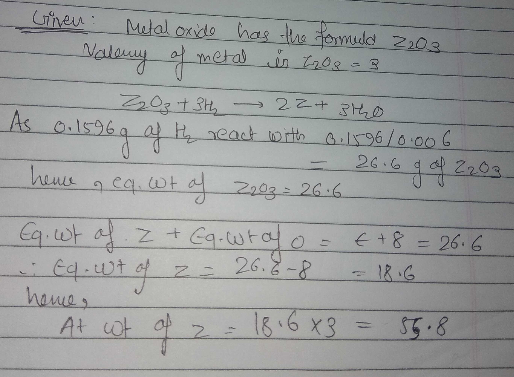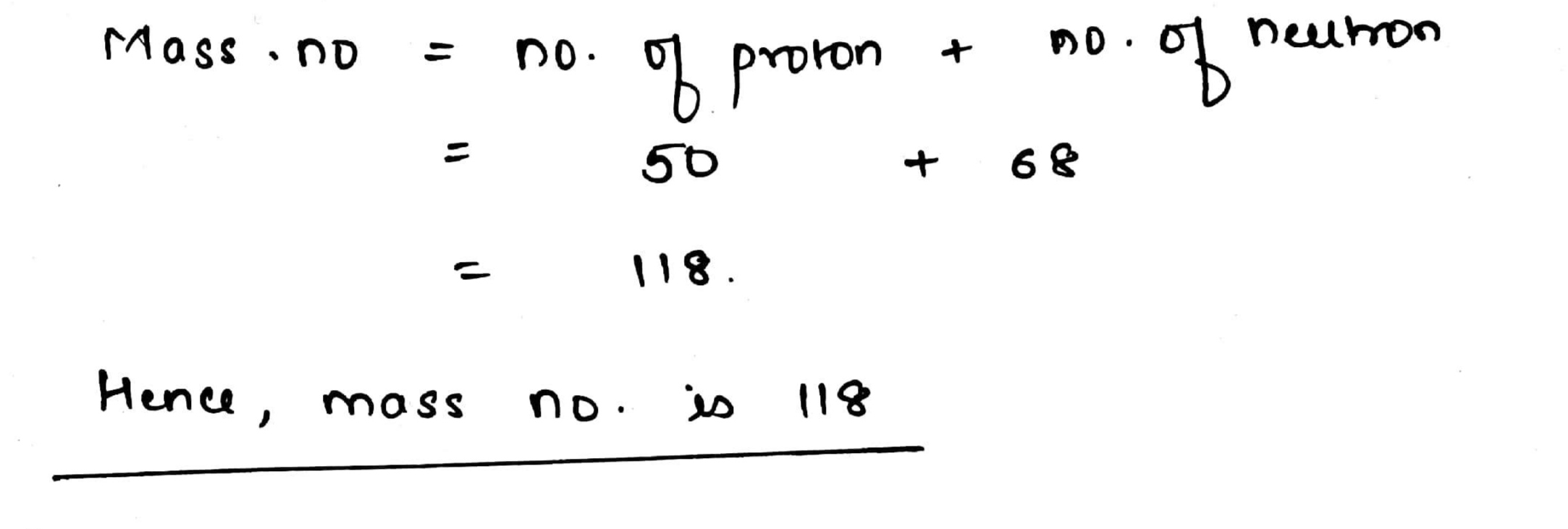Structure Of Atom - Class 11 Engineering Chemistry - Extra Questions
How many of the following are possible? 1p, 2s, 3p, 3f, 3d
Define Aufbau Principle
The atomic masses of three elements $$A$$, $$B$$ and $$C$$ having similar chemical properties are $$7$$, $$23$$ and $$39$$ respectively.
(a) Calculate the average atomic mass of elements $$A$$ and $$C$$.
(b) Compare the average atomic mass with atomic mass of $$B$$.
(c) What could the elements $$A$$, $$B$$ and $$C$$ be?
Calculate the ratio of moles of $$3.6\ g$$ of water and $$4.4\ g$$ of carbon dioxide. Atomic mass of $$C=12u,H=1\ u,O=16u$$.
In the absence of Aufbau rule, what block would K(19), Sc(21) and Ca(20) belong to:
s block
p block
d block
f block
Why $$^{12}\textrm{C}$$ is the basis of the modern atomic mass unit?
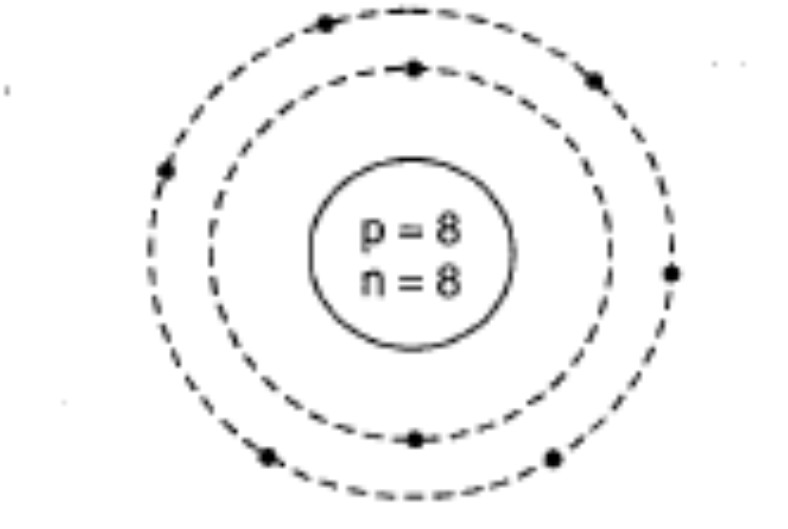
Draw the geometrical representation of the atom of the element $$^{16}_{8}O$$
Write the complete symbol for the atom with the given atomic number (Z) and atomic mass (A).
(i) Z = 17 , A = 35
(ii) Z = 92 , A = 233
(iii) Z = 4 , A = 9
Symbols $$\displaystyle _{ 35 }^{ 79 }{ Br }$$ and $$\displaystyle ^{ 79 }{ Br }$$ can be written, whereas symbols $$\displaystyle _{ 79 }^{ 35 }{ Br }$$ and $$\displaystyle ^{ 35 }{ Br }$$ are not acceptable. Answer briefly:
The mass number of an atom is the sum of the number of protons and number of neutrons in an atom. If it is true enter 1 else 0.
True or False
According to J.J. Thomson model electrons revolve around the nucleus.
Mass numbers of two isotopes of an element differ by $$2$$ units ($$A$$ and $$A+2$$). Average atomic mass is $$0.5$$ more than the lower mass number. What could be the ratio of the percentage abundance two isotopes respectively?
Calculate the atomic number of the element that has $$12$$ neutrons and a mass number of $$23$$.
Calculate energy, frequency and wavelength of the radiation which is corresponding to the spectral line of the lowest frequency in Lyman series in the hydrogen atom spectrum . Also calculate the energy for the corresponding line in the spectrum of $$Li^{2+}$$
($$R_{H} = 1.09677 cm^{-1}, c= 3x10^{8} ms^{-1}, Z=3$$)
The weight of $$350mL$$ of a diatomic gas at $${0}^{o}C$$ and $$2$$ atm pressure is $$1g$$. The weight of one atom is:
State Aufbau's principle.
Mass number is silghtly less than the actual atomic mass. Give reasons.
Find the ratio by mass of the elements present in molecules of hydrogen sulphide $$(H_{2}S)$$. Given that, $$H_2S$$ molecular wt= 34, Atomicity= 2, Atomic wt= 1
Calculate the number of aluminium ions present in 0.051 g of aluminium oxide. (Hint: The mass of an ion is the same as that of an atom of the same element. Atomic mass of Al = 27 u)
A metalo xide has the formula $$X_{2}O_{3}$$.It can be reduced by hydrogen to give free metal and water.0.1596 g of metal oxide requires 6 mg of hydrogen for complete reduction. Calculate the atomic weight of the metal(in amu).
Read the table and answer the question below:
| Elements | $$A$$ | $$B$$ | $$C$$ | $$D$$ | $$E$$ |
| MASS NO. | $$1$$ | $$7$$ | $$14$$ | $$40$$ | $$40$$ |
| Atomic No. | $$1$$ | $$3$$ | $$7$$ | $$18$$ | $$20$$ |
Mass number
Give the following a suitable word/phrase.
The sub-atomic particles with negative charge and negligible mass.
Give the following a suitable word/phrase.
The sum of the number of protons and neutrons of an atom.
What is aufbau principle ?
State and explain the Aufbau principle.
State and explain the 'Aufbau Principle'.
Chlorophyll, the green colouring matter of plants, contains 2.68% of magnesium by mass. Calculate the number of magnesium atoms in 3.00 g of chlorophyll. [Atomic mass of magnesium = 24.3 g]
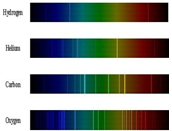
Write short notes on the Atomic spectra or line spectra.
An isotope of $${^{112}_{50}}Sn$$ contains $$68$$ neutrons. What will be its mass number ?
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions