The D-And F-Block Elements - Class 12 Medical Chemistry - Extra Questions
If $$Hg^{2+}$$ salts are coloured enter 1, else enter 0.
The chloride of a copper metal is colourless. Why?
If the following ion(s) is/are coloured enter 1, else enter 0.
$$Zn^{2+}$$
What is Lanthanoid contraction? Mention one of its consequences.
Why do $$Zr$$ and $$Hf$$ have similar properties?
Account for the following :
$$Ti^{3+}$$ is coloured whereas $$Sc^{3+}$$ is colourless in aqueous solution.
Given reasonfor the following in one or two sentences 'Silver bromide is used in photography '
How would you account for the following situations:
The transition metals generally form coloured compounds.
Explain the following:
Transition elements forms coordinate bonds.
Transition elements generally form coloured compounds.Why?
Why $$Sc^{3+}$$ salts are colorless whereas $$Cr^{3+}$$ salt are coloured?
Write the balanced equations in the manufacture of $$K_2Cr_2O_7$$ from Chromite Ore.
Explain the manufacture of potassium dichromate from chromite ore $$(FeCr_2O_4)$$.
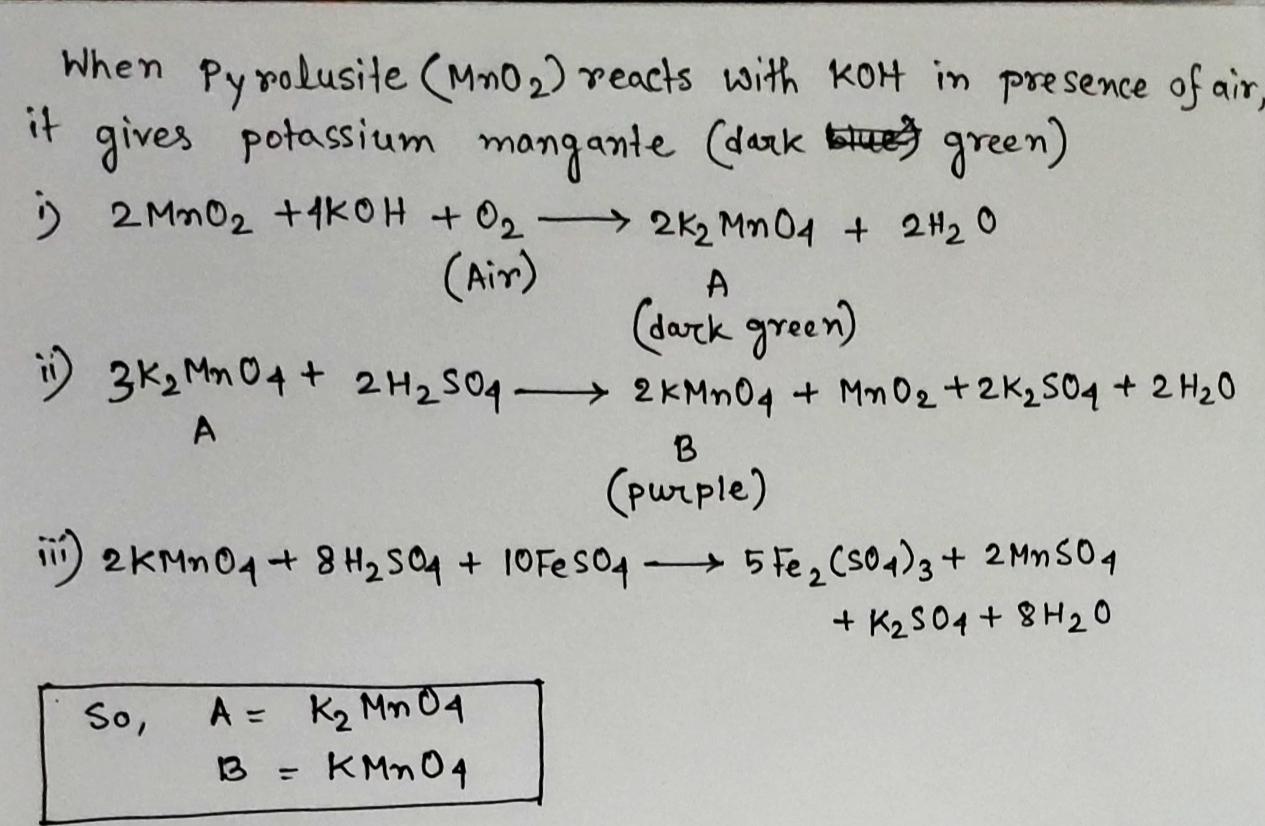
Pyrolusite on heating with $$KOH$$ in the presence of air gives a dark green compound $$(A)$$. The solution of $$(A)$$ on treatment with $$H_2SO_4$$ gives a purple coloured compound $$(B)$$, which gives following reactions.
The colours of the compound $$(B)$$ disappears on treatment with the acidic solution of $$FeSO_4$$. Identify A and B .
All the compounds of $$scandium$$ (Sc) are colourless. Give reason.
What is lanthanoid contraction? Explain
Class 12 Medical Chemistry Extra Questions
- Alcohols, Phenols And Ethers Extra Questions
- Aldehydes, Ketones And Carboxylic Acids Extra Questions
- Biomolecules Extra Questions
- Chemical Kinetics Extra Questions
- Chemistry In Everyday Life Extra Questions
- Coordination Compounds Extra Questions
- Electrochemistry Extra Questions
- General Principles And Processes Of Isolation Of Elements Extra Questions
- Haloalkanes And Haloarenes Extra Questions
- Organic Compounds Containing Nitrogen Extra Questions
- Polymers Extra Questions
- Solutions Extra Questions
- Surface Chemistry Extra Questions
- The D-And F-Block Elements Extra Questions
- The P-Block Elements Extra Questions