Polymers - Class 12 Medical Chemistry - Extra Questions
Define polymerisation.
Define thermoplastics and thermosetting polymers with two examples of each.
In which classes, the polymers are classified on the basis of molecular forces?
What is a biodegradable polymer? Given an example of biodegradable aliphatic polyester.
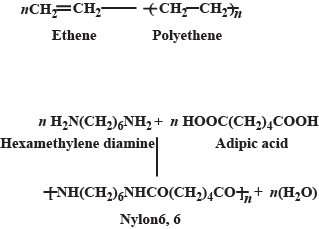
Name the type of polymerisation (addition or condensation) and name the monomers in polyethylene.
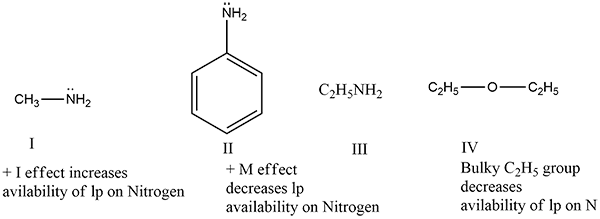
(i) Arrange the following compounds in the ascending order of their basic strength and give reasons for your answer:
Methylamine, Aniline, Ethylamine, Diethyl ether
(ii) Name the monomers and the type of polymerization in each of the folloiwng polymers:
(a) Polyester (b) Bakelite
Explain with example, branched and linear polymers.
Write any two difference between addition and condensation polymerisation.
The Equations of a chemical reaction are given in the figure:
(a) To which type this chemical reaction belongs?
(b) Which is the monomer in this reaction?
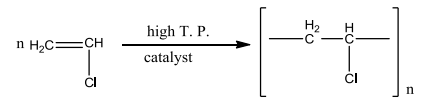
(b) Which is the monomer in this reaction?
What is polymerization? Give an example of a polymerization reaction.
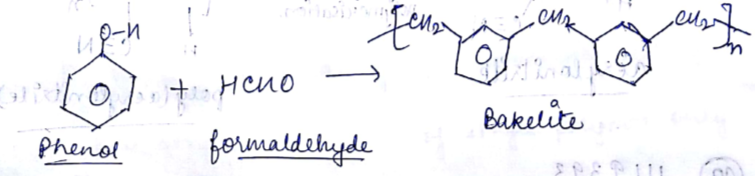
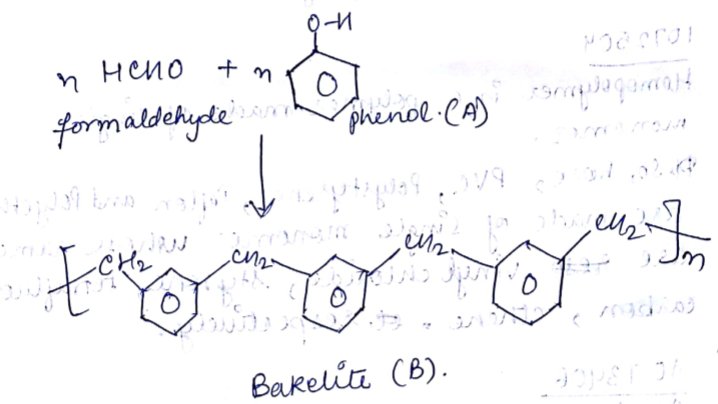
Draw the structure of Phenol formaldehide polymer.
Which type of polymer based on structure is obtained when phenol reacts with form aldehyde?
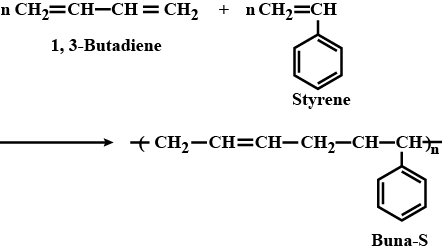
Write the structures of the monomers of the following polymer :
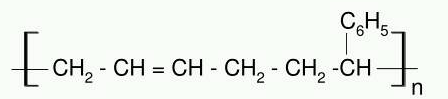
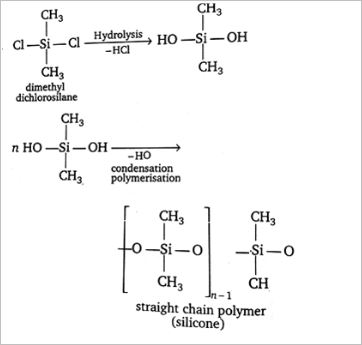
What is straight chain polymer? How is it formed?
How are polymers classified on the basis of molecular forces?
Arrange the following polymers in decreasing order of their intermolecular forces:
Bakelite, Polythene, Buna-S, Nylon-6,6
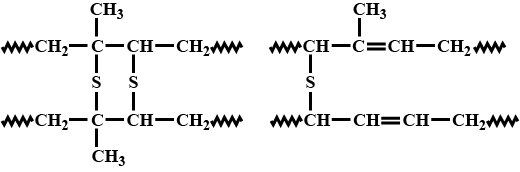
Describe how Thiokol is synthesised?
Explain Chain growth polymerization.
Arrange the following polymers in the increasing order of their intermolecular forces:
Terylene, Polythene, Neoprene
Arrange the following polymers in the increasing order of their intermolecular forces:
Polystyrene, Terylene, Buna-S
Give one example each of
i). Addition polymers
ii). Condensation polymers
iii). Copolymers.
Identify the polymer given below:
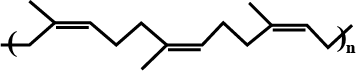
Identify the type of polymer.
$$-A-A-A-A-A-A-$$
Out of chain polymerization and step-growth polymerization, which type will you place the following
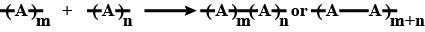
Identify the type of polymer.
$$-A-B-B-A-A-A-B-A-$$
On the basis of the source of how polymers are classified? Give one example of each?
Classify polymers on the following basis of the type of polymerization.
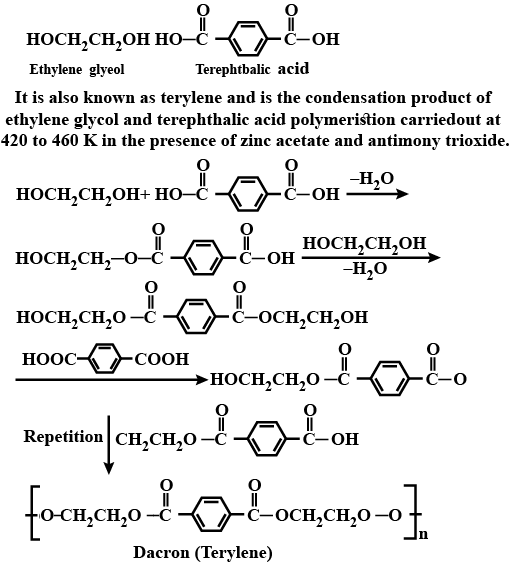
Write the process of polymerization of dacron.
Classify polymers on the following basis of molecular forces.
Match the column.
Match the column I (polymer) with column II (its type):
How can you differentiate between addition and condensation polymerisation?
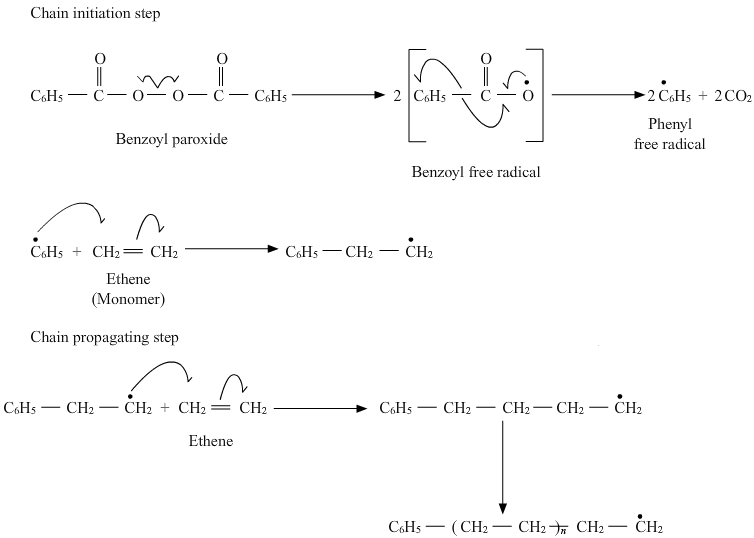
(i) What is the role of $$\text{t}$$-butyl peroxide in the polymerization of ethene?
(ii) Identify the monomers in the following polymer:
$${ \left[ -NH-{ \left( C{ H }_{ 2 } \right) }_{ 6 }-NH-CO-{ \left( C{ H }_{ 2 } \right) }_{ 4 }-CO- \right] }_{ n }$$
(iii) Arrange the following polymers in the increasing order of their intermolecular forces: Polystyrene, Terylene, Buna-S(iv) Write the mechanism of free radical polymerization of ethene.
Write the formula for monomers used in the preparation of dextron.
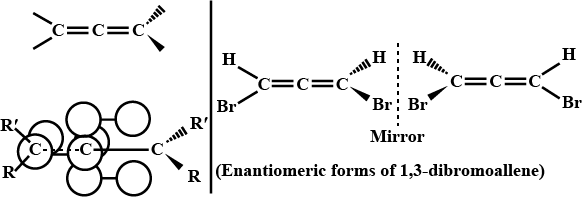
Discuss the anticipated stereochemistry of each of the following compounds.
a) $$BrCH=C=C=CHBr$$
b) $$CH_2=C=C=CHBr$$
c) $$BrCH=C=C=CBr_2$$
The polymerisation of acetonitryl gives .........
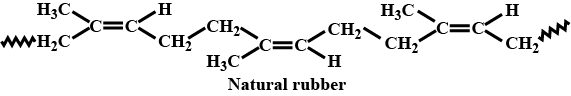
A natural linear polymer of $$2-$$ methyl $$-1, 3-$$ butadiene becomes hard on treatment with sulphur between $$373$$ to $$415\ K$$ and $$-S-S-$$ bonds are formed between chains. Write the structure of the product of this treatment?
Differentiate between rubbers and plastics based on intermolecular forces.
What is the polydispersity index? Explain.
Class 12 Medical Chemistry Extra Questions
- Alcohols, Phenols And Ethers Extra Questions
- Aldehydes, Ketones And Carboxylic Acids Extra Questions
- Biomolecules Extra Questions
- Chemical Kinetics Extra Questions
- Chemistry In Everyday Life Extra Questions
- Coordination Compounds Extra Questions
- Electrochemistry Extra Questions
- General Principles And Processes Of Isolation Of Elements Extra Questions
- Haloalkanes And Haloarenes Extra Questions
- Organic Compounds Containing Nitrogen Extra Questions
- Polymers Extra Questions
- Solutions Extra Questions
- Surface Chemistry Extra Questions
- The D-And F-Block Elements Extra Questions
- The P-Block Elements Extra Questions