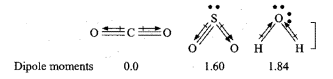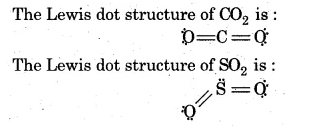Chemical Bonding And Molecular Structure - Class 11 Engineering Chemistry - Extra Questions
Predict which out of the following molecules will have higher dipole moment and why ?
$$CS_{2}$$ and $$OCS$$
Arrange the following in the increasing order of $$C-C$$ bond length $$C_2H_6, C_2H_4, C_2H_2$$.
Fill in the blanks:
For hydrogen bonding to occur, hydrogen atom must be linked to .......... atom.
Explain the hydrogen bonding in molecules of $$HF,$$ $$H_2O$$ and $$NH_3$$.
In $$ CuSO_4.5H_2O $$ all Cu-O bond length are not equal. Find the total number of shorter Cu-O bonds.
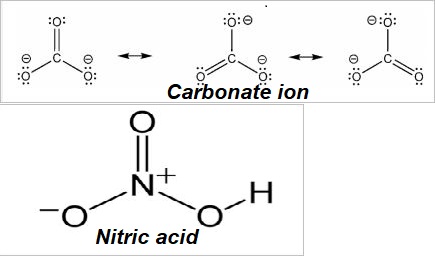
What is the formal charge on atom in carbonate and nitric acid?
On the basis of ground electronic configuration, arrange the following molecules in the order of increasing $$O-O$$ bond lengths: $$ KO_2 ,\ O_2 ,\ O_2[AsF_6] .$$
Although $$ CO_2 $$ has no dipole moment, $$ SO_2 $$ and $$ H_2O $$ have considerable dipole moments-explain.
Name two intermolecular forces that exist between $$HF$$ molecules in a liquid state.
Answer the following question:
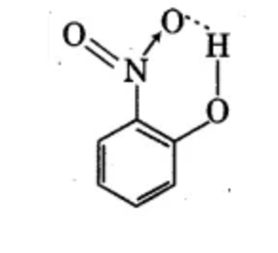
Dipole moment of phenol is smaller than that of methanol. Why?
Write a short note on Hydrogen bond.
Which of the following has maximum dipole moment; $$Na^+, K^+, Li^+$$ and $$Cs^+$$?
Dipole moment of $$CO_{2}$$ molecule is zero where as $$SO_{2}$$ has some dipole moment. Explain the reason.
Fill in the blanks:
Dipole moment of a diatomic polar molecule is the product of .............
Explain how VB theory differs from Lewis concept.
What requirement should a molecule fulfil for the formation of a hydrogen bond ?
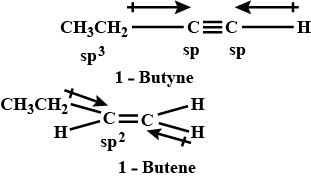
Which of the following has larger dipole moment? Explain.1-Butyne or 1-Butene
Find the number of delta bonding molecular orbital from the following set if z is the inter-nuclear axis :
$$ d_{x^2\,-\,y^2}$$ and $$d_{xz}, \,d_{x^2\,-\,y^2}$$ or $$d_{xz}$$
Assuming all the four valencies of carbon atom in propane pointing towards the corners of a regular tetrahedron; the distance (in $$\dot{A}$$) between the terminal carbon atoms in propane will be: (write the value of the nearest integer)
Given: $$C-C$$ single bond length is $$1.54$$ $$\dot{A}$$.
Write two differences between $$O^{2}$$ and $$O^{2-}$$.
Arrange Halides (MeX) in order of decreasing bond length.
MeF, MeCl, MeBr, MeI.
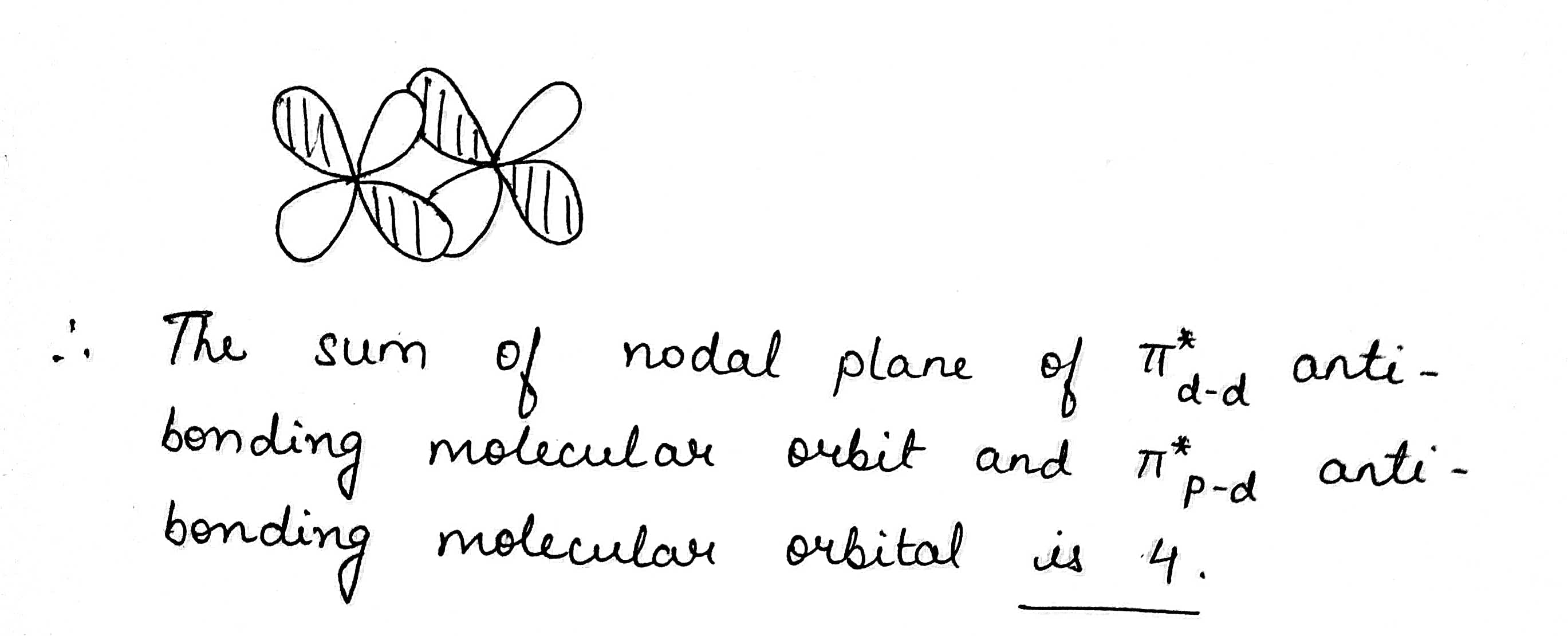
Find the sum of nodal plane of $$ \pi^* _{d-d} $$ anti-bonding molecular orbit and $$ \pi^*_{p-d} $$ anti-bonding molecular orbital.
Why the repulsions between non-bonded orbitals are greater than between the bonded orbitals?
Write a short note on 'Hydrogen bond'.
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions