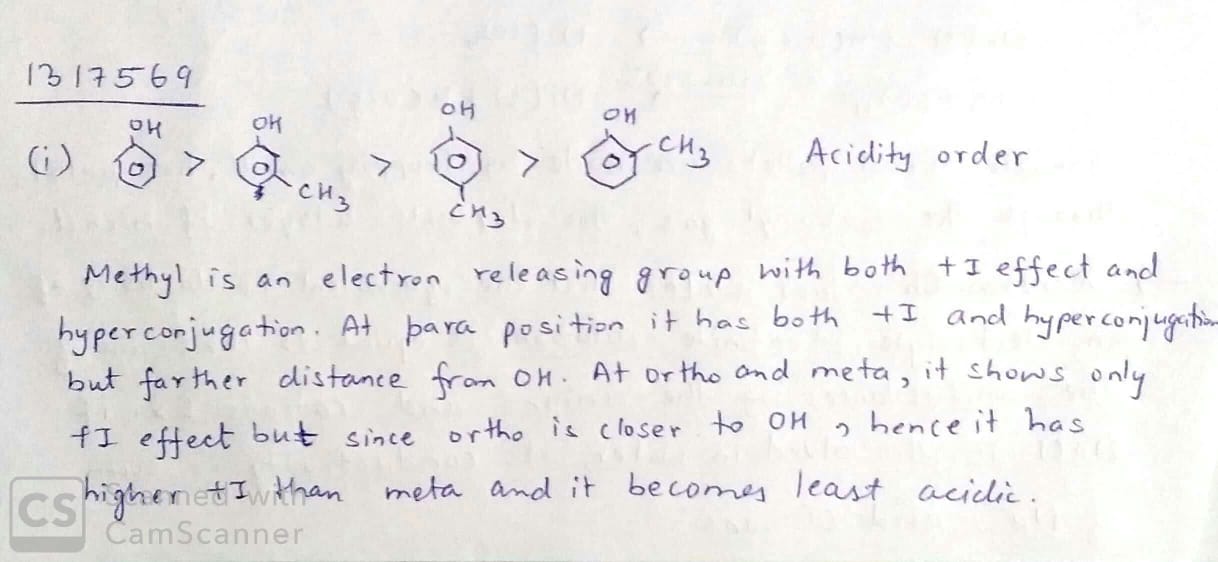
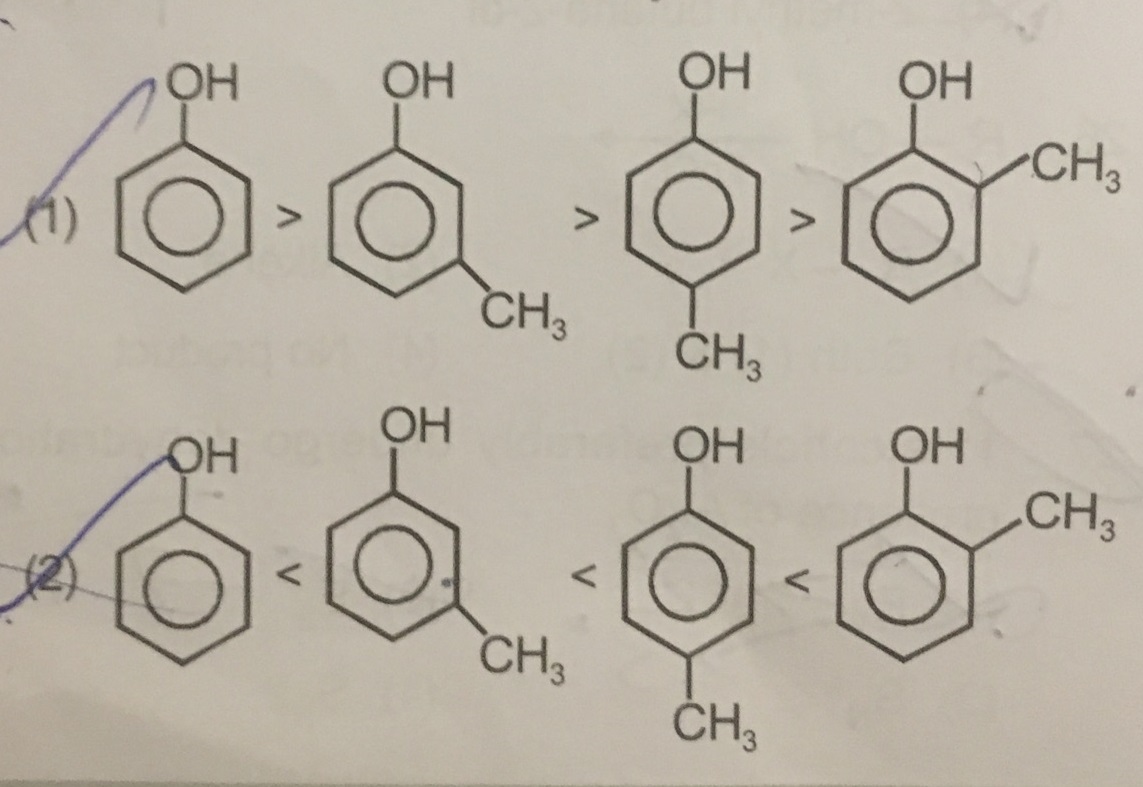
Arrange the following compounds groups in the increasing order of their property indicated:
pnitrophenol,ethanol,phenol (acidic character)
Dipole moment of phenol is smaller than that of methanol. Why?
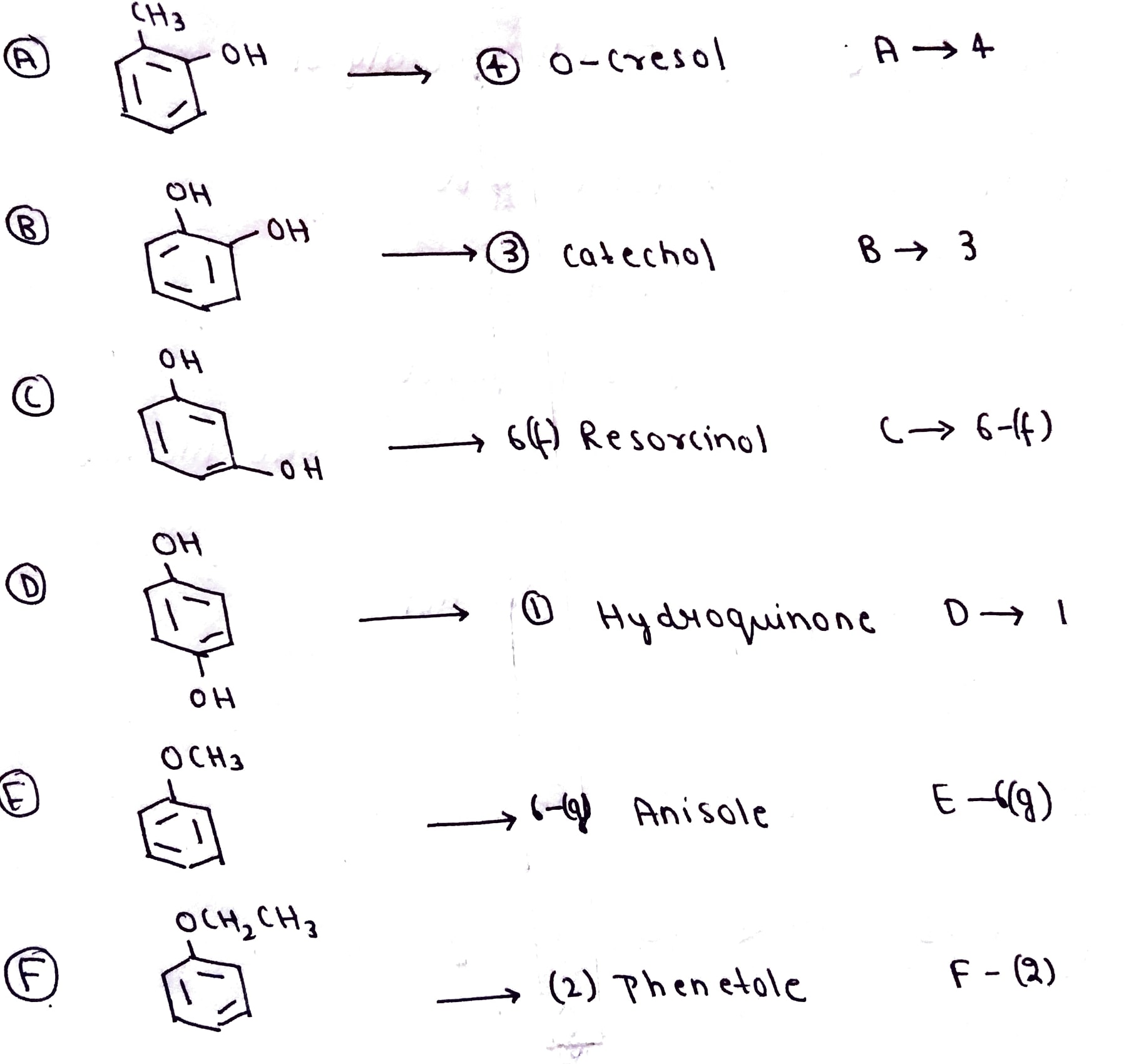
Match the structures of the compound given in Column I with the name of the compounds given in Column II.
Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
What happens when phenol is kept open in the air?
Class 12 Medical Chemistry Extra Questions
- Alcohols, Phenols And Ethers Extra Questions
- Aldehydes, Ketones And Carboxylic Acids Extra Questions
- Biomolecules Extra Questions
- Chemical Kinetics Extra Questions
- Chemistry In Everyday Life Extra Questions
- Coordination Compounds Extra Questions
- Electrochemistry Extra Questions
- General Principles And Processes Of Isolation Of Elements Extra Questions
- Haloalkanes And Haloarenes Extra Questions
- Organic Compounds Containing Nitrogen Extra Questions
- Polymers Extra Questions
- Solutions Extra Questions
- Surface Chemistry Extra Questions
- The D-And F-Block Elements Extra Questions
- The P-Block Elements Extra Questions