MCQ Questions for Class 12 Medical Chemistry Quiz with Answers Chapter Wise PDF Download
CBSE Multiple Choice Type Questions for 12th Class Medical Chemistry PDF formatted study resources are available for free download. These Grade 12 Medical Chemistry CBSE MCQ Mock Test helps you learn & practice the concepts in a fun learning way.
Class 12 Medical Chemistry MCQs Multiple Choice Questions with Answers
Here are the chapterwise CBSE MCQ Quiz Test Questions for Class 12th Medical Chemistry in pdf format that helps you access & download so that you can practice online/offline easily.
- Alcohols, Phenols And Ethers Class 12 Medical Chemistry MCQ Questions
- Aldehydes, Ketones And Carboxylic Acids Class 12 Medical Chemistry MCQ Questions
- Biomolecules Class 12 Medical Chemistry MCQ Questions
- Chemical Kinetics Class 12 Medical Chemistry MCQ Questions
- Chemistry In Everyday Life Class 12 Medical Chemistry MCQ Questions
- Coordination Compounds Class 12 Medical Chemistry MCQ Questions
- Electrochemistry Class 12 Medical Chemistry MCQ Questions
- General Principles And Processes Of Isolation Of Elements Class 12 Medical Chemistry MCQ Questions
- Haloalkanes And Haloarenes Class 12 Medical Chemistry MCQ Questions
- Organic Compounds Containing Nitrogen Class 12 Medical Chemistry MCQ Questions
- Polymers Class 12 Medical Chemistry MCQ Questions
- Solutions Class 12 Medical Chemistry MCQ Questions
- Surface Chemistry Class 12 Medical Chemistry MCQ Questions
- The D-And F-Block Elements Class 12 Medical Chemistry MCQ Questions
- The P-Block Elements Class 12 Medical Chemistry MCQ Questions
Alcohols, Phenols And Ethers Class 12 Medical Chemistry MCQ Quiz



Alcohols, Phenols And Ethers Questions and Answers
| Alcohols, Phenols And Ethers Quiz Question | Answer |
|---|---|
The IUPAC name of 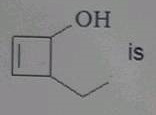 |
|
Identify: |
|
Which of the following is the best set of reagents to perform the above conversion? 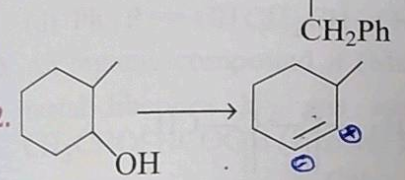 |
ThO2,ΔThO2,Δ |
Major product of the following reaction is 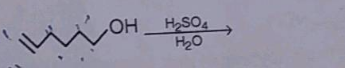 |
|
| The order of melting point of ortho, para, meta-nitrophenol is | p>m>op>m>o |
The IUPAC name of 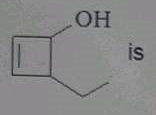 |
|
| Propene is subjected to acidic hydration and hydroboration oxidation in two different experiments. The products obtained respectively are | isopropyl alcohol, n-propyl alcohol |
What is the major product A?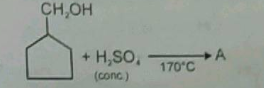 |
|
| What is the reagent used and appropriate temperature required to prepare ethene from ethyl alcohol? |
Concentrated H2SO4,170∘CH2SO4,170∘C |
| Dehydration of alcohol to alkene involves all the steps, except:- | loss of proton from carbonium ion from the ββ-position |
Aldehydes, Ketones And Carboxylic Acids Class 12 Medical Chemistry MCQ Quiz



Aldehydes, Ketones And Carboxylic Acids Questions and Answers
| Aldehydes, Ketones And Carboxylic Acids Quiz Question | Answer |
|---|---|
| Which of the following carboxylic acid is most acidic in character - | Benzoic acid |
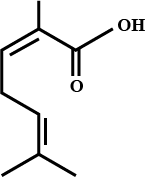 |
2,6-dimethylhepta-2,5-dienoic acid |
| Which of the following forms a stable hydrate | HCHOHCHO |
| CH3COOHLiAlH4→XCu/300oC→Ydil.NaOH→ZCH3COOHLiAlH4−−−−→XCu/300oC−−−−−−→Ydil.NaOH−−−−−−→Z in the above reaction ZZ is | Aldol |
| 1-butyne reacts with cold alkaline KMnO4KMnO4 to produce | CH3CH2COOH+CO2CH3CH2COOH+CO2 |
| Vinegar is present in acetic acid. Curd contains ____ acid. | lactic acid |
The number of carbon atoms in the principle chain of the given compound are : 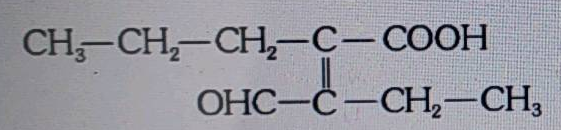 |
55 |
| Citric acid is present in lemon. Apple contains: | malic acid |
| To get DDT , chlorobenzene has to react with which of the following compounds in the presence of concentrated sulphuric acid | Trichloroacetaldehyde |
| The correct structure representation of carboxylate ion is |
Biomolecules Class 12 Medical Chemistry MCQ Quiz



Biomolecules Questions and Answers
| Biomolecules Quiz Question | Answer |
|---|---|
Glucose is carbohydrate. |
True |
| Enzymes are different from inorganic catalysts in | Being proteinaceous in nature. |
| An enzyme can be synthesised by chemically bonding together molecules of | Amino acids |
| Kmm is related to | ES complex |
Incorrect statement about given carbohydrate is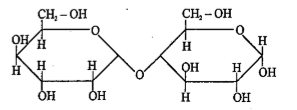 |
Above compound is a reducing sugar |
| Guanine is an example of: | a nitrogenous base |
| Raffinose on hydrolysis forms_________ |
All of these |
| Fructose is present in sperm. | True |
| State whether the following statements are True or False: Raffinose is a trisaccharide. On hydrolysis, it gives three molecules of glucose. |
False |
| State whether the following statements are True or False: Vitamin C is synthesised in skin cells in sunlight. |
False |
Chemical Kinetics Class 12 Medical Chemistry MCQ Quiz



Chemical Kinetics Questions and Answers
| Chemical Kinetics Quiz Question | Answer |
|---|---|
| For a first order reaction, A→P,t1/2(half -life)A→P,t1/2(half -life) is 1010 days. The time required for 14th14th conversion of AA(in days) is : (ln2 = 0.693, ln3 = 1.1)(ln2 = 0.693, ln3 = 1.1) | 4.14.1 |
| for a zero order reaction- | t12∝at12∝a |
| Rate of the reaction A⟶PA⟶P is 0.5M s−10.5M s−1 at time t=15 st=15 s, then rate at time t=5 st=5 s will be:(inM−s−1)(inM−s−1) | (0.5)2(0.5)2 |
A graph plotted between log t50%t50% vs log of concentration is a straight line. What conclusion can you draw from the given graph?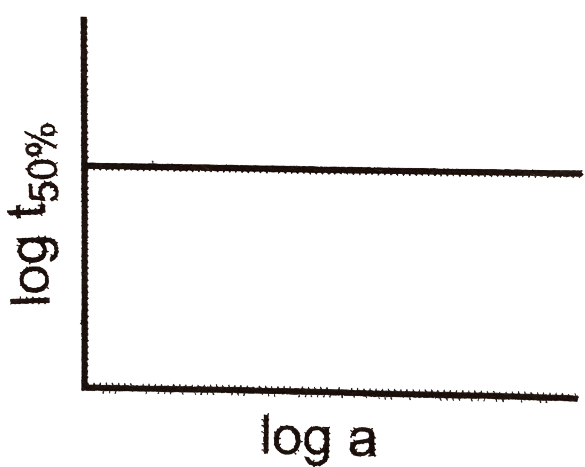 |
n=1, t1/2=0.693/kn=1, t1/2=0.693/k |
| Which one of the following formula represents a first-order reaction? | K=2.303tlog10a(a−x)K=2.303tlog10a(a−x) |
| For the reaction 4NH3+5O2→4NO+6H2O4NH3+5O2→4NO+6H2O, the rate of reaction with respect to NH3NH3 is 2×10−3Ms−12×10−3Ms−1. Then the rate of the reaction with respect to oxygen is _________ Ms−1Ms−1. | 2.5×10−32.5×10−3 |
| For the first order reaction 2N2O5(g)⟶4NO2(g)+O2(g)2N2O5(g)⟶4NO2(g)+O2(g) |
the reaction proceeds to 99.6% completion in eight half-life duration. |
| For a gaseous equilibrium : 2A(g)⇌2B(g)+C(g) Kp has a value 1.8 at 700 K. The value of Kc for the equilibrium : 2B(g+C(g)⇌2A(g) at that temperature is about _________. | 0.031 |
| In the following first order competing reaction: A+Reagent ⟶ Product B+Reagent ⟶ Product The ratio of K1/K2 if only 50% of B will have been reacted when 94% of A has been reacted is: |
4.06 |
| Which among the following reaction is an example of a zero order reaction? | 2NH3(g)Pt→N2(g)+3H2(g) |
Chemistry In Everyday Life Class 12 Medical Chemistry MCQ Quiz



Chemistry In Everyday Life Questions and Answers
| Chemistry In Everyday Life Quiz Question | Answer |
|---|---|
| Which of the following is used as an antipyretic medicine? | Paracetamol |
| Drugs that bind to the receptor sites and inhibit its natural function, are called: |
antagonist |
| The chemical substances which relieve the pain either completely or partially are called laxatives. |
False |
| On treating roll sulphur with an aqueous solution of calcium hydroxide : | milk of sulphur is prepared |
| Antacids are _____ in nature. | basic |
| Which one of the following is employed as a tranquilizer? | Equanil |
| Which of the following is not a classification of drugs? | None |
| Which of the following is an antacid? | All of the above |
| Which of the following is an antihistamine? | Brompheniramine |
| Tincture of iodine is - | solution of I2 in aqueous KI. |
Coordination Compounds Class 12 Medical Chemistry MCQ Quiz



Coordination Compounds Questions and Answers
| Coordination Compounds Quiz Question | Answer |
|---|---|
| Which compound to use for treatment of tumor? | Cis[PtCl2(NH3)2] |
| Which of the following represent E isomer? | |
| A complex of iron and cynide is 100% ionised at 1m if its elevation in boiling point is 2.08o (Kb=0.52omol/kg) then the complex is: | K3[Fe(CN)6] |
| Select the correct statement(s) about "Ziese's salt'', K[PtCl3(η2−C2H4)]. | All of these |
| The total number of possible isomers for the square planar [Pt(Cl)(NO2)(NO3)(SCN)]2− is : | 12 |
| In the following reaction the major complex product is [PtCl4]2−+2NH3→ [PtCl2(NH3)2]+2Cl− | cis |
| Number of ions present in K4[Fe(CN)6] | 5 |
| What is the pairing energy of the following completes? A=(Fe(NH3)6)3+ B=[Co(ox)3]3− C=CoF3−6 |
Pairing energy of A≠B≠C |
| Which of the following complexes is(are) paramagnetic ? |
[Cr(NH3)6]3+ |
[Co(NH3)4Cl2] possesses : |
Octahedral geometry |
Electrochemistry Class 12 Medical Chemistry MCQ Quiz



Electrochemistry Questions and Answers
| Electrochemistry Quiz Question | Answer |
|---|---|
Faraday laws hold good : |
in all concentrations |
| Which of the following statements is incorrect? |
Conductance of an electrolytic solution decreases with dilution. |
| 1 faraday = ? | 96500 C |
| Which of the following methods is employed to prevent rusting? | both (b) and (c) |
| In electrolysis | positive ions move towards the negative electrode and negative ions towards the positive electrode. |
| The U shaped vessel used in Nelson cell is lined inside with asbestos which: |
acts as an insulator. |
| In which cell the free energy of a chemical reaction is directly converted into electricity | Concentration cell |
| Which of the following is correct? | Ecell is an intensive property while △rG of cell reaction is an extensive property |
| A 250.0 ml sample of a 0.20 M Cr3+ is electrolysed with a current of 96.5 A. If the remaining [Cr3+] is 0.1 M the duration of process is ? | 75 sec |
The compounds which decompose on passage of an electric current through them are known as : |
electrolytes |
General Principles And Processes Of Isolation Of Elements Class 12 Medical Chemistry MCQ Quiz



General Principles And Processes Of Isolation Of Elements Questions and Answers
| General Principles And Processes Of Isolation Of Elements Quiz Question | Answer |
|---|---|
| During electrolytic refining of Alumina, the molten electrolyte used contains _______ . |
All of the above |
| During electrolytic reduction of alumina, ___________. |
Al is formed at cathode and oxygen is formed at anode |
| The composition of copper matte is: | Cu2S+FeS |
| During calcination: |
carbonate ores are changed into oxides |
| Metal oxide can be reduced by __________. | C |
| Copper matte contains ___________ . | Cu2S,FeS |
| Which of the following two substances react to produce blister copper? | Cu2S(s) and Cu2O(s) |
| In the metallurgy of zinc, the zinc dust obtained from roasting and reduction of zinc sulphide contains some ZnO. How is this removed? | Smelting |
| Gravity separation method is used to concentrate which type of ores? | Oxide ores |
| During the extraction of copper from pyrite slag is mainly made up of | FeSiO3 |
Haloalkanes And Haloarenes Class 12 Medical Chemistry MCQ Quiz



Haloalkanes And Haloarenes Questions and Answers
| Haloalkanes And Haloarenes Quiz Question | Answer |
|---|---|
| Compound (B) is: | |
| Which of the following show optical isomerism? | |
| Which of the following reactions will yield 2,2−dibromo−propane? | CH3C≡CH+2HBr |
| What is the minimum quantity (in grams) of methyl bromide required for preparing one mole of ethane by wurtz reaction? | 188 |
| Which of the following alkyl halides will undergo SN1 reaction most readily? | (CH3)3C−I |
The compounds are optically inactive because |
Compound I has the plane of symmetry, while compound II has the centre of symmmtry. |
| Compound (F) is: | 1,3,5− Trimethylbenzene |
| The reaction of an aromatic halogen compound with an alkyl halides in presence of sodium and ether is called | Wurtz-fittig reaction |
| Which of the following statements is correct about halogens? | they are all diatomic and form univalent ions |
The major product of the following reaction is :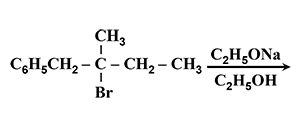 |
Organic Compounds Containing Nitrogen Class 12 Medical Chemistry MCQ Quiz



Organic Compounds Containing Nitrogen Questions and Answers
| Organic Compounds Containing Nitrogen Quiz Question | Answer |
|---|---|
| Which of the following is the most stable diazonium salt? | C6H5N+2X− |
| Which of the following amines yields foul smelling product with haloform and alcoholic KOH? | Ethylamine |
| Which of the following is a secondary amine ? | Diphenyl amine |
| Which of the following will not undergo diazotisation ? |
Benzyl amine |
Which of the N atoms is least basic in the given compound ?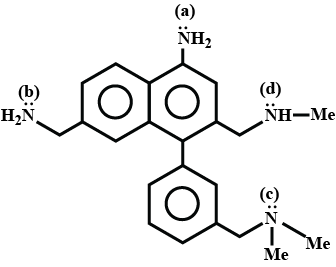 |
a |
| Which of the following is the least basic? | |
| Which is most basic? | |
| Which of the following compound is expected to be most basic | Ethylamine |
| Which of the following is obtained by reducing methyl cyanide with Na+C2H5OH? | Ethyl amine |
| What is the_decreasing order of basicity of pri., sec., tert., ethyl amines and NH3? | (C2H5)2NH>C2H5NH2>NH3>(C2H5)3N |
Polymers Class 12 Medical Chemistry MCQ Quiz



Polymers Questions and Answers
| Polymers Quiz Question | Answer |
|---|---|
| Nylon is an example of: | polyamide |
| Which of the following fibres are made of polyamides? | Nylon |
| Which of the following has extreme resistance to abrasion? | Both A and B |
| Chloroprene is the monomer of: | Neoprene |
| Bakelite is ___. | brittle plastic |
| Which one of the following is a linear polymer? | Amylose |
| The plastic product which can be remoulded is | polyethene |
| Fishing nets are made of | nylon |
| Nylon-6,6 is a: | polyamide |
| The natural rubber is the polymer of: | isoprene |
Solutions Class 12 Medical Chemistry MCQ Quiz



Solutions Questions and Answers
| Solutions Quiz Question | Answer |
|---|---|
| On a certain day, the vapour pressure is 24 mm of Hg for water vapour in air at 300 K. The saturated vapour pressure is 26.463 mm. How many moles of water vapour per liter of air would be required to saturate the air at this temperature? | 0.00013 |
| One mole of non-volatile solute is dissolved in two moles of water. The vapour pressure of the solution relative to that of water is: | 23 |
| Volume occupied by one molecule of water (density = 1 gcm−3) is: | 3.0×10−23 cm3 |
| The vapour pressure of a pure liquid solvent (X) is decreased to 0.60 atm from 0.80 atm on the addition of a non-volatile substance (Y). The mole fraction of Y in the solution is: | 0.25 |
| One mole of calcium phosphide on reacting with excess of water gives: | two moles of phosphine |
| Among the following, the substance with greater lowering of vapour pressure is | Equal in all cases |
| Relative lowering in vapour pressure of a solution containing 1 mole K2SO4 in 54 g H2O is: ( K2SO4 is 100 % ionised ) | 34 |
| The mass of CaO obtained by heating 200 kg of limestone (CaCO3) which is 95% pure is: | 106.4 kg |
| There are two oxides of sulphur. They contain 50% and 60% of oxygen respectively by weight. The weights of sulphur which combine with 1 gm of oxygen are in the ratio of: | 3 : 2 |
| 18g glucosse (C6H12O6) is added to 178.2 g water. The vapor pressure of water (in torr) for aqueous solution is |
752.4 |
Surface Chemistry Class 12 Medical Chemistry MCQ Quiz



Surface Chemistry Questions and Answers
| Surface Chemistry Quiz Question | Answer |
|---|---|
| Adsorption played an important role in: | heterogeneous catalysis |
| The catalyst used in the reduction of perisulphate to sulphate with iodide ion is | Both 1 and 2 |
| Physical adsorption | involves the weak attractive interaction between the adsorbent and adsorbate |
| In physical adsorption the gas molecules are held on solid surface by | Vander waal's forces |
| Which is wrong about a positive catalyst? |
It makes the reaction more exothermic or endothermic |
| Identify an emulsion from among the following. |
Milk |
| Which of the following cannot be used to prepare an emulsion of benzene in water? |
(CH3)4+NBr− |
| Which one of the following is an incorrect statement for physisorption? | It requires activation energy |
| If some gelatin is mixed in collodial solution of gold, then it does | Protection of gold sol |
| Colloidal solution of arsenious sulphide can be prepared by | Double decompositon |
The D-And F-Block Elements Class 12 Medical Chemistry MCQ Quiz



The D-And F-Block Elements Questions and Answers
| The D-And F-Block Elements Quiz Question | Answer |
|---|---|
| Titanium oxide is added in interior paints for walls of rooms, halls and galleries to give : | a whiter 'white' |
| The correct statement for d-block element is: | It has tendency of formation of colored ions |
| Lanthanides and actinides resemble in: | electronic configuration |
| The atomic numbers of elements of second inner transition elements lie in the range of : |
90 to 103 |
| An atom of element has 2K,8L and 3M electrons. Then the element belongs to : | IIIA group |
| Which element among the Lanthanides has the smallest atomic radius ? |
Lutetium |
| The transition metal ions responsible for color in Ruby and Emerald are, respectively : | Cr3+ and Cr3+ |
| Assertion is true, but reason is false. | |
| The correct order of spin-only magnetic moments among the following is: (Atomic number: Mn=25,Co=27,Ni=28,Zn=30). |
[MnCl4]2−>[CoCl4]2−>[NiCl4]2−>[ZnCl4]2− |
| The correct order of the spin-only magnetic moment of metal ions in the following low spin complexes, [V(CN)6]4−,[Fe(CN)6]4−, [Ru(NH3)6]3+, and [Cr(NH)3)6]2+, is: | V2+>Cr2+>Ru3+>Fe2+ |
The P-Block Elements Class 12 Medical Chemistry MCQ Quiz



The P-Block Elements Questions and Answers
| The P-Block Elements Quiz Question | Answer |
|---|---|
| What is the atomic number (Z) of the noble gas that reacts with fluorine? |
54 |
| The word Argon represents the term_______ |
Lazy |
| Halogens means: |
sea salt products |
| The element that oxidizes water to O2 with a large evolution of heat is: |
F2 |
| Zero group elements are known as____________. | inert gases |
| Ionic radius (in Ao) of As3+,Sb3+ and Bi3+ follow the order : |
Bi3+>Sb3+>As3+ |
| Which is the most thermodynamically stable allotropic form of phosphorus? | Black |
| Among these ores the highest phosphorus content is in : | phosphorite |
| Which one of these group off elements is also called the halogen family? | Group 17 |
| Which of the following does not contain fluorine? |
Chile saltpeter |
Medical Chemistry MCQ Questions for Class 12 - Practice Test with Solutions
Do you want to overcome your drawbacks while attempting the quizzes or MCQ tests like time consumption, approaching questions, etc.? Take the advantage of practicing with MCQExams.com MCQ Questions for Standard 12 Medical Chemistry Test. As it is a time-based approach and also provides answers to all questions.
One should practice the MCQs in this way for a better assessment of their preparation level. All chapters CBSE Class 12 Medical Chemistry MCQ Quiz Questions with Solutions PDF free download links are available for easy access & quick reference.
How to Use MCQExams.com Chapterwise 12th Medical Chemistry MCQ Interactive Quiz?
Guys do you love to share your practice hacks and tips with your friends? If yes, then our 12tth standard CBSE Medical Chemistry MCQ interactive quiz help you do the same. Excited to know the process then jump into the below steps right away:
- Go with the respective chapter class 12 Medical Chemistry MCQ quiz link from the above
- Now, you will find the MCQ quiz boxes for the Chemical Kinetics chapter along with the interactive quiz windows.
- Click on the CBSE 12th Class Chemical Kinetics MCQ Interactive Quiz and it will redirect you to another window where it displays the questions with options in stories format.
- Answer the question one after another and learn the answers right away this helps you to do a quick assessment of your knowledge.
- You can also share this cool MCQ Interactive Quiz Questions of Plus Two Medical Chemistry topicwise with your friends by just tapping on the send arrow located at the top left corner of the story.
- After clicking the button, you can opt for the copy link option and easily paste the link on your friend's chat or else in your whatsapp story too. Isn’t it cool!!
- Keep passing this interesting approach of practicing Plus Two CBSE Medical Chemistry Chemical Kinetics MCQ Questions to your co-students and help them in attempting the entrance exams like JEE & NEET.