Metals And Non Metals - Class 10 Chemistry - Extra Questions
Enumerate some of the physical properties of non-metals.
What is a sickle that is used to cut sugarcane made from? Why?
Generally, non-metals are not lustrous. Name a non-metal which is lustrous.
State the conditions under which the following metals react with water. Give equations for each reaction.
(i) Na
(ii) Mg
(iii) Fe
Based on the following reactions, arrange the metals involved in the following reactions in decreasing order of reactivity:
(i) Zn+CuSO4→ZnSO4+Cu
(ii) Zn+FeSO4→ZnSO4+Fe
(iii)Cu+2AgNO3→Cu(NO3)2+2Ag
(iv) Fe+CuSO4→FeSO4+Cu
A silver article generally turns black when kept in the open for a few days. The article when rubbed with toothpaste again starts shining. Silver articles turn black when kept in the open for a few days because of _________.
What is corrosion? Explain whether gold ornaments corrode or not.
Define the reactivity series of metals.
Is there any reaction between copper and dilute hydrochloric acid? Give reasons for your answer.
A copper spoon had fallen into a container containing dil. HCl. What would happen to it in three days time?
Can you store lemon pickle in an aluminium utensil? Explain.
Copper can be extracted by hydrometallurgy but not zinc. Explain.
Ionic compounds are generally soluble in ________.
A copper coin is kept immersed in a solution of silver nitrate for some time. What will happen to the coin and the colour of the solution?
A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument, the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Explain why iron sheets are coated with zinc during galvanizing.
Why does the colour of copper sulphate change when an iron nail is kept in it? Justify your answer.
Chemical equation for the reaction between CuSO4 solution and an iron nail is given below.
CuSO4+Fe→FeSO4+Cu
(a) Write the reduction reaction taking place here.
(b) Give reason for the displacement of Cu by Fe from CuSO4 solution.
Name the following:
i) The ion formed by the loss of an electron from its neutral atom
ii) The ion formed by the gain of an electron from its neutral atom
iii) An element that loses an electron to form cationiv) An element that gains an electron to form an anion
v) Ion(s) involved in the formation of ionic compounds.
v) Ion(s) involved in the formation of ionic compounds.
Why are bells made up of metals and not from wood?
What is meant by reactivity series of metals?
Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg and Zn
Give an example of a metal which can be easily cut with a knife.
Name two metals which exist in a free state in nature.
Name the metal which is used to make foils for the packing of food materials.
What is occurrence of metals?
Radha kept milk for curdling in a copper vessel while Priya used steel vessel for the same. Which of the two would be edible and why?
Write chemical equation for the following reaction:
Cinnabar is heated in the presence of air.
Cinnabar is heated in the presence of air.
Write balanced chemical equations for each of the following:
(i) Reaction of iron with chlorine.
(ii) Addition of silver nitrate solution to sodium chloride solution.
(iii) Addition of zinc to sodium hydroxide solution.
Give balanced chemical equation for the following conversions A,B, and C FeA⟶FeCl3B⟶FeCO3C⟶Fe(NO3)2
The compound A is
Why curd and pickles are not stored in metallic boxes ?
Write a chemical equation for the following reaction.
Zinc metal reacts with aqueous hydrochloric acid to produce a solution of zinc chloride and hydrogen gas.
Explain the following giving proper reason:
In moist air, copper objects acquire a green layer on the surface.
In moist air, copper objects acquire a green layer on the surface.
You are given samples of three metals - sodium, magnesium and copper. Suggest any two activities to arrange them in order of their decreasing reactivities.
What is the action of water on (a) sodium (b) magnesium and (c) aluminium? Write the equations of the chemical reactions involved.
Name the metal which has been placed :
(a) at the bottom of the reactivity series.
(b) at the top of the reactivity series.
(c) just below copper in the reactivity series.
Name the non-metal which is used :
(a) To convert vegetable oil into vegetable ghee(solid fat)
(b) As a rocket fuel (in liquid form)
(c) To preserve food materials
(a) Name the most abundant metal in the earth's crust.
(b) Name the most abundant non-metal in the earth's crust.
(a) What is meant by the reactivity series of metals? Arrange the following metals in an increasing order of their activities towards water.
Zinc, iron, magnesium, Sodium
(b) Name one metal more reactive and another less reactive than hydrogen.
(c) Name one metal which displaces copper from copper sulphate solution and one which does not.
(d) Name one metal which displaces silver from silver nitrate solution and one which does not.
How would you show that silver is chemically less reactive than copper ?
What is meant by "brittleness"? Which type of elements usually show brittleness: metals or non-metals?
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
Name two metals which react violently with cold water. Write any three observations you would make when such a metal is dropped into water. How would you identify the gas evolved if any during the reaction?
In a solution of lead acetate, a strip of metal M was dipped, after sometime lead from the solution was deposited on the metal strip. Which metal is more reactive M or lead?
A student has found black coating on his silver coins and green coating on his copper coins. Which chemical phenomenon is responsible for this? Write the chemical name of these coatings.
(a) Name a metal for each case:
(i) It does not react with cold and hot water but reacts with steam.
(ii) It does not react with water.
(b) When calcium metal is added to water, the gas evolved does not catch fire but the same gas evolved on addition of sodium metal to water catches fire. Why is it so?
Name any three objects (or structures ) which are gradually damaged by the corrosion of iron and steel.
What do you mean by malleability and ductility of a metal ?
How are highly reactive metals reduced?
Name two metals that do not react with water at all.
What changes in the colour of iron nails and copper sulphate solution do you observe after keeping the iron nails dipped in copper sulphate solution for about 30 minutes?
How are metals which are in the middle of the reactivity series reduced?
Given reasons why :(a) Copper metal is used for making electric wires.
(b) Gold and Silver is used for making jewellery
(b) Gold and Silver is used for making jewellery
What happens when calcium is treated with water?
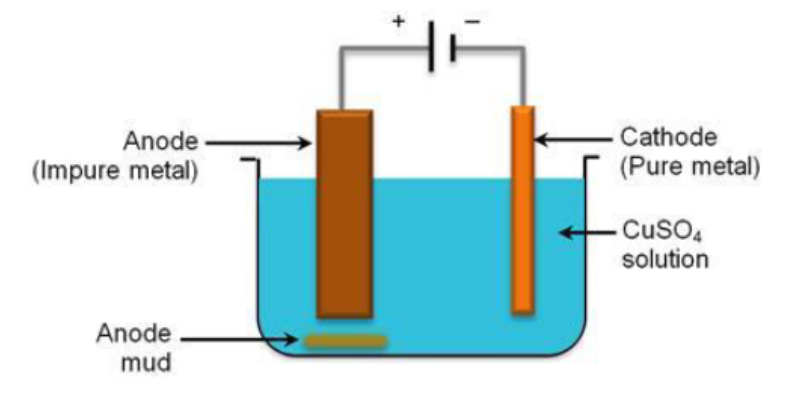
During extraction of metal, electrolytic refining is used to obtain pure metals.
Suggest a suitable electrolyte also.
When calcium metal is added to water the gas evolved does not catch fire but the same gas evolved on adding sodium metal to water catches fire. Why is it so?
During extraction of metals, electrolytic refining is used to obtain pure metals.
(a) Which material will be used as anode and cathode for refining of silver metal by this process?
(b) Suggest a suitable electrolyte also.
(c) In this electrolytic cell, where do we get pure silver after passing electric current?
Paheli prepared a blue coloured solution of copper sulphate in beaker A and placed an iron nail in it. Boojho prepared a yellowish green solution of ferrous sulphate in beaker B and placed a copper wire in it. What changes will they observe in the two beakers after an hour?
Iqbal treated lustrous, divalent element M with sodium hydroxide. He observed the formation of bubbles in reaction mixture. He made the same observations when this element was treated with hydrochloric acid. Suggest how can he identify the produced gas. Write chemical equations for both the reactions.
During the reaction of some metals with dilute hydrochloric acid, following observations were made.
(a) Silver metal does not show any change
(b) The temperature of the reaction mixture rises when aluminium (Al) is added.
(c) The reaction of sodium metal is found to be highly explosive
(d) Some bubbles of gas are seen when lead (Pb) is reacted with the acid.
Explain these observations giving suitable reasons.
Properties of the elements arc given below. Where would you locate the following elements in the periodic table?
(a) A soft metal stored under kerosene
(b) An element with variable (more than one) valency stored under water.
(c) An element which is tetravalent and forms the basis of organic chemistry
(d) An element which is an inert gas with atomic number
(e) An element whose thin oxide layer is used to make other elements corrosion resistant by the process of "anodising"
Name a metal that can be cut with knife.
Explain the following
(a) Reactivity of Al decreases if it is dipped in HNO3.
(b) Carbon cannot reduce the oxides of Na or Mg.
(c) NaCl is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state.
(d) Iron articles are galvanised. (e) Metals like Na, K, Ca and Mg are never found in their free state in nature.
Non-metals are usually poor conductors of heat and electricity. They are non- lustrous, non-sonorous, non-malleable and are coloured. Name a non-metal other than carbon which shows allotropy.
Out of non-metals and metals, which is hard, ductile, sonorous and malleable?
Give the formulae of the stable binary compounds that would be formed by the combination of following pairs of elements.
(a) Mg and N2
(b) Li and O2
(c) Al and Cl2
(d) K and O2
What is the sheet used for roofing made from? Why?
Name a non-metal that is hardest naturally occurring substance.
Under what condition iron reacts with water.
Name the following:
(a) A non-metal that is lustrous.
(b) A metal that is liquid at room temperature.(c) An allotrope of carbon that conducts electricity.(d) An alkali metal that can be cut with a knife.
What is corrosion? What are the necessary conditions for corrosion?
Complete and balance the following equation.
Na+H2O→−−+−−−
Which gas is bubbled on the surface of sodium metal?
Aluminum utensils are used for cooking. Which property of aluminum is used?
What is a screwdriver made from? Why?
Write products of reaction of acid with metal.
Differentiate between metals and non-metals on the basis of their physical properties.
Which are the metals found in free state? Why?
Name two metals which react with cold water.
How many of the following substances do not shine?
Sulphur, Copper, Diamond, Lead, and Iodine
Some properties are listed in the following table. Distinguish between metals and non-metals based on these properties.
| Properties | Metals | Non-metals |
| Appearance | ||
| Hardness | ||
| Malleability | ||
| Ductility | ||
| Heat Conduction | ||
| Conduction of Electricity |