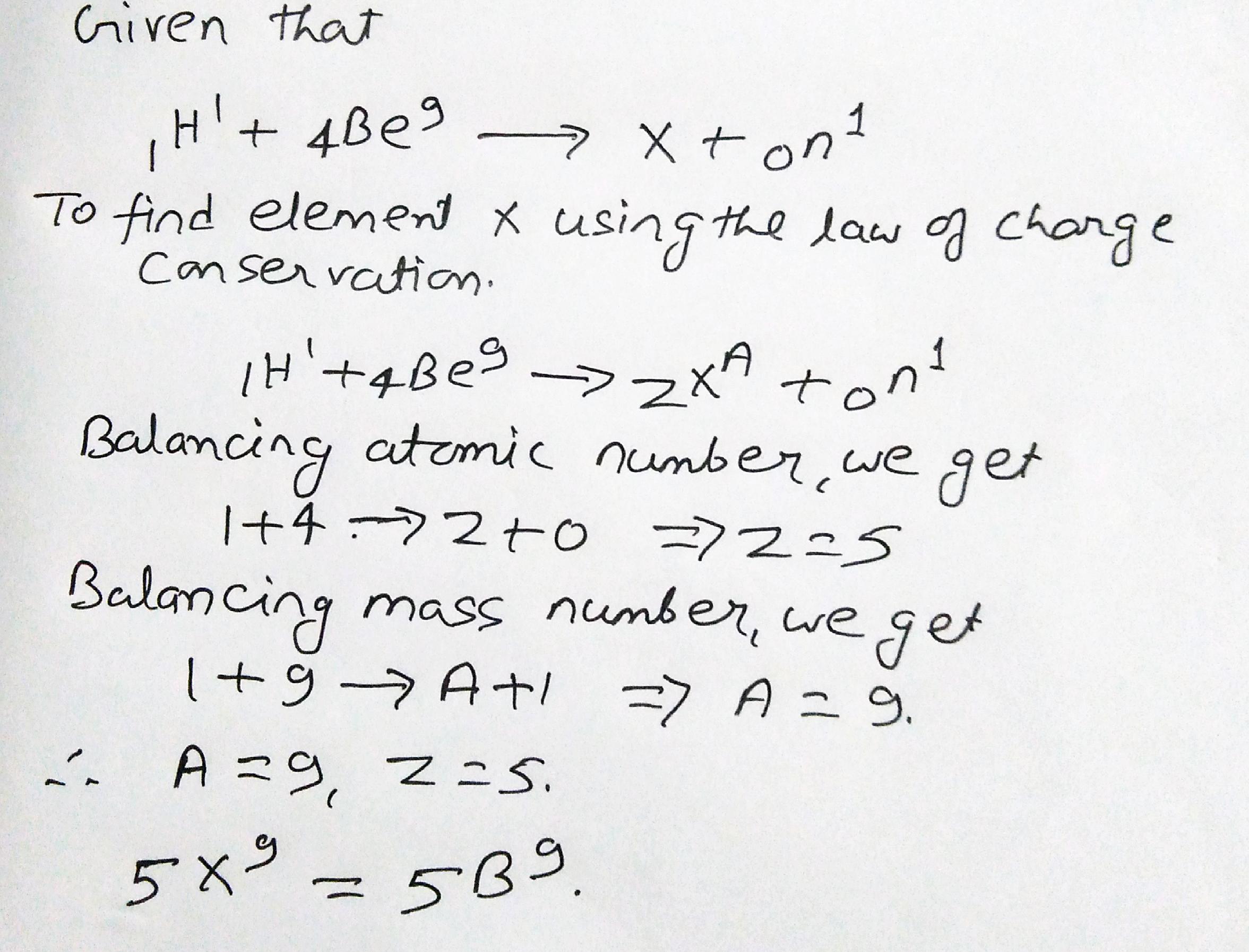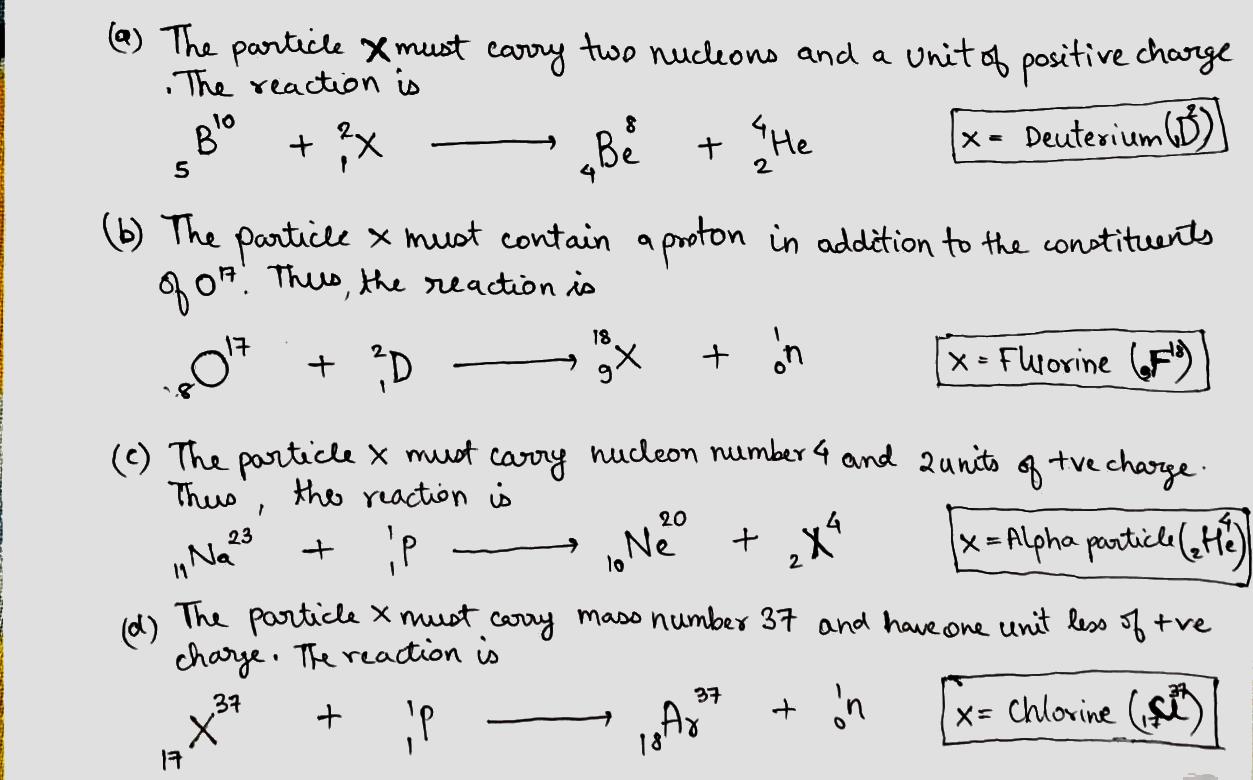Nuclei - Class 12 Engineering Physics - Extra Questions
Give a reason for your answer.
Write down the Einsteins mass-energy equivalence relation, explaining the meaning of
each symbol used in it.
What would be the element X in the following nuclear reactions using the law of charge conservationH1+Be9=X+n1
What would be the element X in the following nuclear reactions using the law of charge conservation
8N15+1H1→X+2He4
What would be the element X in the following nuclear reactions using the law of charge conservation
6C12+1H1→X,
What is the number of photons and neutrons in 2215X?
A−radioactive element whose mass number is 218 and atomic number is 84 emits $$\beta -$ particle. What would be the mass number and atomic number of element after decay?
By which process energy is produced in stars?
Consider a radioactive nucleus A which decays to a stable nucleus C through the following sequence:
A→B→C
Here B is an intermediate nuclei which is also radioactive. Considering that there are N0 atoms of A initially, plot the graph showing the variation of number of atoms of A and B versus time.

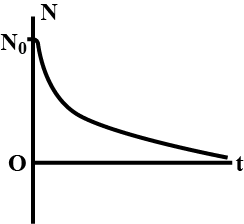
State the law of Radioactive disintegration.
Einstein's equation is given as-
Energy released in a nuclear reaction is given by ________.
State radioactive decay law. Prove that radioactive decay is exponential in nature.
Obtain the exponential law of radioactive disintegration. Explain the decay curve.
Atoms having the same ________ but different _____ are called isotopes.
When four hydrogen nuclei combine to form a helium nucleus in the interior of sun, the loss in mass in 0.0625 amu. How much energy is released?
what is the physical significance of negative energy of electron in atom?
Write any one equation representing nuclear fusion reaction.
Give two examples of nuclear fusion.
If both the number of protons and the number of neutrons are conserved in each nuclear reaction in what way is mass converted into energy (or vice-versa) in a nuclear reaction?
ZXAB+vedecay→ ?
In a nuclear fusion reaction hydrogen atoms combine to create a helium atom. But in a hydrogen atom there are no neutrons, then where does the neutrons in helium nucleus come from ?
Complete the following decay schemes.
(a) 22688Ra→α+
(b) 198O→199F+
(c) 2513Al→2512Mg+
Write two distinguishing feature of nuclear forces.
In a typical nuclear reaction, e.g.
21H+21H⟶32He+n+3.27MeV,
although number of nucleons is conserved, yet energy is released.How?Explain.
Initial mass of a radioactive substance is 3.2 mg. It has a half-life of 4 h. Find the mass of the substance left undecayed after 8 h.
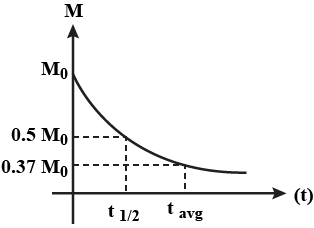
For a radioactive substance, show the variation of the total mass disintegrated as a function of time t graphically.
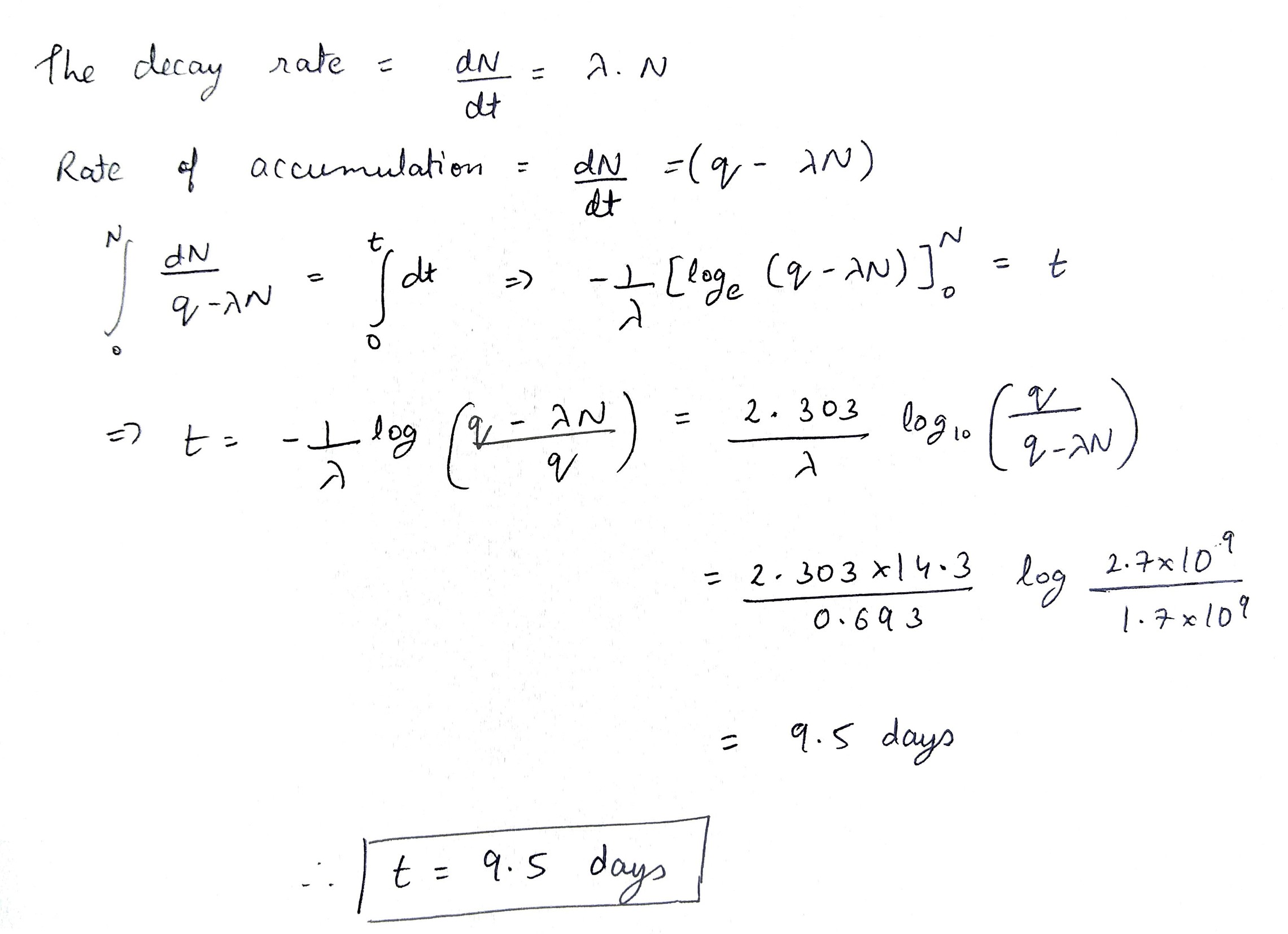
Nuclei of a radioactive element A are being produced at a constant rate α. The element has a decay constant λ. At time t=0, there are N0 nuclei of the element
(a) Calculate the number N of nuclei of A at time t
(b) If α=2N0λ, calculate the number of nuclei of A after one half-life of A, and also the limiting value of N as t→∞.
Show that 23092U does not decay by emitting a neutron or proton.Given: M(23092U)=230.033927a.m.u; M(22992U)=229.033496a.m.u; M(22992Pa)=229.032089a.m.u;
m(n)=1.008665a.m.u; m(p)=1.007825a.m.u
m(n)=1.008665a.m.u; m(p)=1.007825a.m.u
Identify X in the following nuclear reactions:
15N+ 1H→ 4He+X. Appendix F will help.
Identify X in the following nuclear reactions:
12C+ 1H→X;
At what temperature does the thermal energy equal the
energy separation?
What minimum energy is needed to remove this electron from the atom?
State the law of radioactive decay. Plot s graph showing the number (N) of undebased nuclei as a function of time (t) for a given radioactive sample having half-life T1/2. Depict in the plot the number of undecayed nuclei at
(i) t=3T1/2 and
(ii) t=5T1/2.

Answer the following question:
A radioactive nucleus 'A' undergoes a series of decays as given below:
Aα⟶A1β⟶A2α⟶A3γ⟶A4
Write the basic nuclear processes underlying β+ and β− decays.
Answer the following question:
(i)State the law of radioactive decay. Write the SI unit of 'activity'.
(ii) There are 4√2×106 radioactive nuclei in a given radioactive sample. If half-life of the sample is 20s, how many nuclei will decay in 10s?
1He3 and 2He3 nuclei have the same mass number. Do they have the same binding energy?
State Soddy-Fajan's displacement laws for radioactive transformations.
What happens to the position of an element in the periodic table when its nucleus emits a β particle? Give reason for your answer.
What happens to the atomic number of the nucleus when an β particle is emitted?
What changes occur in a nucleus of a radioactive element when it emits a beta particle? Give one example in support of your answer.
What happens to the mass number of the nucleus when an β particle is emitted?
The ratio of recoil of residual nucleus of Th232 to the velocity of a particle x emitted out is 1 :Find the mass of particle x emitted.
Calculate the separation between the particles of a system in the ground state, the corresponding binding energy and wavelength of first line in Lyman series of such a system is positronium consisting of an electron and a positron revolving round their common center.
Centain theories predict that the proton is unstable, with a half life of about 1023 years. Assuming that this is true, calculate the number of proton decays you would expect to occur in one year in the water of Olympic-sized swimming pool holding 4.32×105 L water.
Answer the following question:
Write the process of β− decay. How can radioactive nuclei emit β−particles even though they do not contain them ? Why do all electrons emitted during β−decay not have the same energy ?
The radionuclide 11C decays according to 116C→115B+e++v;T1/2=20.3min
A P32 radionuclide with half-life T=14.3 days is produced in a reactor at a constant rate q=2.7⋅109 nuclei per second. How soon after the beginning of production of that radionuclide will its activity be equal to A=1.0⋅109 dis/s ?
The specific activity of a preparation consisting of radioactive Co58 and nonradioactive Co59 is equal to 2.2⋅1012 dis/(s ⋅ g). The half life of Co58 is 71/3 days. Find the ratio of the mass of radioactive cobalt in that preparation to the total mass of the preparation (in per cent).
Write missing symbols , denoted by x, in the following nuclear reactions:
(a) B10(x,α)Be8;
(b) O17(d,n)x
(c) Na23(p,x)Ne20;
(d) x(p,n)Ar37.
Find the kinetic energy of the recoil nucleus in the positronic decay of Na13 nucleus for the case when the energy of positrons is maximum.
Taking the values of atomic masses from the tables, calculate the kinetic energies of a positron and a neutrino emitted by C11 nucleus for the case when the daughter nucleus does not recoil.
Class 12 Engineering Physics Extra Questions
- Alternating Current Extra Questions
- Atoms Extra Questions
- Current Electricity Extra Questions
- Dual Nature Of Radiation And Matter Extra Questions
- Electric Charges And Fields Extra Questions
- Electromagnetic Induction Extra Questions
- Electromagnetic Waves Extra Questions
- Electrostatic Potential And Capacitance Extra Questions
- Magnetism And Matter Extra Questions
- Moving Charges And Magnetism Extra Questions
- Nuclei Extra Questions
- Ray Optics And Optical Instruments Extra Questions
- Semiconductor Electronics: Materials,Devices And Simple Circuits Extra Questions
- Wave Optics Extra Questions