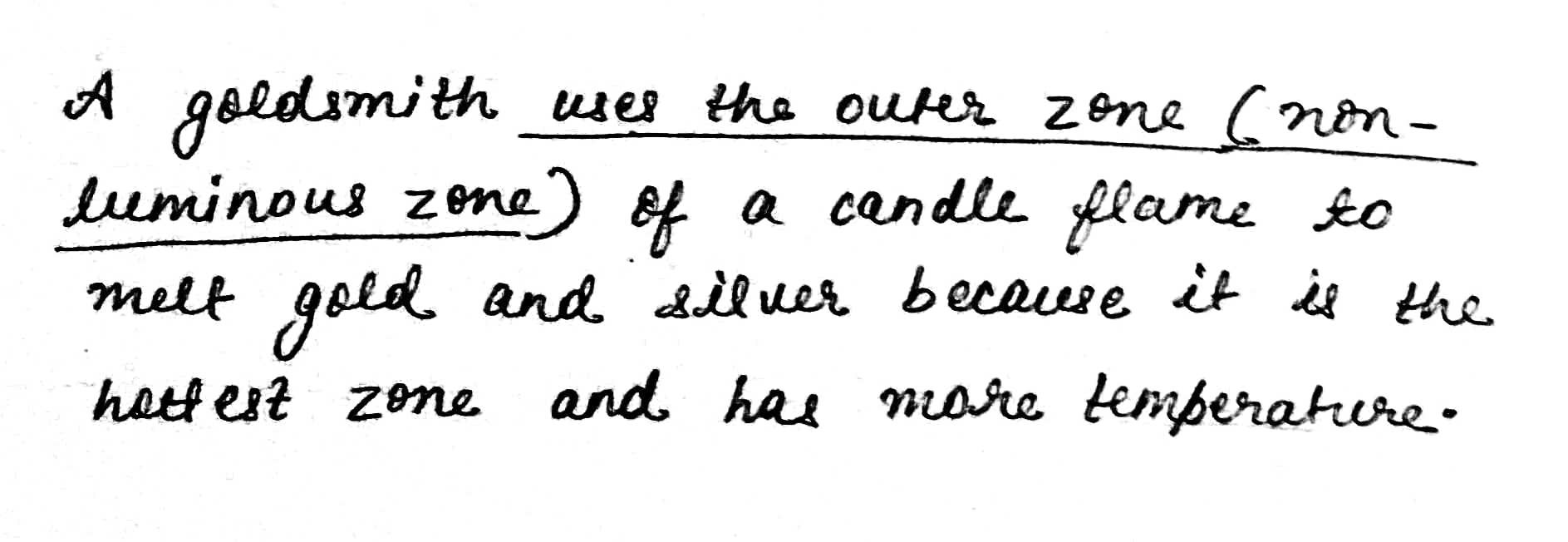Combustion And Flame - Class 8 Chemistry - Extra Questions
Estimate the maximum possible temperature of a Bunsen burnur flame.
_________ is a substance that reacts with oxygen and burns to release energy.
Explain the principle on which a fire extinguisher works.
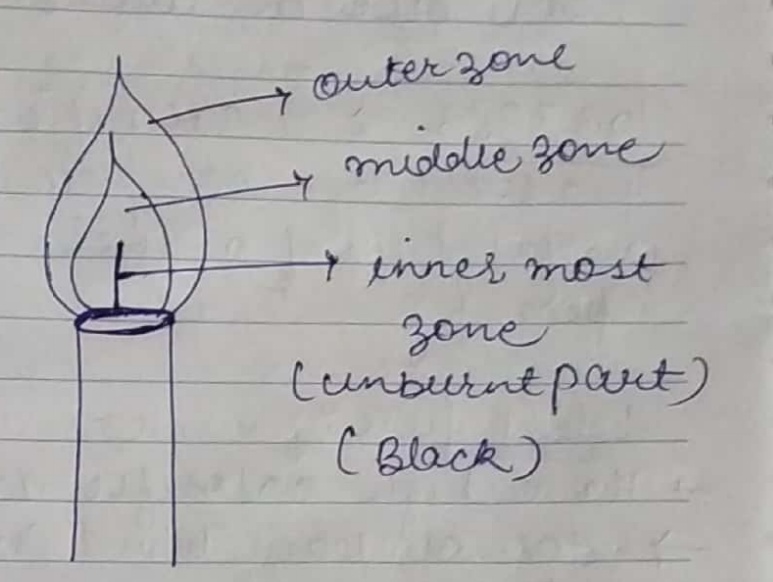
Draw a labelled diagram of a candle flame and explain what happens in each zone.
When fewer amounts of oxygen are present, carbon combines with it to give ________ gas.
Two glass jars A and B are filled with carbon dioxide and oxygen gases, respectively. In each jar a lighted candle is placed simultaneously. In which jar will the candle remain lighted for a longer time and why?
A fuel should have high ________ (Calorific /Octane) value.
Write your answer in form of 1 or 2.
Light a candle. Keep one hand above the flame and one hand on the side of the flame. Do your hands feel equally hot?
Give reasons why respiration is called slow combustion?
Is it possible to heat a liquid or gas from above?
Prateek took a piece of burning charcoal and collected the gas evolved in a test tube. Write down word equations of all the reactions taking place in this process?
Carbon dioxide is used in fire extinguishers. Why?
What is ignition temperature?
Make a labelled diagram of a candle flame.
How $$CO_2$$ is able to control fire? Explain.
List conditions under which combustion can take place.
Fill in the blanks:
(a) Burning of wood and coal causes _____ of air.
(b) A liquid fuel, used in homes is _____ .
(c) Fuel must be heated to its _____ before it starts burning.
(d) Fire produced by oil cannot be controlled by ______ .
Explain how the use of CNG in automobiles has reduced pollution in our cities.
Compare LPG and wood as fuels.
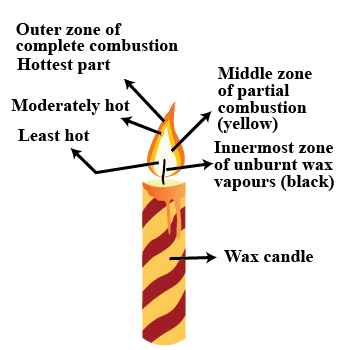
Which zone of a flame does a goldsmith use for melting gold and silver and why?
Why, water cannot be used to extinguish petrol fires?
A fuel should have high ________ value.
Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick of the candle flame. Ramesh kept the beaker on the outermost part of the flame. Whose water will get heated in a shorter time?
Name the process of converting crude oil obtained from seeds into commercially useful fuel.
When the material P mined from the earth is processed, a black solid Q is produced which consists mainly of carbon. When another material R obtained from trees is heated in an insufficient supply of air, it produces another solid fuel S which also consists mainly of carbon. Name P, Q, R and S.
Why CNG is considered a better fuel?
Explain the different zones of a candle flame.
Explain how $$CO_2$$ is able to control fire.
Compare the usefulness of solid, liquid and gaseous fuels.
What is the difference between combustible and non-combustible substances?
A wax candle burns with yellow flame. Give reason.
How are fuels classified based on their physical state? Give at least two examples of each.
A wet cotton handkerchief soaked in alcohol will not catch fire even it is set to fire. Why?
Give any two advantages of hydrogen as a fuel.
Why is it more difficult to burn some combustible substances than others?
During a fire accident, when you are not supported with any fire extinguisher, it is advisable to evacuate the building and close the doors and windows. Why do you think we should close the doors and windows? (Hint : Recall the conditions necessary for combustion)
How can you make a flame thrower with orange?
During an explosion , a large amount of ____ is given out.
What are the characteristics of a good fuel?
Why are gaseous fuels better than the solid and liquid ones?
Give example of spontaneous combustion.
Which zone of candle flame is uses by gold smith to melt gold and silver and why?
What is spontaneous combustion?
State two disadvantages of incomplete combustion.
Write characteristics of an ideal fuel.
Write the type of heat of combustion.
What is indicated by the blue flame of a bunsen burner?
What type of fuels:
(a) burn with a flame?
(b) burn without a flame?
Write short notes on fire extinguisher.
Write short notes on gaseous fuels.
Why are holes provided at the bottom of a bunsen burner?
Mrs. Anita Pandey observed that her cooking utensils are becoming black in colour and the flame of her gas stove is yellowish in colour. She complains about it in the gas company and got the gas stove repaired.
Answer the following question based on the above situation:
How is this problem harmful for our environment?
Give reason for the following observation:
(a) Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame.
A _______________ process in which a substance reacts with ___________ to give off heat is called combustion.
Slags is a non-combustible material.
Seldie is a combustible substance.
Mrs. Anita Pandey observed that her cooking utensils are becoming black in colour and the flame of her gas stove is yellowish in colour. She complains about it in the gas company and got the gas stove repaired.
Answer the following question based on the above situation:
What values are promoted by Mrs. Pandey regarding this situation?
The __________ temperature at which a substance catches fire is called its ___________ temperature.
The substances which have a very __________ ignition temperature and can easily catch fire with a flame are called __________ substances.
When the clothes of a person catch ________, the person is covered with a __________ to extinguish fire.
Chittsmack does not burn by itself.
Some substances on combustion produce thea and htlig.
Wood, paper, CNG are __________ substances.
A chemical process in which a substance reacts with oxygen to give off heat is called _____________.
Match the following for the flame of a candle
| Column A | Column B (zone) | Column C (colour) |
| (a) hottest part | (i) innermost zone | (x) blue of |
| (b) moderately hot | (ii) middle zone of | (y) black partial combustion |
| (c) least hot | (iii) outer zone of | (z) yellow |
The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its ficalroic value.
Boojho wants to separate the following materials as combustible and non-combustible. Can you help him? Charcoal, chalk, stone, iron rod, copper coin, straw, cardboard, glass, paper, candle, wood.
Anu wants to boil water quickly in a test tube. On observing the different zones of the flame, she is not able to decide which zone of the flame will be best for boiling water quickly. Help her in this activity.
If you hold a piece of iron wire with a pair of tongs inside a candle flame or a Bunsen
burner flame, what will you observe? Will it produce a flame?
The substances which have very low ___________ temperature and can easily catch fire with a flame are called __________ substances.
The lowest temperature at which a substance catches fire is called its__________________ temperature.
What do you understand by fuel efficiency?
The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its ____________.
The lowest temperature at which a substance catches fire is called its ___________ temperature.
Cracker on ignition produces sound. Why?
You are provided with three watch glasses that contain milk, petrol, and mustard oil, respectively. Suppose you have brought a burning candle near these materials, which material(s) will catch fire instantly and why?
The calorific values of petrol and CNG are 45,000 kJ/kg and 50,000 kJ/kg, respectively. If you have a vehicle which can run on petrol as well as CNG, which fuel will you prefer and why?
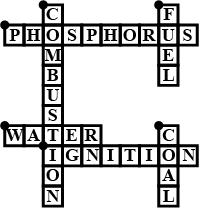
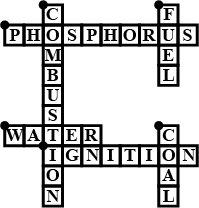
Complete the crossword Figure with the help of the clues:
Across
Non-metal which catches fire if exposed to air (10)
The lowest temperature at which a substance catches fire is called its ________ (8)
The most common fire extinguisher. (5)
Down-
A chemical process in which a substance reacts with oxygen to give off heat. (10)
Petrol is used as ________ in automobiles. (4)
It is as hard as stone and black in color. (4)
Fill in the blanks:The supporter of combustion in air is ______.
Give two examples each for a solid, liquid, and gaseous fuel along with some important uses.
Give short answers:
What is a fuel?
Give two examples of fuel.
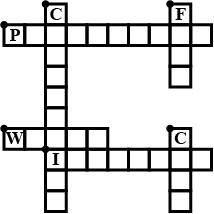
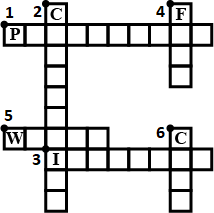
Complete the crossword Figure with the help of the clues:
Across
Non-metal which catches fire if exposed to air. (10)
The lowest temperature at which a substance catches fire is called its ________ .(8)
The most common fire extinguisher. (5)
Down-
A chemical process in which a substance reacts with oxygen to give off heat. (10)
Petrol is used as ________ in automobiles.(4)
This fossil fuel is hard as stone and black in colour. (4)
Although wood has a very high calorific value, we still discourage its use as a fuel. Explain.
What are the three essential requirements to produce fire? How fire extinguisher is useful for controlling the fire?
Which fuel has the highest calorific value?
Why are carbon and its compounds are used as fuels for most applications?
What are the limitations of using hydrogen as a fuel?
A candle brought near the mouth of a jar containing hydrogen gas starts burning but is extinguished when pushed inside the jar.
What is combustion?
Why is water not used to put off the fire caused due to electricity?
Complete the statement by filling in the blank/s with the correct words.
Water is added to the atmosphere by _________and ________ .
................ gas is necessary for combustion.
Define combustion. List the substance undergoing complete and incomplete combustion.
What will you do if a fire breaks out in the house of your neighbor?
What did you see? Make note of the observation made. Keep it for some time as it is. Make note of the observation made.

Give two examples of two solid fuels.
Why do we have holes in the formation of lantern / primus/gas stove?
Explain the three zones of a candle flame.
Prepare a list of combustible substances.
Prepare a list of some combustible and non combustible substances.
In which places do we find 'fire extinguishers'?
Name the types of fossil fuels that are found in nature.
For cooking, why are L.P.G./P.N.G. better than the other fuels?
Is the heat produced by the combustion of different fuels the same? Discuss
Observe the fast and complete combustion of flat paper. Understand that the combustion of a crumbled paper makes more smoke.
List the conditions for the complete combustion.
Observe the fast and complete combustion of flat paper. Understand that the combustion of a crumbled paper makes more smoke.
How does partial combustion cause environmental pollution?
Name the gas produced during the complete combustion of fuels.
Answer the following questions:(a) Which is the main constituent of LNG? (b) What are the uses of CNG?
Name the main constituent of CNG.
Write the full form of LNG and CNG.
Take three papers of the same size. Keep one stretched. Crumble the next. Make the third paper wet using water. Burn each of them over a candle flame using pincers. Compare the burning of each.

What are the drawbacks of partial combustion?
Filling in the blanks.
Inflammable tendency is higher in ________ fibres.
Write down any two examples of combustible substances.
Write down three essential conditions for the combustion of fuels.
It is advisable to keep stirring a heap of waste while burning it. How does the stirring help the combustion? Explain.
List out some of the hazards of incomplete combustion of fuels.
Write down disadvantages of partial combustion.
Making a volcano.Heap up some ammonium dichromate powder on a tile. Deposit on it the chemical present on a match stick, and ignite. Write down the changes happening there.
Change in colour: ____
Change in amount: ______
Exchange of energy: ______
Give reasons
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Write any four characteristic of an ideal fuel.
I: When a hydrocarbon is combusted in air, carbon monoxide is the major product
II: Air contains much more nitrogen than oxygen.
What kinds of fire extinguishers are most often used for extinguishing an electrical fire?
What is the use of oxyacetylene flame?
Why do goldsmiths use outermost flame to burn gold and silver?
What is meant by rapid combustion ? Give an example.
Mrs. Anita Pandey observed that her cooking utensils are becoming black in colour and the flame of her gas stove is yellowish in colour. She complains about it in the gas company and got the gas stove repaired.
Answer the following question based on the above situation:
What can be the reason for this sooty flame?
Explain combustion reaction with example.
Why does coal not burn by itself in air, but once initiated by a flame, continues to burn?
Manu was heating oil to fry potato chips. The cooking oil all of a sudden caught fire, he poured water to extinguish the fire. Do you think this action was suitable? If yes, why? If not, why not? In such a condition what should Manu have done?
Certain gases are given below.
Hydrogen, oxygen, nitrogen, chlorine, carbon dioxide
a)Which is a combustible gas?
b)Which gas support combustion?
c)Which gas has the tendency to limit combustion?
d)Which gas resists combustion?
Light a candle and observe its flame.
- How many parts are visible?
- Which are they?
- Which are they?
Forest fire produces a lot of air pollution. Write in brief about the reasons for forest fires.