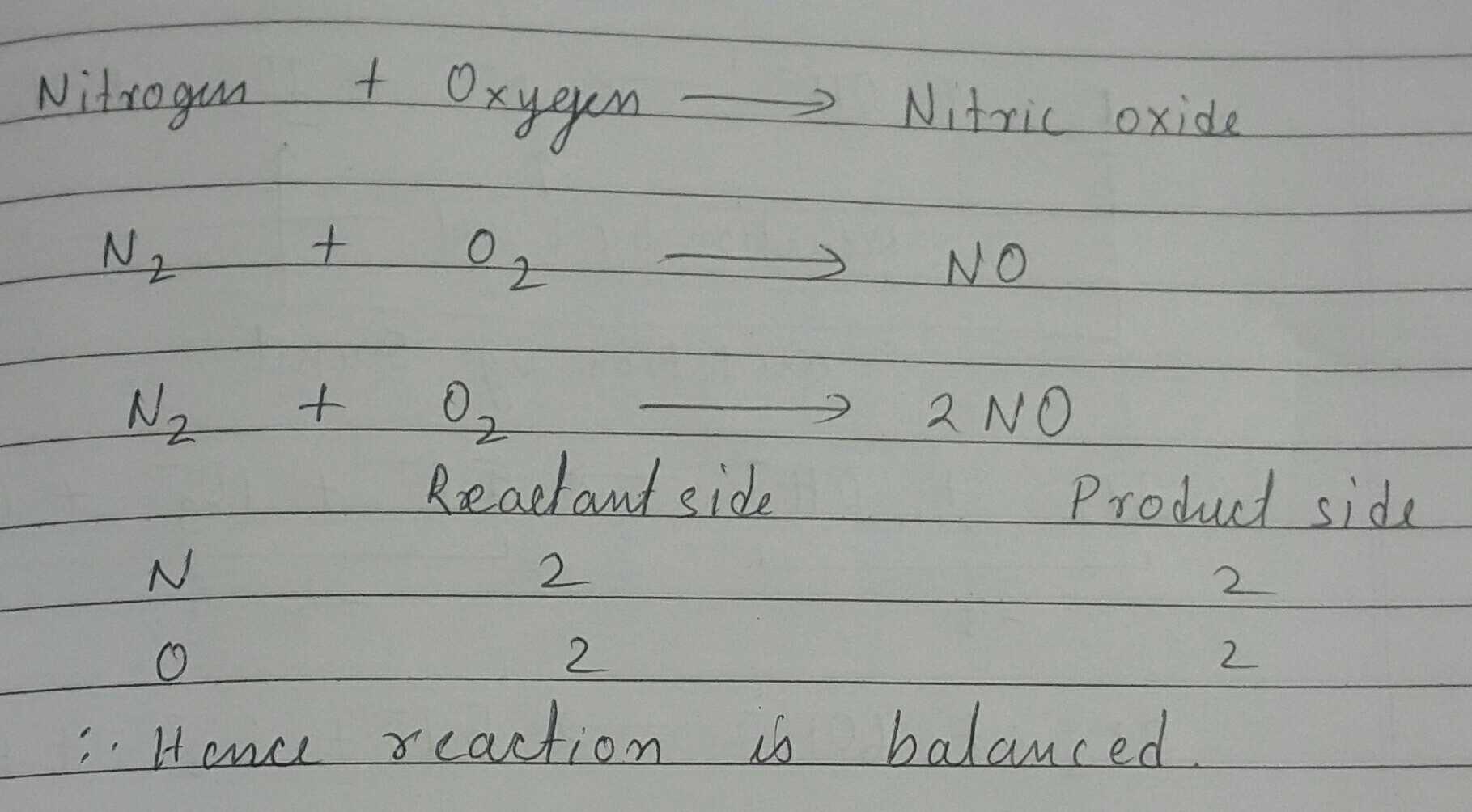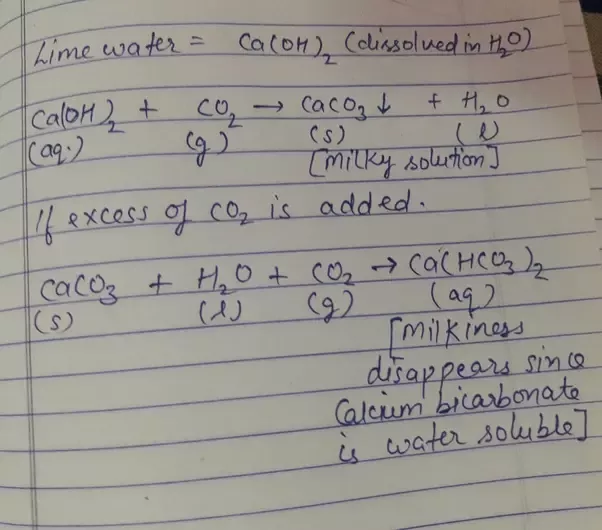Chemical Reactions And Equations - Class 10 Chemistry - Extra Questions
Complete the statement by filling the gaps using the appropriate terms.
An ...... is drawn in between the reactants and products while writing the equation for a chemical reaction.
Give the reason for the following:
Respiration is an exothermic reaction.
Mention the types of chemical reactions.
A decomposition reaction taking place due to the passage of electric current is called ___________.
The arrow mark between the products and reactants of a chemical equation shows __________ of the reaction.
What is a combination reaction? Write combination reaction of quick lime with water.
Write balanced chemical equations for the following word equation:
Nitrogen + Oxygen $$\rightarrow $$ Nitric Oxide
Write a balanced chemical equation for each of the following:
Action of dilute hydrochloric acid on Iron
Name the substance oxidized and reduced in the given reaction
$$Cl_2 + H_2S\rightarrow 2HCl + S$$
Write a balanced chemical equation for each of the following:
Action of heat on calcium bicarbonate
Write equations for the following reactions:
(i) Aluminium oxide and Sodium hydroxide.
(ii) Zinc and dilute sulphuric acid.
A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.a) What changes do you expect?b) Are these changes chemical in nature?c) Write a word equation for the chemical change, if any.
Write the essential condition for the following reaction to take place:
$$2AgBr\rightarrow 2Ag + Br_2$$
Write an application of this reaction.
An iron salt A reacts with NaOH to form a green precipitate. Another iron salt B reacts with NaOH to form a brown precipitate. Identify the iron salts, A and B along with their colours and write the reactions involved.
Activity:
Take lead nitrate solution in a test tube.
Add potassium iodide solution to this.
What do you observe ?
What is a redox reaction? Identify the substances oxidized and the substances reduced in the following reactions.
1) $$2PbO + C\rightarrow 2Pb+CO_2$$
2) $$MnO_2 + 4HCl\rightarrow MnCl_2 + 2H_2O + Cl_2$$
| A student has mixed the solutions of lead $$ (II) $$ nitrate and potassium iodide. (i) What was the colour of the precipitate formed? Can you name the compound? (ii) Write the balanced chemical equation for this reaction. (iii) What type of reaction is it? |
Classify each of the following reactions as a combination, decomposition, displacement, or double displacement:
$$CaO+H_{2}O\rightarrow Ca\left(OH\right)_{2}$$
$$\text{Name the organic compound prepared by each of the following reactions}:$$
$$CH_{3}COONa + NaOH \xrightarrow[\Delta]{CaO}$$
When ammonium chloride is dissolved in water in a test tube, the test tube becomes cold why?
Classify each of the following reactions as a combination, decomposition, displacement, or double displacement:
$$BaCl_{2}+Na_{2}SO_{4}\rightarrow BaSO_{4}+2NaCl$$
2 g of ferrous sulphate crystals are heated in a dry boiling tube. Name the type of chemical reaction taking place.
Classify each of the following reactions as a combination, decomposition, displacement, or double displacement:
$$2KClO_{3}\rightarrow 2KCl+3O_{2}$$
Name the gas that evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
2 g of ferrous sulphate crystals are heated in a dry boiling tube.Is this also a double displacement reaction ?
Why can copper displace silver from silver nitrate and silver cannot displace copper from copper sulphate solution? Give example.
Identify the following reactions as
i) combination ii) decomposition iii) displacement reactions and double
displacement reaction
$$CaCO_{3}(s) \rightarrow CaO(s) + CO_{2}(g)$$
Identify the following reactions as
i) combination ii) decomposition iii) displacement reactions and double
displacement reaction
$$ Fe_{2}O_{3} + Al \rightarrow Al_{2}O_{3} + 2Fe $$
Identify the following reactions as
i) combination ii) decomposition iii) displacement reactions and double displacement reaction
$$Pb(s) + CuCl_{2} \rightarrow PbCl_{2} + Cu(s)$$
Identify the following reactions asi) combination ii) decomposition iii) displacement reactions and double displacement reaction
$$NaCl + AgNO_{3} \rightarrow AgCl + NaNO_{3} $$
$$NaCl + AgNO_{3} \rightarrow AgCl + NaNO_{3} $$
Name the brown coloured gas evolved when lead nitrate crystal are heated in dry test-tube.
$$Zn + H_{2}SO_{4} \rightarrow ZnSO_{4} + H_{2}$$
Write the ionic equation for the reaction.
Identify the following reaction as
i) combination ii) decomposition iii) displacement reactions and double displacement reaction.
$$ZnCO_{3}(s) \rightarrow ZnO(s) + CO_{2}(g)$$
Identify the following reactions as
i) combination ii) decomposition iii) displacement reactions and double
displacement reaction
$$3H_{2}(g)+ N_{2}(s) \rightarrow 2NH_{3} $$
Identify the following reactions as
i) combination ii) decomposition iii) displacement reactions and double displacement reaction
$$H_{2}(g)+ Cl_{2} \rightarrow 2HCl$$
Following observations are recorded during a number of experiments on the substance P. When the substance P is heated, a reddish brown gas is evolved along with a gas which relights glowing wooden splint. A yellow residue is left in the test tube.
Name the gas which relights the glowing splint.
Mention the physical states of the reactants and products of the following chemical reactions and balance the
equation.
$$NH_{3} + Cl_{2} \rightarrow N_{2} + NH_{4}Cl$$
$$ \bullet $$ Take about 100 ml of water in a beaker and dissolve a small quantity of sodium sulphate $$(Na_{2}SO_{4})$$.
$$ \bullet $$Take about
100ml of water in another beaker and dissolve a small quantity of barium chloride $$(BaCl_{2})$$. observe the colours of the
solutions obtained.
(a)What are the colours of the above solutions?
(b) Can you name the solutions obtained?(Add $$Na_{2}SO_{4}$$ solution to $$BaCl_{2}$$ solution and observe.)
(c)Do you observe any change on mixing these solutions?
Following observations are recorded during a number of experiments on the substance P. When the substance P is heated, a reddish brown gas is evolved along with a gas which relights glowing wooden splint. A yellow residue is left in the test tube.
Name the reddish brown gas,
What is decomposition reaction? Explain it with suitable example.
Burning magnesium ribbon in the air to form magnesium oxide is an example of _______ (combination/decomposition) reaction.
Digestion of food is an example of ______ (physical/chemical) change.
Respiration is an example of ____________ (physical/chemical) change.
Heat is released in _______ (endothermic/exothermic) reactions.
________ reactions called the opposite of combination reactions.
In a solution of silver nitrate, a copper plate was dipped. After some time, silver from the solution was deposited on the copper plate. Which metal is more reactive, copper or silver?
Balance the following chemical equations:
(a) $$HN{ O }_{ 3 }+Ca{ \left( OH \right) }_{ 2 }\rightarrow Ca{ \left( N{ O }_{ 3 } \right) }_{ 2 }+{ H }_{ 2 }O$$
(b) $$NaOH+{ H }_{ 2 }S{ O }_{ 4 }\rightarrow { Na }_{ 2 }S{ O }_{ 4 }+{ H }_{ 2 }O$$
(c) $$NaCl+AgN{ O }_{ 3 }\rightarrow AgCl+NaN{ O }_{ 3 }$$
(d) $$Ba{ Cl }_{ 2 }+{ H }_{ 2 }S{ O }_{ 4 }\rightarrow BaS{ O }_{ 4 }+HCl$$
(a) $$HN{ O }_{ 3 }+Ca{ \left( OH \right) }_{ 2 }\rightarrow Ca{ \left( N{ O }_{ 3 } \right) }_{ 2 }+{ H }_{ 2 }O$$
(b) $$NaOH+{ H }_{ 2 }S{ O }_{ 4 }\rightarrow { Na }_{ 2 }S{ O }_{ 4 }+{ H }_{ 2 }O$$
(c) $$NaCl+AgN{ O }_{ 3 }\rightarrow AgCl+NaN{ O }_{ 3 }$$
What do you see when a magnesium ribbon is burnt? Is magnesium oxidized or reduced in this reaction?
Write word equations and then balanced equations for the reaction taking place when:
(a) Dilute sulphuric acid reacts with zinc granules.
(b) Dilute hydrochloric acid reacts with magnesium ribbon.
(c) Dilute sulphuric acid reacts with aluminium powder.
(d) Dilute hydrochloric acid reacts with iron filings.
What is the difference between displacement and double displacement reactions? Write equations for these reactions?
Which reaction is this?$$2NaHCO_{3}\xrightarrow {heat} Na_{2}CO_{3} + H_{2}O + CO_{2}$$
What causes rancidity? Name an antioxidant which prevents rancidity.
Write one difference between displacement and double displacement reactions.
What happens when:
i) $$CuS{ O }_{ 4 }\left( aq \right) +Fe\left( s \right) \rightarrow $$?
ii) $$Zn\left( s \right) +{ H }_{ 2 }S{ O }_{ 4 }\left( l \right) \rightarrow $$?
iii) $$Na\left( s \right) +{ O }_{ 2 }\left( g \right) \rightarrow $$?
Explain rancidity.
Identify $$X$$, $$Y$$ and $$Z$$ in the following equations :
i) $$Cu + C{O}_{2} \xrightarrow {\quad moisture\quad }$$ Green compound $$ \left( X \right)$$
ii) $$ Ag + Y \xrightarrow {\quad air\quad }$$ Black compound $$ \left( { Ag }_{ 2 }S \right) $$
iii) $$Fe S{ O }_{ 4 } \xrightarrow { \quad heat\quad } { Fe }_{ 2 }{ O }_{ 3 }+S{ O }_{ 3 }+Z$$
Give the name of the gas that evolved when sodium hydroxide reacts with zinc metal. Write the equation also.
Write the balanced chemical equation when zinc reacts with dilute nitric acid.
(a) What is a displacement reaction?
(b) Identify A in the following reactions.
(i) $$Zn+CuSO_4\rightarrow A+Cu$$
(ii) $$Na_2SO_4+BaCl_2\rightarrow A+2NaCl$$.
Choose the correct answer from the four alternatives given in the brackets:
$$Zn+2HCl\longrightarrow Zn{ Cl }_{ 2 }+{ H }_{ 2 }\uparrow $$
The above reaction is an example of _______.
(Combination reaction, Double displacement reaction, Displacement reaction, Decomposition reaction)
Explain an experiment with a chemical equation to establish that iron is more reactive than silver.
What type of chemical reaction takes place when:
(a) lime stone is heated.
(b) a magnesium ribbon is burnt in air.
Write the balanced chemical reaction for the following and identify the type of reaction in each case.
(A) $$Magnesium_{(a)} + Iodine_{(g)} \rightarrow Magnesium\ iodide_{(s)}$$
(B) $$Zinc_{(s)} + Hydrochloric\ acid_{(aq)} \rightarrow Zinc\ chloride_{(aq)} + Hydrogen_{(g)}$$
Divide the following redox reactions into oxidation and reduction half reactions.
$$Zn + Cu^{2+} \rightarrow Zn^{2+} + Cu$$
What is observed when a solution of sodium sulphate is added to a solution of barium chloride taken in a test tube? Write equation for the chemical reaction involved and name the type of reaction in this case.
State the law which is followed in balancing a chemical equation.
Taking into consideration the relationship in the first pair, complete the second pair.
$$2H_2+O_2\rightarrow 2H_2O :$$ Combination Reaction $$:$$ $$:$$ $$CaCO_3\rightarrow CaO+CO_2$$ $$:$$ ___________.
Give an example of a double displacement reaction.
Write a balance chemical equation of the following:
$$FeSO_4(s) \xrightarrow{heat} Fe_2O_3(s) + SO_2(g) + SO_3(g)$$
Write balanced chemical equations for the following word equation:
Zinc + Steam $$\rightarrow $$ Zinc oxide + Hydrogen
State an important use of decomposition reaction.
Potassium metal reacts with water to give potassium hydroxide and hydrogen gas. Write balanced chemical equation for this reaction.
Complete the following reactions and give name of the products.
$$CuSO_{4}(aq)+Pb(s)\longrightarrow .......+.........$$
When silver nitrate solution is added to a solution of $$A$$, a white precipitate, insolube in nitric acid is formed.
State any one observation for each of the following :
(i) Dilute Hydrochloric acid is added to Silver nitrate solution.
(ii) Concentrated Nitric acid id added to Copper turnings.
(iii) Mixture of Ammonium Chloride and Sodium Hydroxide is heated.
(iv) Ammonium hydroxide solution is added in excess to copper sulphate solution.
(v) NaOH solution is added to calcium nitrate solition.
Explain Balanced equation with examples.
(a) A white powder is added while baking breads and cakes to make them soft and fluffy. Write the name of the powder. Name the main ingredients. Explain the function of each ingredient. (b) Write the chemical reaction taking place when the powder is heated during baking.
Fill in the blanks:
In some chemical reactions an insoluble ................... is formed when two solutions are mixed.
Write balanced chemical equations for the following word equation:
Potassium chlorate $$\rightarrow $$ Potassium chloride + Oxygen
When ammonium chloride is mixed with barium hydroxide in a beaker, it becomes cold.
Write balanced chemical equations for the following word equation:
Copper +Oxygen $$\rightarrow $$ Copper (II) oxide
How to balance a reaction?
Give an example of a double displacement reaction other than the reaction of barium chloride with sodium sulphate.
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
We can store copper sulphate solution in a silver vessel but not silver nitrate solution in a copper vessel. Give reason.
What is the products formed when zinc reacts with dilute nitric acid?
What does the symbol ($$aq$$) represent in a chemical equation?
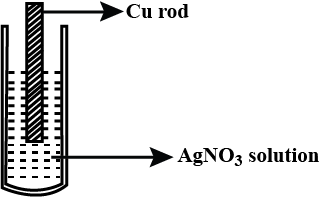
Observe the diagram showing a copper rod kept immersed in silver nitrate solution.
a. What is the colour change of the solution?
b. Write the balanced chemical equation for the reaction.
Write symbolic representation for the following word equations and balance them:
Calcium oxide + Water $$\rightarrow$$ Calcium hydroxide
Which of the following reactions will not occurs? Why not?
(a) $$ MgSO_4 (aq) + Cu (s) \rightarrow CuSO_4(aq) +Mg (s) $$
(b) $$ CuSO_4(aq) +Fe(s) \rightarrow FeSO_4(aq) +Cu(s) $$
(c) $$ MgSO_4(aq) +Fe(s) \rightarrow FeSO_4(aq) +Mg(s) $$
Correct and balance the following equation:
$$Ca+H_2O\rightarrow CaOH+H$$
$$Ca+H_2O\rightarrow CaOH+H$$
Aluminium hydroxide reacts with sulphuric acid to form aluminium sulphate and water. Write a balanced equation for this reaction.
a) Why are decomposition reactions called the opposite of combination reactions? Explain with equations of these reactions.
b) Express the following facts in the form of a balanced chemical equation:
"When a strip of copper metal is placed in a solution of silver nitrate, metallic silver is precipitated and a solution containing copper nitrate is formed".
"When a strip of copper metal is placed in a solution of silver nitrate, metallic silver is precipitated and a solution containing copper nitrate is formed".
Give one example of a chemical reaction.
Fill in the blank with suitable words:
Anti-oxidants are often added to fat-containing foods to prevent .......... due to oxidation.
Which of the following is a combination and which is a displacement reaction?
a) $$Cl_2+2KI \rightarrow 2KCl+I_2$$
b) $$2K+Cl_2 \rightarrow 2KCl$$
Correct and balance the following equation:$$N+H_2 \rightarrow NH_3$$
Balance the following chemical reaction:
$$MnO_2+HCl \rightarrow MnCl_2+Cl_2+H_2O$$
$$MnO_2+HCl \rightarrow MnCl_2+Cl_2+H_2O$$
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(i) Name the iron salt and name the type of reaction that takes place during the heating of iron salt.
(ii) Write a chemical equation for the reaction involved.
Define rancidity.
Why does food spoil when kept for a very long time?
Why is photosynthesis considered an endothermic reaction?
What is meant by rancidity and how it can be prevented?
Name the type of chemical reaction represented by the following equation:
(i)$$CaO+H_{2}O \longrightarrow Ca(OH)_{2}$$
(ii)$$3BaCl_{2}+Al_{2}(SO_{4})_{3} \longrightarrow 2AlCl_{3}+3BaSO_{4}$$
(iii)$$2FeSO_{4} \xrightarrow[]{Heat} Fe_{2}O_{3}+SO_{2}+SO_{3}$$
"We need to balance skeletal chemical equation." Give reason to justify this statement.
Identify the oxidising agent (oxidant) in the following reactions
$$\text { (a) } P b_{3} O_{4}+8 H C l \rightarrow 3 P b C l_{2}+C l_{2}+4 H_{2} O$$
Write the balanced chemical equations for the following reaction and identify the type of reaction.
Thermit reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
Identify the reducing agent in the following reaction-
$$ F e_{2} O_{3}+3 C O \rightarrow 2 F e+3 C O_{2}$$
Identify the oxidising agent (oxidant) in the following reactions
$$\text { (e) } 3 F e+4 H_{2} O \rightarrow F e_{3} O_{4}+4 H_{2}$$
Identify the oxidising agent (oxidant) in the following reactions
$$\text { (c) } C u S O_{4}+Z n \rightarrow C u+Z n S O_{4}$$
Identify the oxidising agent (oxidant) in the following reactions
$$\text { (b) } 2 M g+O_{2} \rightarrow 2 M g O$$
Identify the reducing agent in the following reactions
$$2 H_{2}+O_{2} \rightarrow 2 H_{2} O$$
Write a balanced chemical equation for each of the following reactions and also classify them.
(b) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
Give the characteristic tests for the following gas.
$$CO_{2}$$
Give the characteristic tests for the following gas.
$$O_{2}$$
Give the characteristic tests for the following gas.
$$SO_{2}$$
On heating blue coloured powder of copper (II) nitrate in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed.
Identify the type of reaction.
Give the characteristic tests for the following gas.
$$H_{2}$$
You are provided with two containers made up of copper and aluminium. You are also provided with solutions of dilute HCl, dilute $$H N O_{3}, Z n C l_{2}$$ and $$H_{2} O .$$ In which of the above containers these solutions can be kept?
On heating blue coloured powder of copper (II) nitrate in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed.
Write a balanced chemical equation of the reaction.
A magnesium ribbon is burnt in oxygen to give a white compound X accompanied by emission of light. If the burning ribbon is now placed in an atmosphere of nitrogen, it continues to burn and forms a compound Y.
(a) Write the chemical formulae of X and Y.
(b) Write a balanced chemical equation, when X is dissolved in water.
Identify the oxidising agent (oxidant) in the following reactions
$$\text { (f) } C u O+H_{2} \rightarrow C u+H_{2} O$$
Write symbolic representation for the following word equations and balance them:
Aluminium + Chlorine $$\rightarrow$$ Aluminium chloride
On heating calcium carbonate gets converted into calcium oxide and carbon dioxide. Can you prepare one acidic and one basic solution by using the products formed in the above process? If so, write the chemical equation involved.
A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
Give the following a suitable word.
The smallest unit of matter which takes part in a chemical reaction.
What are the chemical reactions?
What information do you get from the equation $$H_2 + Cl_2 \rightarrow 2HCl$$ ?
Write the balanced chemical equation for the following word equation:
Potassium permanganate + Hydrochloric acid $$\rightarrow$$ Potassium chloride + Manganese chloride + Chlorine + Water
Write the balanced chemical equations of the following word equation:
Aluminium + sodium hydroxide + water $$\rightarrow$$ sodium meta aluminate + hydrogen
Write the balanced chemical equations of the following word equation:
Potassium dichromate + Hydrochloric acid $$\rightarrow$$ Potassium chloride + Chromium chloride + Water + Chlorine
Write the balanced chemical equations of the following word equation:
Barium chloride + Sulphuric acid $$\rightarrow$$ Barium Sulphate + Hydrochloric acid
Balance the following equations
$$P + HNO_3 \rightarrow NO_2 + H_2O + H_3PO_4$$
Divide the following redox reactions into oxidation and reduction half reactions.
$$Cl_{2} + 2Br^{-} \rightarrow Br_{2} + 2Cl^{-}$$
Divide the following redox reactions into oxidation and reduction half reactions.
$$2Cu^{+} \rightarrow Cu + Cu^{2+}$$
Write the balanced chemical equations of the following word equation:
Aluminium sulphate + sodium hydroxide $$\rightarrow$$ sodium sulphate + sodium meta aluminate + water
Write the balanced chemical equations of the following word equation:
Aluminium carbide + water $$\rightarrow$$ aluminium hydroxide + methane
Addition of oxygen is known as __________.
Write the balanced chemical equation for the following word equation:
Sulphur + Nitric acid $$\rightarrow$$ Sulphuric acid + Nitrogen dioxide + Water
What kind of chemical reaction is synthesis? Support your answer by an example.
Divide the following redox reaction into oxidation and reduction half reactions.
$$Zn+Cu^{2+}\rightarrow Zn^{2+}+Cu$$
Write the balanced chemical equations of the following word equation:
Sodium chloride + manganese dioxide + sulphuric acid $$\rightarrow$$ sodium hydrogen sulphate + manganese sulphate + water + chlorine
Write balanced equation and give your observations the following metals react:
Sodium with cold water
Write an equation to represent a chemical change.
Give one example of decomposition reaction in which a solid and a gas are the two products obtained.
Define corrosion, rusting and rancidity.
What is rancidity?
Name the type of reaction in which two or more than two reactants form a single compound.
Define a chemical equation.
Define chemical reaction and write its characteristics .
Name the type of reaction for the following:
Vegetable matter changing into compost.
Name the chemical reaction responsible for rancidification of food.
Write the steps of writing a chemical equation. What are the characteristics of a chemical equation?
Suggest some measures to avoid rancidity of oils.
Name the type of reaction for the following:
Burning of natural gas.
State any two observations in an activity that may suggest that a chemical reaction has taken place. Give examples to support your answer.
Identify the substances that are oxidized and the substances that are reduced in the following reactions.
$$ 4Na (s) + O_2 (g) \rightarrow 2Na_2O (s) $$
Identify the substances that are oxidized and the substances that are reduced in the following reactions.
$$ CuO(s) + H_2(g) \rightarrow Cu(s) + H_2O (l) $$
Discuss oxidation and reduction in the chemical process given below:
$$H_2 + CuO \rightarrow Cu + H_2O$$
$$H_2 + CuO \rightarrow Cu + H_2O$$
Some incomplete equations are given below:
$$MgSO_4 +BaCl_2 \to ..... +MgCl_2$$
Complete the equation.
Mention the formula, name and physical state of the products of following reactions :
$$AgNO_{3} (aq) + NaCl (aq) \rightarrow AgCl (s) + NaNO_{3} (aq)$$
Write a balanced chemical equation with state symbols for the following reactions.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution(in water) reacts with hydrochloric acid solution(in water) to produce sodium chloride solution and water.
Mention the formula, name and physical state of the products of following reactions :
$$Na_{2}SO_{4} (aq) + Ba(OH)_{2} (aq) \rightarrow$$
Mention the formula, name and physical state of the product of following reactions :
$$CO_{2} (g) + H_{2}O (l) \rightarrow H_{2}CO_{3} (aq)$$
What is meant by reduction?
Name the substance oxidised and reduced and also identify oxidising agents and reducing agents in the
following reactions :
$$1)Fe_{2}O_{3} + 3CO \rightarrow 2Fe + 3CO_{2} $$
$$2)MnO_{2} + 4Al \rightarrow 3Mn + 2Al_{2}O_{3} $$
$$3)H_{2}S + SO_{2} \rightarrow S+ H_{2}O $$
Explain different types of decomposition reactions.
A compound 'A' is used in the manufacture of cement. When dissolved in water, it evolves a large amount of heat and forms compound 'B'.
i) Identify A and B.
ii) Write chemical equation for the reaction of A with water.
iii) List two types of reaction in which this reaction may be classified.
What is meant by skeletal type chemical equation? How can it be represented? Using the equation for electrolytic decomposition of water, differentiate between a skeletal chemical equation and a balanced chemical equation.
Divide the following redox reactions into oxidation and reduction half reactions.
$$Zn + Pb^{2+} \longrightarrow Zn^{2+} + Pb$$
How is sodium hydroxide manufactured in industries ? Name the process. In this process a gas X is formed as byproduct. This gas reacts with lime water to give a compound Y, which is used as a bleaching agent in the chemical industry. Identify X and Y and write the chemical equation of the reactions involved.
Rearrange the table with correct pairing.
Reactants Products Types of Reactions $$Fe + S$$ $$NaCl + H_2O$$ Oxidation $$CuSO_4 + Zn$$ $$2CuO$$ Neutralization $$2Cu + O_2$$ $$ZnSO_4 + Cu$$ Displacement $$HCl + NaOH$$ $$FeS$$ Combination
| Reactants | Products | Types of Reactions |
| $$Fe + S$$ | $$NaCl + H_2O$$ | Oxidation |
| $$CuSO_4 + Zn$$ | $$2CuO$$ | Neutralization |
| $$2Cu + O_2$$ | $$ZnSO_4 + Cu$$ | Displacement |
| $$HCl + NaOH$$ | $$FeS$$ | Combination |
Give reason:
White-colored $$AgCl$$ turns grey when kept in sunlight.
Write the balanced chemical equation for the following and identify the type of reaction in each case:
(a) Potassium bromide(aq) + Barium iodide(aq) $$\rightarrow$$ Potassium iodide(aq) + Barium bromide(s)
(b) Zinc carbonate(s) $$\rightarrow$$ Zinc oxide(s) + Carbon dioxide(g)
(c) Hydrogen(g) + Chlorine(g) $$\rightarrow$$ Hydrogen chloride(g)
(d) Magnesium(s) + Hydrochloric acid(aq) $$\rightarrow$$ Magnesium chloride(aq) + Hydrogen(g)
(a) Potassium bromide(aq) + Barium iodide(aq) $$\rightarrow$$ Potassium iodide(aq) + Barium bromide(s)
(b) Zinc carbonate(s) $$\rightarrow$$ Zinc oxide(s) + Carbon dioxide(g)
(c) Hydrogen(g) + Chlorine(g) $$\rightarrow$$ Hydrogen chloride(g)
(d) Magnesium(s) + Hydrochloric acid(aq) $$\rightarrow$$ Magnesium chloride(aq) + Hydrogen(g)
Translate the following statements into chemical equations and then balance them:
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Write the balanced chemical equations for the following reactions.
(a) Calcium hydroxide + Carbon dioxide $$\rightarrow$$ Calcium carbonate + Water
(b) Zinc + Silver nitrate $$\rightarrow$$ Zinc nitrate + Silver
(c) Aluminium + Copper chloride $$\rightarrow$$ Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate $$\rightarrow$$ Barium sulphate + Potassium chloride
(b) Zinc + Silver nitrate $$\rightarrow$$ Zinc nitrate + Silver
(c) Aluminium + Copper chloride $$\rightarrow$$ Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate $$\rightarrow$$ Barium sulphate + Potassium chloride
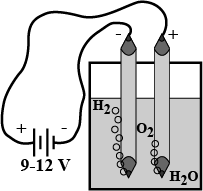
What are the materials required for the experiment to show the chemical decomposition of water? Write the procedure of the experiment. Name the products which we get in this reaction.
What happens when:
(a) dilute sulphuric acid is poured on a copper plate?
(b) iron nails are placed in copper sulphate solution?
Write word equations of the reactions involved.
Four metals $$A$$, $$B$$, $$C$$ and $$D$$ are in turn added to the following solutions one by one. The observations made are tabulated below.
| Metal | Iron (II) sulphate | Copper (II) sulphate | Zinc sulphate | Silver nitrate |
| A | No reaction | Displacement | - | - |
| B | Displacement | - | No reaction | - |
| C | No reaction | No reaction | No reaction | Displacement |
| D | No reaction | No reaction | No reaction | No reaction |
(i) Which is the most reactive metal? Why?
(ii) What would be observed, if '$$B$$' is added to a solution of Copper (II) sulphate and why?
(iii) Arrange the metals $$A$$, $$B$$, $$C$$ and $$D$$ in order of increasing reactivity.
(iv) Which one among $$A$$, $$B$$, $$C$$ and $$D$$ metals can be used to make containers that can be used to store any of the above solutions safely?
Do you expect different products in solution when aluminium (III) chloride and potassium chloride treated separately with (i) normal water (ii) acidified water, and (iii) alkaline water? Write equations wherever necessary.
Write the word equations and the balanced equations for the reaction taking place when:
Dilute sulphuric acid reacts with zinc granules.
Ammonium chloride, a sublimable solid undergoes _____ (synthesis/dissociation) on heating.
Complete and balance the following reaction:
$$CaCN_2 +H_2O (steam) \to CaCO_3 +.....$$
Give one example each of a chemical reaction characterised by
Evolution of a gas.
Change in colour.
Formation of a precipitate.
Change in temperature.
Change in state.
Evolution of a gas.
Change in colour.
Formation of a precipitate.
Change in temperature.
Change in state.
Give two examples of a reaction which is both endothermic and decomposition in nature.
Give an example of a reaction where heat is involved.
Answer the following questions:
a) Explain the term "rancidity". What damage is caused by rancidity?
b) What type of chemical reaction is responsible for causing rancidity?
c) State and explain the various methods for preventing or retarding the rancidity of food.
Match the following
| Column I | Column II | ||
| a) | $$Zn_{(s)} + H_2SO_{4(aq)} \to ZnSO_{4(aq)} + H_{2(g)}$$ | i) | Photochemical decomposition |
| b) | $$2AgCl(s) \xrightarrow{sunlight} 2Ag(s) + Cl_2(g)$$ | ii) | Thermal decomposition |
| c) | $$2KCl \xrightarrow{electricity} 2K + Cl_2$$ | iii) | Displacement reaction |
| d) | $$2Hg_{(s)} \xrightarrow{\Delta} 2Hg_{(s)} + O_2$$ | iv) | Electrolytic decomposition |