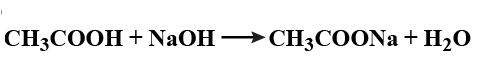Carbon And Its Compounds - Class 10 Chemistry - Extra Questions
Even though both are oxidation processes, combustion and respiration are different in many aspects. Explain those differences.
Where is ethanol used?
Carbon in hydrocarbons forms bonds by _________ of electrons.
Name five compounds of carbon.
Write the main characteristics of the carbon atom.
After completing the chemical reactions, write down to which category they belong.
CH4+2O2→.....+H2O
CH4+2O2→.....+H2O
Covalent compounds have low melting point. Justify.
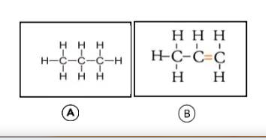
At least one carbon bond in an unsaturated hydrocarbon is ..................
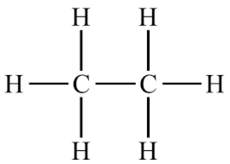
Name the saturated hydrocarbon containing two carbon atoms.
Fill in the blank:
The Latin name of carbon is____.
Name the organic compound prepared by each of the following reactions:
C2H5OH+CH3COOHCon.H2SO4→Δ
The phenomenon of the existence of a chemical element in two or more forms differ in physical properties but of the same chemical nature is known as Allotropy. Carbon has two major allotropes diamond and graphite and the third one is Buckminsterfullerene. Diamond has a three-dimensional network of covalent bonds and is hard. In graphite, the carbon atoms are arranged in flat parallel layers as regular hexagons resulting in soft and ________ nature.
What is the term defined below?
A bond is formed by a shared pair of electrons, and each bonding atom contributes one electron to the pair.
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Why are carbon and its compounds used as fuels for most applications?
Give a test that can be used to differentiate chemically between butter and cooking oil.
Write the number of covalent bonds in the molecule of ethane.
State the reason why carbon can neither form C4+ cations nor C4− anions but forms covalent compounds. Also, state reasons to explain why covalent compounds:
(i) are bad conductors of electricity?
(ii) have low melting and boiling points?
Allotropy is a property shown by which class substance: elements, compounds or mixtures?Explain allotropy with suitable examples.
Give the structural formula of Ethanol:
What are allotropes? Name any two allotropic forms of carbon. Give one use of it.
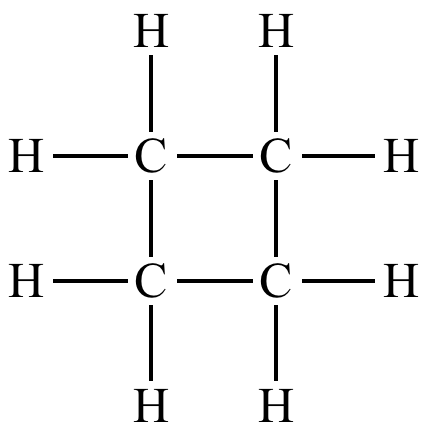
What do you mean by saturated hydrocarbons and unsaturated hydrocarbons? State their examples.
Define :
(a) Saturated hydrocarbon
(b) Unsaturated hydrocarbon
(c) Catenation
Diamond is the hardest allotrope of carbon. Give reason for its hardness.
Write three allotropes of carbon.
(a) Write the dehydration reaction of ethanol and write the name of dehydrating agent.
(b) Choose correct series for the following.
| Compund | Series |
| (i) Ethane | (a) unsaturated hydrocarbon |
| (ii) Benzene | (b) long chained saturated hydrocarbon |
| (iii) Hexane | (c) hydrocarbon having odd atom |
| (iv) Methyl alcohol | (d) saturated hydrocarbon |