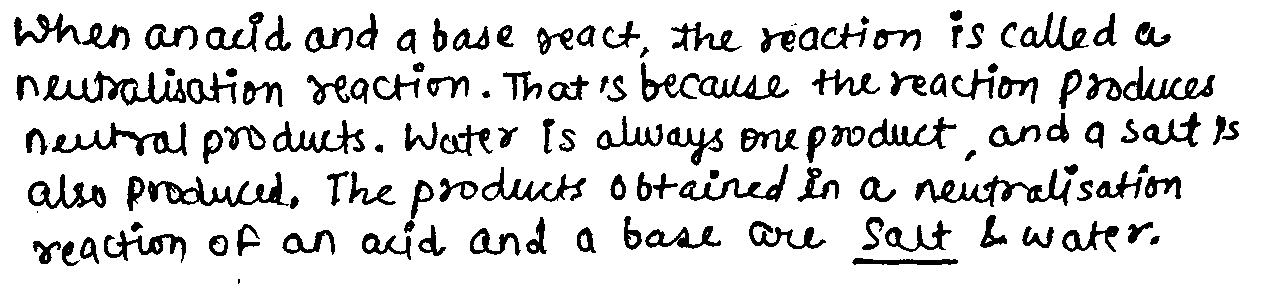Acids, Bases And Salts - Class 10 Chemistry - Extra Questions
Give one word or phrase for the following statement.
The property by which certain hydrated salts, when left exposed to atmosphere, lose their water of crystallization and crumble into powder.
Do acids produce ions only in an aqueous solution? Give reasons for your answer.
Give the reaction of Potassium Hydroxide ($$KOH$$) with water.
Complete the following table:
| $$Acid + Base \rightarrow Salt + water$$ |
| $$HNO_3 + .............. \rightarrow KNO_3 + H_2O$$ |
| $$............. +2NH_4OH \rightarrow (NH_4)_2SO_4 + .............$$ |
| $$............. + KOH \rightarrow KBr + .............$$ |
Name a salt which does not contain water of crystallization.
Fill in the following table
| Name of the salt | Formula | Salt obtained from Base | Salt obtained from Acid |
| (i) Ammonium Chloride | NH$$_4$$Cl | NH$$_4$$OH | - |
| (ii) Copper sulphate | - | - | H$$_2$$SO$$_4$$ |
| (iii) Sodium chloride | NaCl | NaOH | - |
| (iv) Magnesium Nitrate | Mg(NO$$_3$$)$$_2$$ | - | HNO$$_3$$ |
| (v) Calcium nitrate | Ca(NO$$_3$$)$$_2$$ | Ca(OH)$$_2$$ | - |
Complete and balance the following chemical reaction:$$CaCO_{3}+HCl\rightarrow $$
Give a suitable chemical term for the following:A definite number of water molecules bound to a salt.
Mention the types of solutions based on the $$pH$$ value.
Describe the process of neutralization with the help of an example.
State one relevant observation for the following.
Anhydrous calcium chloride is exposed to air for some time.
Dry ammonia has no action on litmus paper, but a solution of ammonia in water turns red litmus paper blue. Why?
Balance the following equation:
$$Mg(OH)_{2}+HCl \longrightarrow MgCl_{2}+H_{2}O$$
State the number of water molecules present in crystals of washing soda and plaster of Paris. What are these molecules called?
Conc. $$H_2SO_4$$ is a good drying agent, yet it is not used to dry $$NH_3$$.
Which colour is shown by a neutral solution on a $$pH$$ scale?
When a solution is added to cloth strip treated with onion extract, then the smell of onion cannot be detected. State whether the given solution contains an acid or a base.
When a solution is added to vanilla extract, then the characteristics smell of vanilla cannot be detected. State whether the given solution is an acid or a base.
15ml of water and 10 ml of sulphuric acid are to be mixed in a beaker.
i] State the method that should be followed for mixing with appropriate reasons.
ii] What is this process called?
i] State the method that should be followed for mixing with appropriate reasons.
ii] What is this process called?
Sodium hydrogen carbonate is a basic salt. Justify the statement.
Which is more basic (or more alkaline)? A solution of pH = 8 or a solution of pH = 11?
Two drinks P and Q gave acidic and alkaline reactions, respectively. One has a pH value of $$9$$ and the other has a pH value of $$3$$. Which drink has a pH value of $$9$$?
Fill in the blanks in the following sentences:
(a) Acids have a ....... taste and they turn ....... litmus to .......
(b) Substances do not show their acidic properties without .......
(c) Acids produce ....... ions on dissolving in water.
(d) Those substances whose smell (or odour) changes in acidic or basic solutions are called .......
(e) Onion and vanilla extract are .......
List the important products of the Chlor-alkali process. Write one important use of each.
(a) What happens when dilute hydrochloric acid is added to sodium carbonate? Write a balanced chemical equation of the reaction evolved.
(b) Which gas is liberated when dilute hydrochloric acid reacts with sodium carbonate? How will you test for the presence of this gas?
What is an olfactory indicator? Name two olfactory indicators. What is the effect of adding sodium hydroxide solution to these olfactory indicators?
Which is more acidic: a solution of pH = 2 or a solution of pH = 6?
What happens when an acid reacts with a base? Explain by taking the example of hydrochloric acid and sodium hydroxide. Give the chemical reaction which takes place. What is the special name of such a reaction?
Write the name and formula of one salt each which contains:
(a) two molecules of water of crystallisation
(b) five molecules of water of crystallisation
(c) ten molecules of water of crystallisation
Two solutions $$A$$ and $$B$$ have pH of $$5$$ and $$8$$ respectively. Which solution will be basic in nature?
Arrange the following in the increasing order of acidic strength.
Acetic acid, water and hydrochloric acid
(a) What is meant by "water of crystallisation" in a substance? Explain with an example.(b) How would you show that blue copper sulphate crystals contain water of crystallisation?(c) Explain how anhydrous copper sulphate can be used to detect the presence of moisture (water) in a liquid.
Which of the following salt has a blue colour and why?
$$CuSO_4.5H_2O$$ or $$ CuSO_4.$$
How many molecules of water of crystallization (per formula unit) are present in:
(a) copper sulfate crystals?
(b) washing soda?
(c) gypsum?
The pH values of five solutions A, B, C, D and E are given below:
| A | 1 |
| B | 5 |
| C | 7 |
| D | 11 |
| E | 13 |
Which solution is:
weakly alkaline
neutral
strongly acidic
strongly alkaline, and
weakly acidic?
weakly alkaline
neutral
strongly acidic
strongly alkaline, and
weakly acidic?
What is an olfactory indicator? Name two such indicators.
Fill in the blanks:
Sodium hydroxide or potassium hydroxide is found in ________.
Sodium hydroxide or potassium hydroxide is found in ________.
A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, when a gas G obtained at the anode during electrolysis of brine is passed on dry Y, it gives a compound Z, used for disinfecting drinking water. Identity X, Y, G, and Z.
How would you distinguish between baking powder and washing soda by heating?
In one of the industrial processes used for the manufacture of sodium hydroxide, a gas X is formed as a by-product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B, and C.
Classify the following as physical or chemical property Most metal oxides form alkalis on interacting with water.
Water turns the colour of anhydrous copper sulphate to _______.
Fill in the blanks :
The number of molecules of water of crystallization in washing soda is .........
What are salts?
Give the $$pH$$ value of pure water. Does it change if common salt is added to it?
What would you observe when red litmus paper is introduced in a sodium carbonate solution?
Write the name, method of production and uses of following:
$$NaOH$$
What are olfactory indicators? Give examples.
Take $$5mL$$ water in a test tube and slowly add concentrated sulphuric acid to it. Touch the bottom of the test tube. What do you feel? Is the reaction exothermic or endothermic?
A student heated a few crystals of copper sulphate in a dry boiling tube.
(a) What will be the colour of the copper sulphate after heating?
b) Will you notice water droplets in the boiling tube?
(c) Where do these water droplets come from?(d) After adding $$2-3$$ drops of water on the sample of copper sulphate obtained after heating. What do you observe? Is the blue colour of copper sulphate restored?
(i) Dry pellets of a base ‘$$X$$’ when kept in open, absorb moisture and turn sticky. The compound is also formed by chlor-alkali process. Write the chemical name and formula of X. Describe chlor-alkali process with a balanced chemical equation. Name the type of reaction that occurs when X is treated with dilute hydrochloric acid. Write the chemical equation.
(ii) While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Hydrated calcium sulphate has the formula of $$CaSO_{4}.2H_{2}O$$.
(i) What is the name given to the water molecules present in the salt?
(ii) Calculate the percentage of water molecules in hydrated calcium sulphate.
[Ca = 40; S = 32; O = 16; H = 1]
A cloth strip dipped in onion juice is used for testing a liguid $$\mathrm{X}$$. The liquid '$$\mathrm{X}$$' changes its odour. Which type of an indicator is onion juice? The liquid '$$\mathrm{X}$$' turns blue litmus red. List the observations the liquid '$$\mathrm{X}$$'
will show on reacting with the following:
(a) Zinc granules
(b) Solid sodium carbonate
Write the chemical equations for the reactions involved.
Bleaching powder is produced
when chlorine reacts with?
The products obtained in a neutralization reaction of an acid and a base are .......... and water.
Read the information given in the table and answer the following questions.
| Solution | pH Value | Reaction with Phenolphthalein solution | Reaction with Methyl orange solution |
| HCl | 1 | No colour change. | Turns into red |
| Distilled water | 7 | No colour change. | No colour change. |
| NaOH | 13 | Turns into pink colour. | Turns into yellow colour. |
| Lemon juice | 2.5 | No colour change. | Turns into red colour. |
| NaCl | 7 | No colour change. | No colour change. |
| Baking Soda | 8 | Turns into pink colour. | Turns into yellow colour. |
(b) What is the nature of the solution which gives pink colour with Phenolphthalein solution?
(c) List out the neutral solutions in the above table.
(d) Name the strongest acid and the strongest base among the given solutions.