Periodic Classification Of Elements - Class 10 Chemistry - Extra Questions
According to Newlands' assumption, what is the total number of elements in nature?
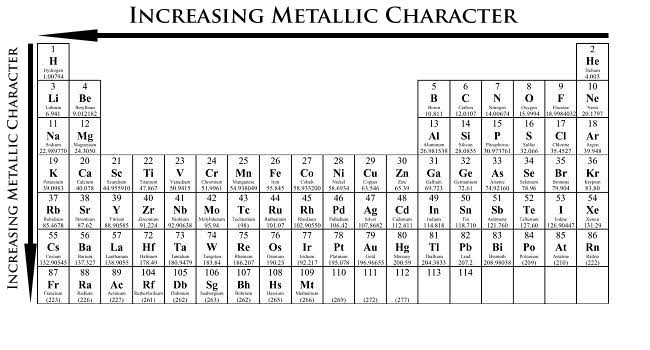
How and why does the atomic size vary as you go:
(i) from left to right along a period?
(ii) down a group?
State Newland's Law of Octaves. Write the name of the element having similar properties to the following:
(a) Nitrogen
(b) Lithium
S, Se, Te are the elements of a triad. If the atomic weight of Sulphur is 32 and Tellurium is 127.5, the atomic mass of Selenium is ________.
Write down five major differences between Mendeleev's periodic table and Modern periodic table.
Which will be the third element among the given elements to complete the given Doberiener triad?
(Atomic mass of $$\text {Be= 9, Na= 23, Mg= 24, Si= 28, Cl= 35, Ca= 40} $$)$$\text {Na, __ , Cl}$$
Which will be the third element among the given elements to complete the given Doberiener triad?
(Atomic mass of $$\text {Be= 9, Na= 23, Mg= 24, Si= 28, Cl= 35, Ca= 40} $$)
Which will be the third element among the given elements to complete the given Doberiener triad?(Atomic mass of Be 9; Na 23; Mg 24; Si 28; Cl 35; Ca 40)Be, ___, Ca
Which will be the third element among the given elements to complete the given Doberiener triad?
(Atomic mass of Be 9; Na 23; Mg 24; Si 28; Cl 35; Ca 40)
The law used by Newland to arrange elements is known as ___________.
State Newland's law of octaves.
Dobereiner classified elements into groups based on their properties. The number of elements in each group is _______.
The arrangement of elements in a group of three is known as ___________.
Arrange the following as per the instruction given in the bracket:
$$He, Ar, Ne$$ (Increasing order of the number of electron shells).
$$He, Ar, Ne$$ (Increasing order of the number of electron shells).
What are the limitations of Mendeleev's periodic table?
Explain Newlands law of Octaves. Why was the law discarded?
The atomic numbers of five elements $$A, B, C, D$$ and $$E$$ are $$6, 8, 3, 7$$ and $$9$$ respectively.
Which is the element having least metallic character among these elements? Why?
What is valency? Write valency of hydrogen.
Name the scientist who presented 'Law of octave'.
Can the following groups of elements be classified as Dobereiner's triad:
(a) Na, Si, Cl
(b) Be, Mg, Ca
Justify the answer in each case.
[Atomic mass(in u) of $$Be-9; Na-23; Mg-24; Si-28; Cl-35.5; Ca-40$$]
(a) Na, Si, Cl
(b) Be, Mg, Ca