Materials Metals And Non Metals - Class 8 Chemistry - Extra Questions
(i) Write the chemical equation for the reaction between gallium(III) oxide and dilute nitric acid to form a salt and water only.
(ii) The reaction between gallium(III) oxide and sodium hydroxide solution forms only water and a
salt containing the negative ion Ga2O2−4 Write the chemical equation for this reaction.
Give a reaction to show that metals react with base.
Why metallic oxides are called basic oxides and non-metallic oxides are called acidic oxides?
Match the substances in Column A with their applications given in Column B.
Which is lustrous?
Which is a liquid at room temperature?
Malleability is the ability of a metal to be beaten into thin sheets. Explain.
How can two objects that are the same temperature are felt by you as if they are at different temperatures ?
Name the non-metal used to make an antiseptic solution called tincture?
Sodium and potassium are stored under oil. Give reason.
Prateek took a piece of burning charcoal and collected the gas evolved in a test tube. How will he find the nature of the gas?
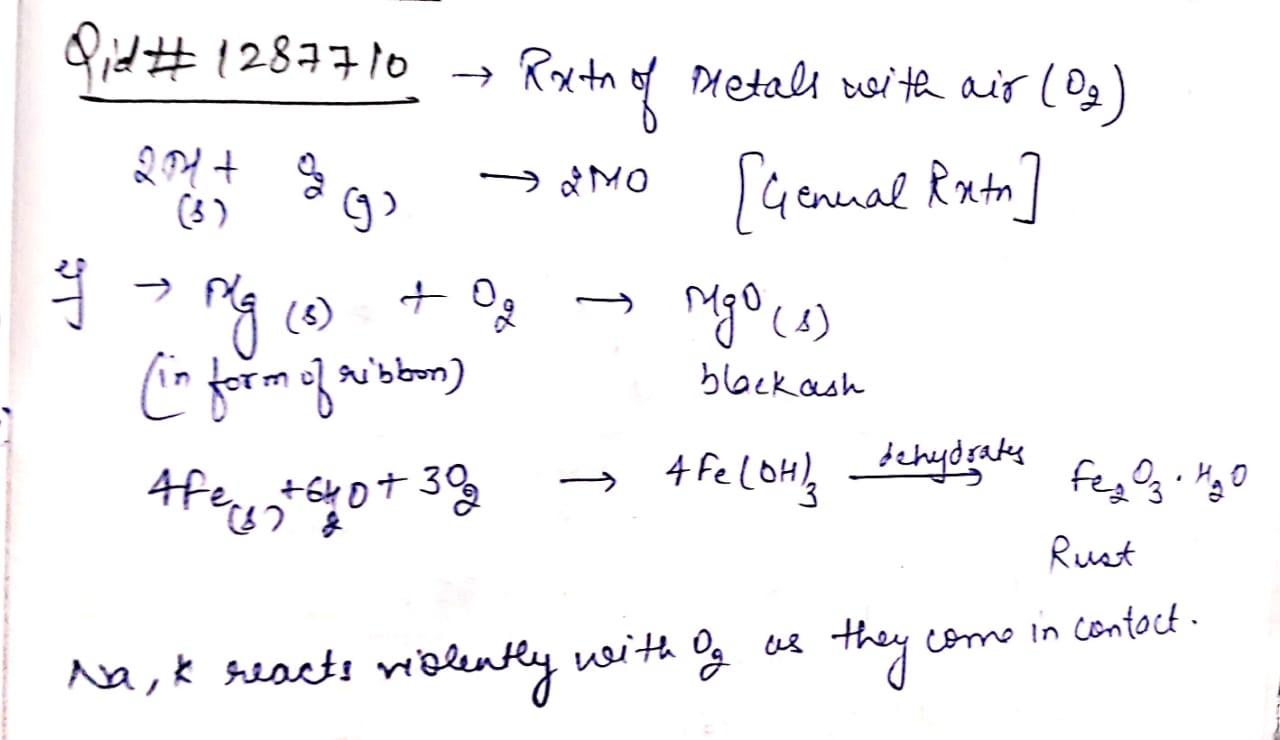
Give an example of activity to show that metals burn in air to form base.
Explain why we don't store lemon pickle in an aluminum utensil.
Name two non-metals whose oxides are acidic in nature.
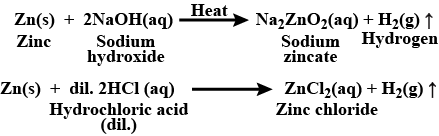
Complete the following reactions:Zn(s)+NaOH(aq)heat→
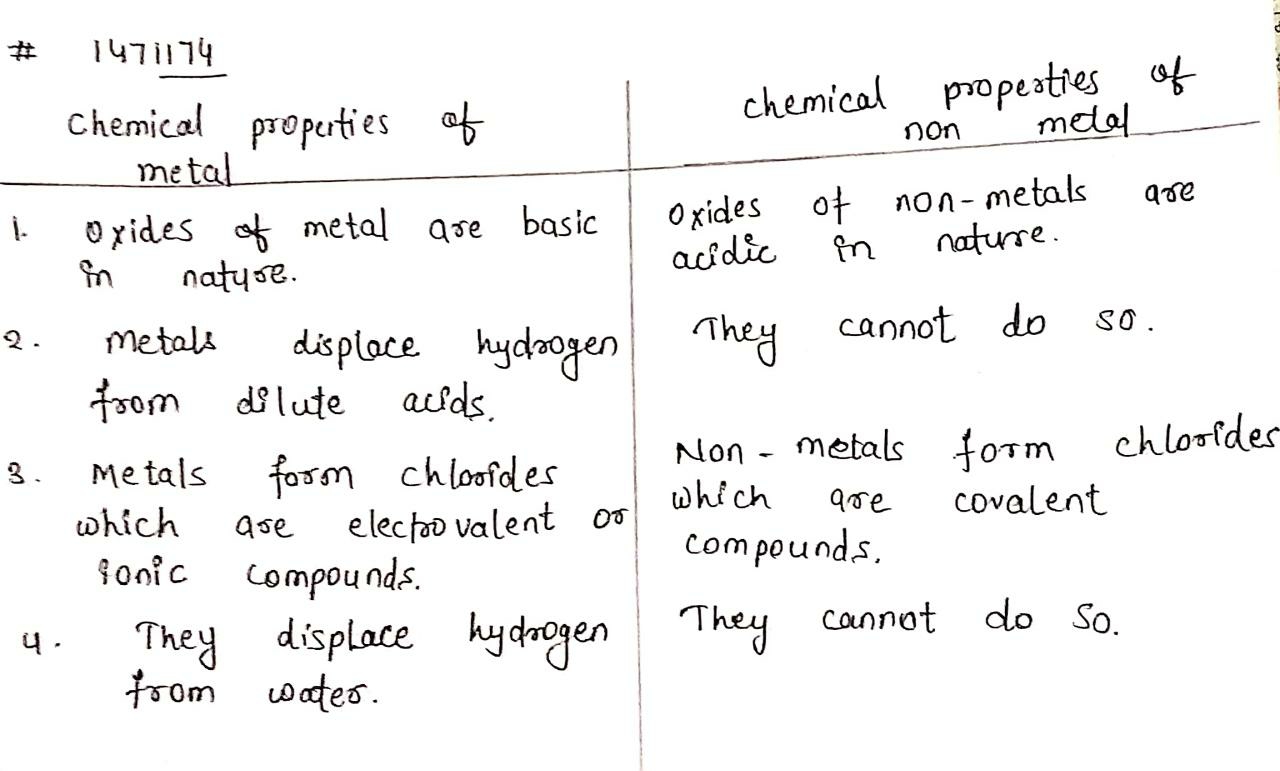
State four differences between metals and non-metals.
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Differentiate between metals and non-metals on the basis of their chemical properties.
Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
What type of oxides are formed when non-metals combine with oxygen?
True or False.
Non-metals have many different colours.
Brain Nurtures
A solution of CuSO4 was kept in an iron pot. After a few days, the iron pot was found to have a number of holes in it. How were these holes formed?
The binary compounds of metallic or non-metallic elements with oxygen are called as __________.
Why do metals generally appear to be dull? How can their brightness be restored?
Solder is used for joining two metal surfaces together. Solder is a mixture of lead and tin. Can two metals be separated by distillation?
Name the following:
The property possessed by metals by which they can be beaten into sheets.
Why is sodium kept immersed in kerosene oil?
True or False:Nonmetals are less dense and have low melting and boiling points.
The number of elements that show ductility among the following is?
Copper, silver, phosphorus, platinum, iodine, aluminium, carbon
Copper, silver, phosphorus, platinum, iodine, aluminium, carbon
Among the following, the number of elements that do not show malleability is:
Zinc, Iron, Sulphur, Copper, Aluminium, Nickel, Chlorine, Iodine
Out of the following elements, phosphorus, iron, copper, sulphur, silver, iodine, boron, titanium, the number of elements that don't show ductility is:
Write any six chemical properties of metals.
Guess who I am?i) I am a cheap metal but highly reactive. Therefore, I sacrifice myself to save the objects made of Iron.
ii) I am a solid solution. Dentists use me to fill cavities.
ii) I am a solid solution. Dentists use me to fill cavities.
Select lustrous materials out of the following substances.Gold, Sand, Silver, Rubber
Name a non-metal that has lustre.
Why aluminum alloys are used to design the body of an aircraft? Give reasons.
Complete the reaction given below:-
2Mg+O2→ ?
2Mg+O2→ ?
Write chemical properties of metal and non-metal.
Name two non-metals used in crackers?
Fill in the blank:
Property of metal by which it can be beaten into sheets is called __________.
Which metal used in making bulb's filament is highly ductile?
Give reason:
Metals forms positive ions.
List any three chemical properties based on which metals and non-metals are differentiated.
Why are metals used for making cooking vessels?
Which non-metal has very high melting and boiling points?
Explain the reactions of metals with air (oxygen)?
Define malleability.
Differentiate between metal and non-metal on the basis of their chemical properties.
Name the most malleable metal in the world.
Metals are _______and plastic are ______of heat.
What is malleability?
Why silver turns into black after a long time? Give the reason.
Give three differences between metals and non-metals.
Why each metal can not react to its own salt (e.g. Cu and CuSO4)?
What name is given to those metal oxides which show basic as well as acidic behaviour?
Give the names of: (a) two acidic oxides, and (b) two basic oxides.
Name one metal and one non-metal which exist in a liquid state at room temperature.
Name the metal which is a very bad conductor of heat.
| (Xi) | (Yi) | (Zi) |
| Constantan | Cu,Al | Measuring tapes |
| Brass | Fe,Cr,Ni | Domestic utensils |
| Rolled gold | Pb,Sn,Sb | Common articles |
| Invar | Cu,Ni | Soldering |
| Stainless steel | Pb,Sn | Airships |
| Solder | Al,Mg | Printing types |
| Type metal | Fe,Ni | Artificial jewellery |
| Magnalium | Cu,Zn | Electrical resistances |
Match the columns Xi, Yi and Zi
where i=1,2,3........8 from top to bottom. Ex- X4 stands for Invar & Z6 stands for Artificial jewellery.
Name two metals which are used :
(a) for making electric wires.
(b) for making domestic utensils and factory equipments.
(c) for making jewellery and to decorate sweets.
(a) What happens when calcium reacts with water? Write the chemical equation of the reaction of calcium with water.
(b) Write the chemical equation of the reaction which takes place when iron reacts with dilute sulphuric acid. What happens when the gas produced is ignited with a burning matchstick?
Name two characteristic physical properties of metals that are not exhibited by non-metals.
Explain why the surface of some metals acquires a dull appearance when exposed to air for a long time.
(a) What type of oxides are formed when non-metals react with oxygen? Explain with an example.
(b) What type of oxides are formed when metals combine with oxygen? Explain with the help of an example.
Name two non-metals that are both brittle and nonductile.
Which property of graphite is utilised in making electrodes?
Which property of copper and aluminium makes them suitable :
(a) for making cooking utensils and boilers?
(b) for making electric wires?
State one use each of the following metals:
Copper, Aluminium, Iron, Silver, Gold, Mercury.
State fives uses of metals.
Name a metal which is soft and a non-metal which is hard.
Name a non-metal which is good conductor of electricity.
What is do we mean by saying that metals are sonorous ?
(a) Define non-metals. Give five examples of non-metals.
(b) Name a non-metal which conducts electricity.
(c) Name a non-metal having lustre (shining surface).
(d) Name a non-metal which is extremely hard.
(e) How do non-metals react with oxygen? Explain with an example. Give equation of the reaction involved. What is the nature of the product? How will you demonstrate it?
State any five physical properties of metals and five physical properties of non-metals.
You are given a dry cell, a torch bulb with holder, wires and crocodile clips. How would you use them to distinguish between samples of metals and non-metals?
What do you mean when you say that metals are lustrous?
What is meant by malleability? Name two most malleable metals.
Compare the properties of metal and non-metals a with respect to: (i) malleability
(ii) ductility
(iii) electrical conductivity
(iii) electrical conductivity
Name two non-metals which are lustrous.
Write three general physical properties of metals.
What is meant by ductility? Name two most ductile metals.
State two physical properties based on which metals can be distinguished from non-metals.
Fill in the following blanks with suitable words:
The elements which are sonorous are called________.
Name two metals which are best conductors of heat.
Why is sodium a metal whereas carbon a non-metal?
Which property of graphite is utilized in making electrodes?
Why will the colour of heated copper powder become black when air is passed over it?
Which of the following metals will melt at body temperature?
Gallium, Magnesium, Caesium, Aluminium
What is the nature of non-metal oxide?
Explain why the surface of same metals acquires a dull appearance when exposed to air for a long time.
Name one metal and non-metal that exist in the liquid state at room temperature. Name two metals having melting point less than 310K(37oC)
Explain the following statement:
At ordinary temperature, the surface of metal such as magnesium, aluminium, and zinc, etc. is covered with a thin layer. What is the composition of this layer? State its importance.
A purple-colored non-metal forms a brown solution in alcohol which is applied on wounds as an antiseptic. Name the nonmetal.
Which liquid metal is used in thermometers?
Why are bells made of metals?
Which non-metal is used to disinfect water?
Name two soft metals which can be cut with a knife.
Name two major non-metals which are present in fertilisers and enhance the growth of plants.
Which non-metal is essential for our life and all living beings which we inhale during breathing?
Which of the following metals can displace the other two metals from their salt solutions?
zinc, iron, copper
A metal A, which is used in thermite process, when heated with oxygen gives an oxide B, which is amphoteric in nature. Identify A and B. Write down the reactions of oxide B with HCl and NaOH.
Iron is more reactive than copper. Write an activity to show this.
Is wood a good conductor of electricity?
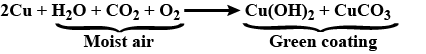
Paheli bought a statue made of copper. To her surprise, it acquired a dull green coating after a couple of months. Explain the reason.
When a mixture of copper sulphate and zinc granules is mixed in water, the blue colour of copper sulphate disappears and a powdery red mass is deposited at the bottom of the beaker. This is which type of reaction?
Which metal generally used for making jewelry?
Property by virtue of which metals can be beaten into thin sheets is called
Give two examples each of the metals that are good conductors and poor conductors of heat respectively.
Name one metal and one non-metal that exist in liquid state at room temperature. Also, name two metals having melting point less than 310K(370C)
Out of Metals and Non-Metals, which is hard, ductile, malleable, and sonorous?
Name a metal which is liquid at room temperature.
Name a metal that reacts with acid as well as base to form hydrogen gas.
Find out the names of three metals and three non-metals from the box given in Fig ;
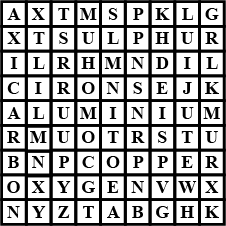
Property of metal by the virtue of which it can be drawn into wires called?
Fill in the blanks to complete the following paragraph.
List some objects made using metal.
Non-metals are usually poor conductors of heat and electricity. They are non- lustrous, non-sonorous, non-malleable, and are colored. Name a non-metal which exists as a liquid at room temperature.
Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non-sonorous, non-malleable and are coloured. Name a lustrous non-metal.
Why do silver articles turn black when kept in the open air for a few days? Name the phenomenon involved.
A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black colour product. Identify M and black coloured product and also explain the reaction of M with oxygen.
Give four examples of non-metallic elements.
Non-metals are usually poor conductors of heat and electricity. They are non- lustrous, non-sonorous, non-malleable, and are colored. The allotropic form of a non-metal is a good conductor of electricity. Name the allotrope.
An element forms an oxide A2O3 which is acidic in nature. Identify A as a metal or non-metal.
Name a non-metal which is required for combustion.
Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non-sonorous, non-malleable, and are colored or colorless. Name a non-metal which is known to form the largest number of compounds.
Give reason :
Gold is mixed with copper and nickel.
Name a metal used in making electric cables.
Name a non-metal that is used for filling electric bulbs.
Give two uses of Tungsten.
Name a metal that is brittle.
Name a non-metal that is used for cancer therapy.
Name a non-metal that is used to kill germs in water.
Give four differences between metals and non-metals with reference to their:
(a) Melting point and boiling point
(b) Conductivity of heat and electricity
(c) Malleability
(d) Solubility
A non-metal used to purify water is _______ .
Fill in the blank:
The most ductile metal is ______.
What do you observe when calcium is heated and then tested with moist blue and red litmus - paper?
Name a gaseous non-metal, having greenish yellow colour.
Name a non-metal which is ductile.
Name a metal with dull appearance.
Name a metal which can be cut with a knife.
Name a non-metal that is found in plants.
State any five physical properties of a non-metal.
Name a metal which is a bad conductor of heat and electricity.
Name a metal which is lighter than water.
Name a metal which is not malleable.
A student has been collecting silver coins and copper coins. One day she observed a black coating on silver coins and a green coating on copper coins. Which chemical phenomenon is responsible for these coatings? Write the names of black and green coatings.
Name two metals which are soft.
Name a metal which lacks ductility.
Name a non-malleable metal.
Name two metals which are liquid at room temperature.
Name a metal that is brittle.
Write balanced equations for the following
Aluminium reacts with fused sodium hydroxide.
Why do gold ornaments look new even after several years of use?
What is the chemical name of the green coating formed on copper when it is exposed to air?
Which metal is used for:
(a) making pipes, buckets, and water tanks
(b) lithographic plants for printing
(c) making face creams
The oxides of non metals are generally ______ in properties.
Write any four uses of metals.
Explain the difference between Metals and non-metals .
Identify the odd term.
Ductility, brittleness, sonority, malleability
Write a scientific reason.
Copper vessels are cleaned with lemon.
Why is the metal good conductor of electricity?
Why is aluminium used for making cooking coil?
Why do we store phosphorus in water?
Thin strips of magnesium, copper and iron are taken.Write down what happens when these metals are treated as follows:
Heated in presence of air
Write the equation of copper with moist air.
What do you mean by malleability?
Give a reason for the following:Iron is used in constructing bridges and houses.
Gold wires are used in ornaments. Which property of gold is used in ornaments?
Write the equation for reaction of copper sulfate with zinc.
Explain the meanings of malleability and ductility.
Give a reason: Lemon pickles should not be stored in aluminum utensils.
Account for: Copper is malleable and ductile while sulphur is not.
Sodium cannot be kept open in atmosphere air, and cannot be stored under water. So, it is stored in kerosene. Give an explanation for the above statements with its chemical equation.
Name the acid formed when sulphur dioxide dissolves in water.
Give reason for the following:
Aluminum is used to make electrical wires.
Some metals are listed below. Complete the table by identifying the different uses and the properties which are responsible for the same.
| Metal | Use | Property |
| Gold | ∙ | |
| Copper | ∙ | |
| Aluminium | ∙ | |
| Zinc | ∙ | |
| Iron | ∙ |
Cut a small sodium metal piece into two, watch it.
What change occured on the surface of sodium metal?
How does oxygen react with non-metals?
C+O2→..........
2H2+O2→.............
Lustre of aluminium utensils disappear after some days. Why?
Give reason for the following,
CuSO4 solution is not stored in iron vessels.
What are the similarities between iron, copper and aluminium?
Metals generally occur in solid-state and are hard. Name a metal that exists in a liquid state and a metal that is soft and can be cut with a knife.
Collect the following materials: an iron nail, an aluminium wire, a pencil lead, a copper wire. Beat them hard using a hammer. Which of them can be flattened? What is your conclusion from this experiment?
Which is the metal used for making filament of bulb? Which property is made use of it?
How will the properties of metals like hardness, malleability, thermal conductivity, electrical conductivity, and ductility be used?
Which metal is seen in liquid state?
Select the metal which does not react with air:
Copper, iron, gold, magnesium, platinum, sodium, silver.
Does any immediate change occur when a bright nail is exposed to air? What happens to it after a few days?
What is the property of metal caused for shining appearance?
Fill the table
| Use | Property |
| To make utensils | ________ |
| To make aluminium foil | ________ |
| To making farming tools | ________ |
| To make electric wire | ________ |
| To make bells | ________ |
Which among the metals is stored in kerosene?
(a) Sodium
(b) Iron
(c) Tungsten
(d) Chromium
Why is it stored in kerosene?
2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the contents are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation of the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with the dilute solution of a strong acid.
Fill in the blanks:
Phosphorus is a very ______ non-metal.
A reddish brown coloured metal, used in electrical wires, when powdered and heated strongly in an open china dish, its colour turns black. When hydrogen gas is passed over this black substance, it regains its original colour. Based on the above information answer the following questions:
(a) Name the metal and black coloured substance formed
(b) Write balanced chemical reactions for both the reactions
Give reasons for the following:
(a) Aluminium foils are used to wrap food items.
(b) Immersion rods for heating liquids are made up of metallic substances.
(c) Copper cannot displace zinc from its salt solution.
(d) Sodium and potassium are stored in kerosene.
Match the elements given, in column I correctly with their respective uses given in column II.
Name :- The non-metal which is lustrous.
- The non-metal which is liquid.
- Non-metal which is conductor of electricity.
- The non-metal which is hardest natural substance known.
- The non-metal which is lustrous.
- The non-metal which is liquid.
- Non-metal which is conductor of electricity.
- The non-metal which is hardest natural substance known.