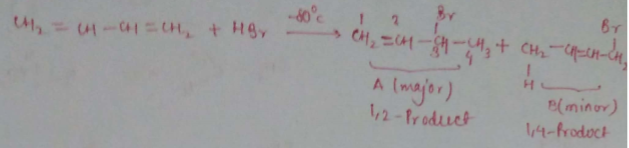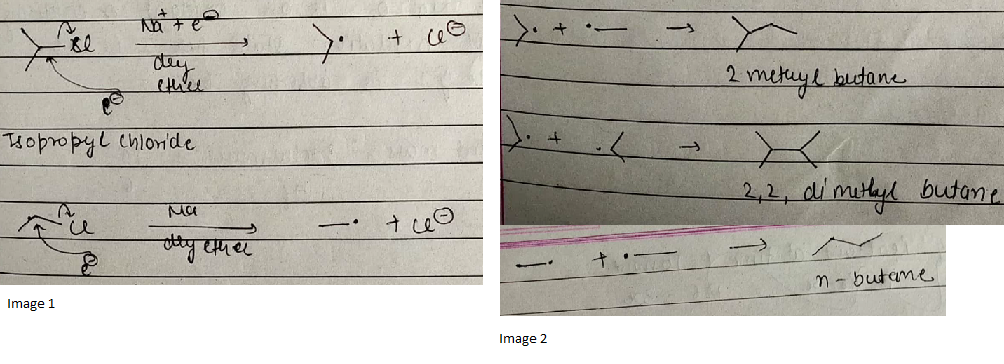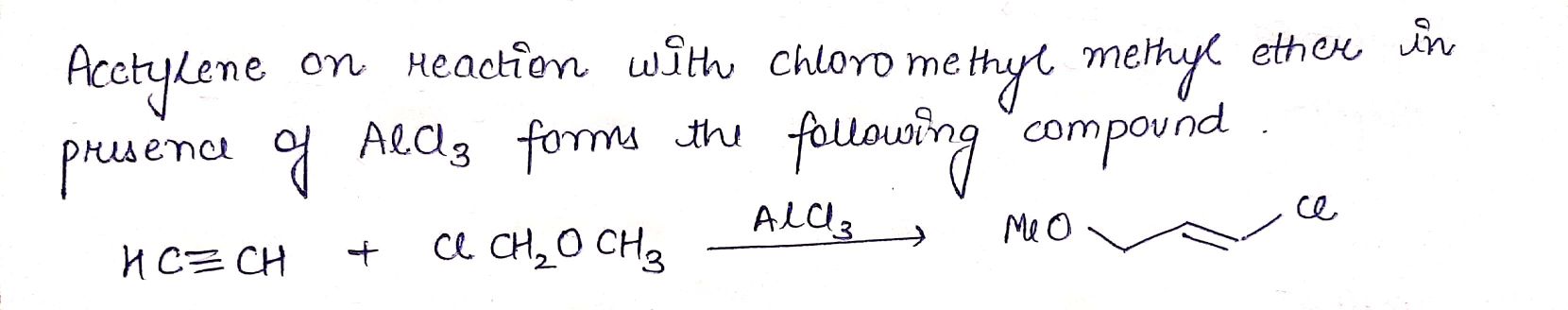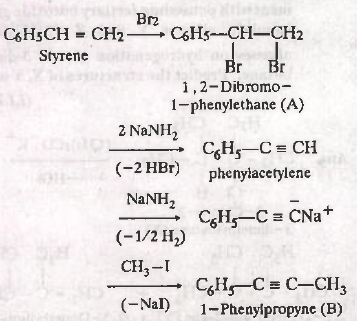Hydrocarbons - Class 11 Engineering Chemistry - Extra Questions
What effect the branching of an alkane has on it melting point?
The direct iodination of alkane fail to product an alkyl iodid. If true enter 1, else 0.
2-butyne is less reactive than butene towards electrophilic addition reaction. If true press 1 else 0.
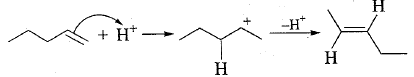
When $$1-$$pentene is treated with an acid, $$trans-2-$$pentene is produced. Propose mechanism of this isomerization reaction and justify.
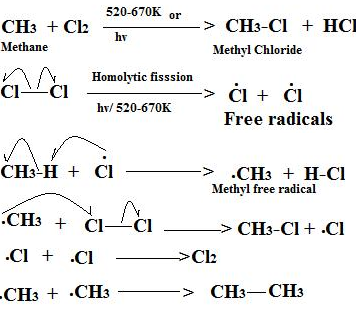
How do you account for the formation of ethane during chlorination of methane?
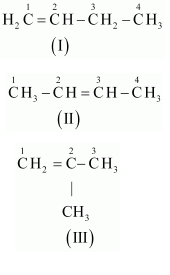
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of the double or triple bond as indicated : (a) $$C_4H_8$$(one double bond) (b)$$C_5H_8$$ (one triple bond)
(b)$$C_5H_8$$ (one triple bond)
Write $$IUPAC$$ names of the following compound:$$CH_3CH = C(CH_3)_2$$
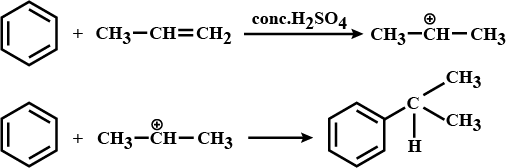
Let us consider the following reaction
$$CH_2=CH-CH=CH_2 + HBr \overset{-80^oC}{\longrightarrow} \underset{(Major)}{A} + \underset{(Minor)}{B}$$.
At which carbon (follow IUPAC nomenclature) bromine is present in the major product?
Identify 'A' and 'B' in the following reactions?
How is chloroform converted into Methane?
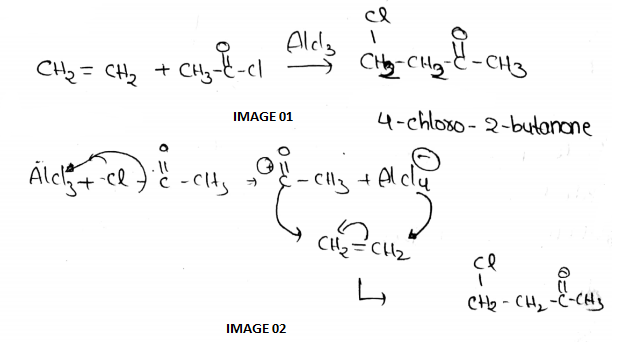
The reaction $$CH_2=CH_2+CH_3COCl \xrightarrow{AlCl_3}$$ gives the product:
Convert Propene into Propan$$-1-ol$$
Heat of Hydrogenation of $$C_6H_6$$ & Cyclohexene is K & Y. Find Resonance energy.
Write the name and formula of the product formed in each case below:
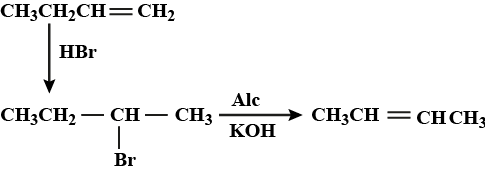
$$H_2C=CH_2+HBr \to ........$$
Bring out the following conversions:
Acetaldehyde to Ethane
Write the name and formula of the product formed in each case below:
$$C_2H_4+Cl_2 \to .................$$
Complete and balanced the following equations. State the conditions wherever necessary.
$$C_{2}H_{4}+HCl\rightarrow ............$$
Give reaction : What happens when :
Passing ethene in cold aqueous solution of alkaline potassium permanganate.
Complete and balanced the following equations. State the conditions wherever necessary.
$$C_{2}H_{2}+Br_{2}\rightarrow ...........$$
Explain with chemical equation what happens when:
Acetylene reacts with bromine water ?
Complete the reaction :
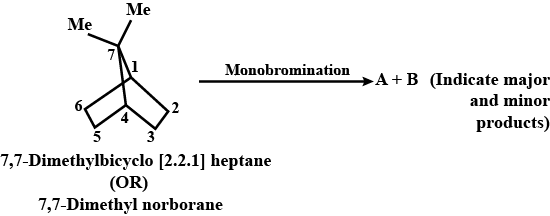
Identify the products in the following reactions:
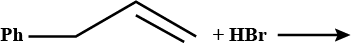
Complete the reaction:
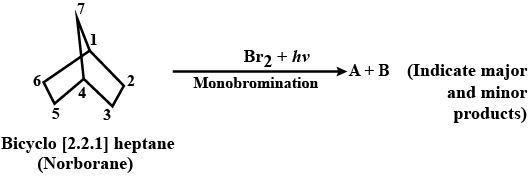
Identify the product when $$(A)$$ reacts with:
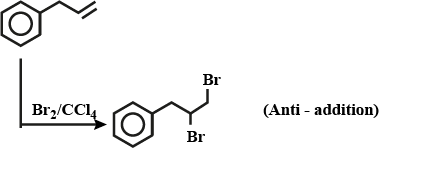
$$Br_2/ CCl_4$$
Among the isomers of pentane $$ (C_{5}H_{12}) $$, Write the one which on photochemical chlorination yields a singe monochloride.
Complete the following, giving the structures of the principal organic products:
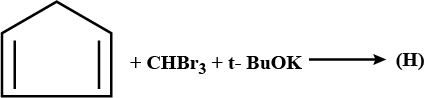
Write the name and formula of the product formed in each case below:
$$CH_2=CH_2 \xrightarrow {alk.\ KMnO_4}...........$$
Ethanol can be converted into ethene which can be changed into ethane. choose the correct word or phrase from the brackets to complete the following sentences.
The conversion of ethene into ethane is an example of _________
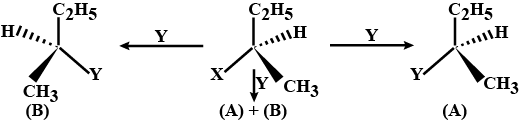
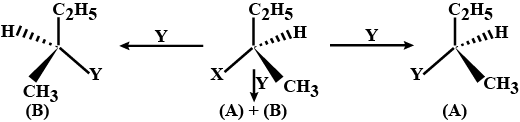
Consider the three types of replacement of group X by group Y as shown here.
This can result in giving compound (A) or (B) or both. What is the process called if
(A) is the only compound obtained
Write the chemical equations for the following conversions ( not more than $$2$$ steps):
Acetone to propene
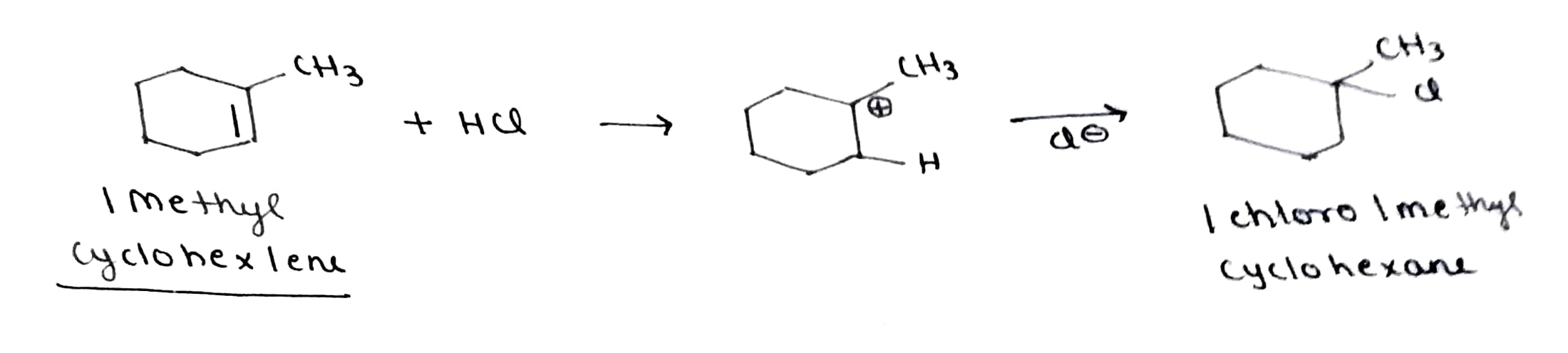
Name the alkene which will yield 1-chloro-1-methylclohexane by the reaction with HCl. Write the reactions involved.
Consider the three types of replacement of group X by group Y as shown here.
This can result in giving compound (A) or (B) or both. what is the process called if
(A) and (B) are formed in equal proportions.
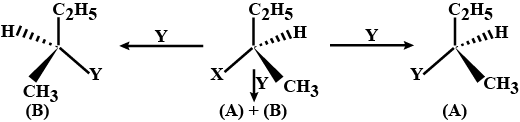
Consider the tree types of replacement of group X by group Y as shown here.
This can result in giving compound (A) or (B) or both. what is the process called if
(B) is the only compound obtained.
What do you observe when ethylene is passed through alkaline $$KMnO_4$$ solution?
Chlorination of ethane to ethy1 chloride is more practicable than the chlorination of n-pentane to 1-chloropentane.
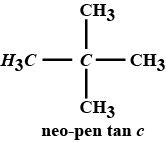
"n-pentane has lesser boiling point than neopentane"
Alkynes are more reactive than alkene towards catalytic hydrogenation. State whether the statement is True or False
"$$Mg_2C_3$$ on reaction with water forms propane."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
Free radical chlorination is a highly reactive process but very less selective, however, free radical bromination is less reactive, require a greater amount of energy very highly selective.
State whether the statement is True or False
Complete the following reaction :
HC $$\equiv$$ CH $$\overset{Hg^{2+}}{\underset{H_2SO_4}{\rightarrow}}(A) \overset{O}{\rightarrow} (B)$$.
Isopropyl chloride and ethyl chloride both react with $$Na$$ in presence of dry ether. How many products are obtained?
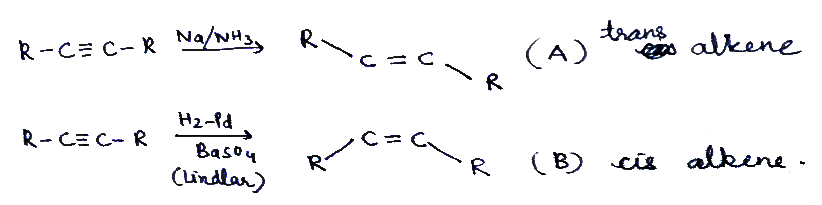
$$B\overset { Liadlar }{ \longleftarrow } R-C\equiv C-R\overset { { Na }/{ { NH }_{ 3 } } }{ \longrightarrow } A$$What is A and B in the given raction repectivley?
Explain the free radical mechanism of chlorination of methane.
What is the number of stereo isomers possbile for the given compound?
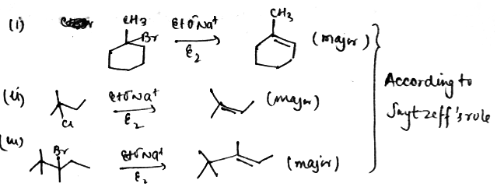
Product all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(i) $$1$$-Bromo-$$1$$-methylcyclohexane
(ii) $$2$$-Chloro-$$2$$-methylbutane
(iii) $$2, 2, 3$$-Trimethyl-$$3$$-bromopentane.
Alkanes are called paraffin's. They undergo substitution reactions with chlorine in present of sunlight. Prove this with suitable example.
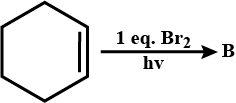
Identify $$B$$.
A and B are:
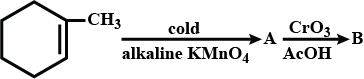
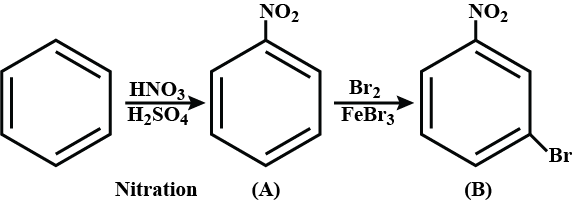
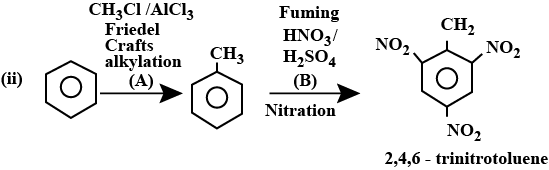
$${ C }_{ 6 }{ H }_{ 6 }\overset { { HNO }_{ 3 } }{ \underset { { H }_{ 2 }{ SO }_{ 4 } }{ \longrightarrow } } A\overset { { Br }_{ 2 } }{ \underset { { FeBr }_{ 3 } }{ \longrightarrow } } B$$, Find the final product B.
How is propyne prepared from an :
$$(i)$$ alkylene dihalide
$$(ii)$$ alkylidene dihalide and
$$(iii)$$ Tetrahaloalkane
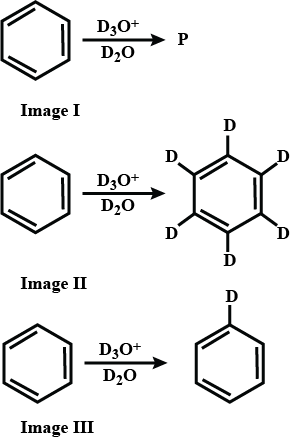
What is the producy 'P'.

Complete the reaction.
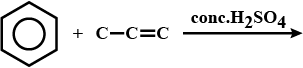
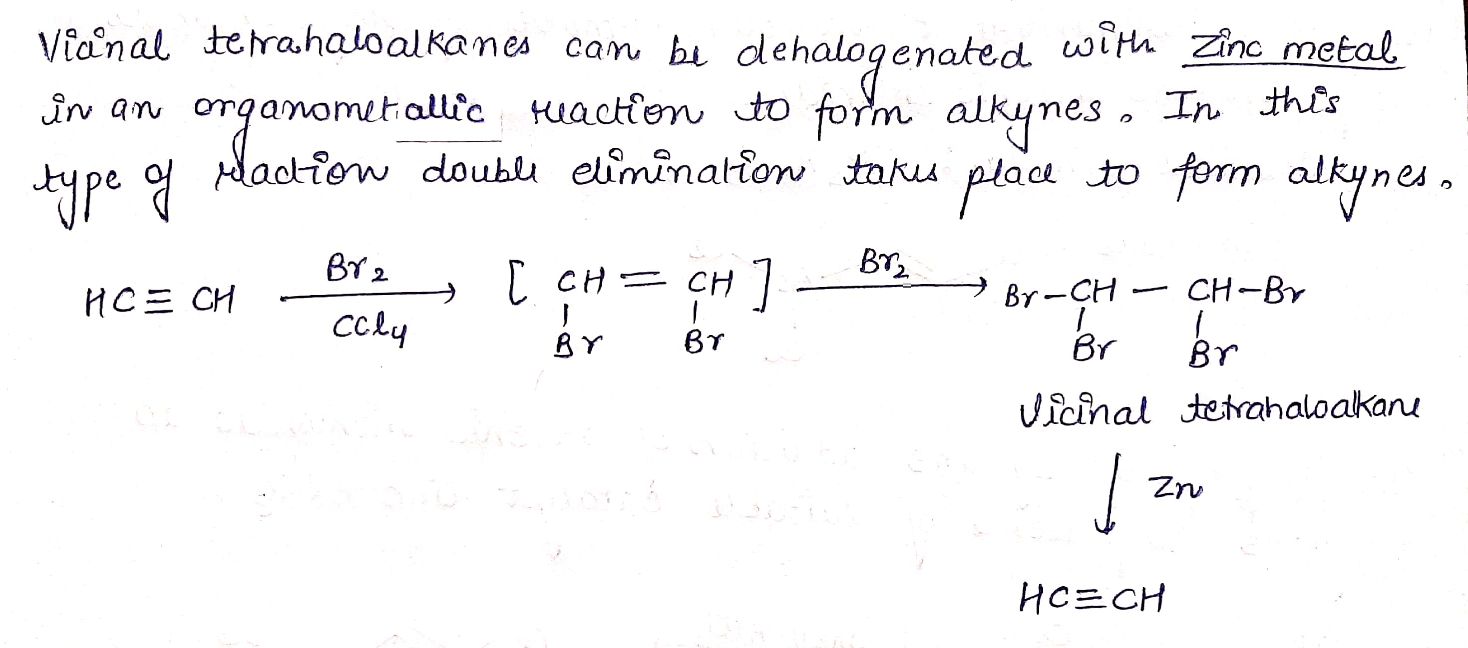
1,1-Dihalides undergo dehydrohalogenation when treated with $$NaN{H_2}$$ to give alkynes .
$$C{H_3}C{H_2}CHC{l_2} \to C{H_3}C \equiv CH$$
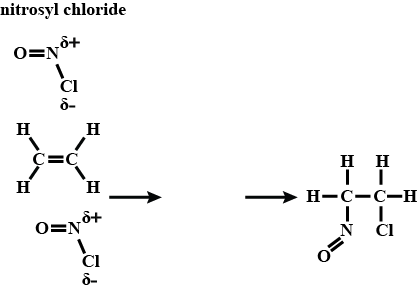
Nitrosyl chloride, $$NOCl$$, is a reactive gas that is sometimes formed when $$NO$$ reacts with $$Cl_{2}$$. $$NOCl$$ is a strong electrophile and readily undergoes an addition reaction with alkenes. Complete the diagram to show the mechanism of the electrophilic addition reaction of $$NOCl$$ with ethene.Include all necessary charges, lone pairs and curly arrows, and the structure of the organic intermediate.
Complete the following table:
| Straight chain of Carbon compound | Structural formula | Molecular formula | Name |
| $$C$$ | $$H - \underset{H}{\underset{|}{\overset{H}{\overset{|}{C}}}} - H$$ | $$CH_4$$ | Methane |
| $$C - C$$ | - | - | Ethane |
| $$C-C-C$$ | - | $$ C_3H_8$$ | - |
| $$C-C-C-C$$ | $$H- \underset{H}{\underset{|}{\overset{H}{\overset{|}{C}}}} - \underset{H}{\underset{|}{\overset{H}{\overset{|}{C}}}} - \underset{H}{\underset{|}{\overset{H}{\overset{|}{C}}}} - \underset{H}{\underset{|}{\overset{H}{\overset{|}{C}}}} - H$$ | - | - |
$$HC \equiv CH \xrightarrow {NaNH_{2}}$$ _______ $$\xrightarrow {CH_{3}Br}$$ ________.
Fill in the blanks :
$$H_{2}C = CH_{2}\underset {CCl_{4}}{\xrightarrow {Br_{2}}}$$ ______ $$\underset {KOH}{\xrightarrow {Alc.}}$$ ______.
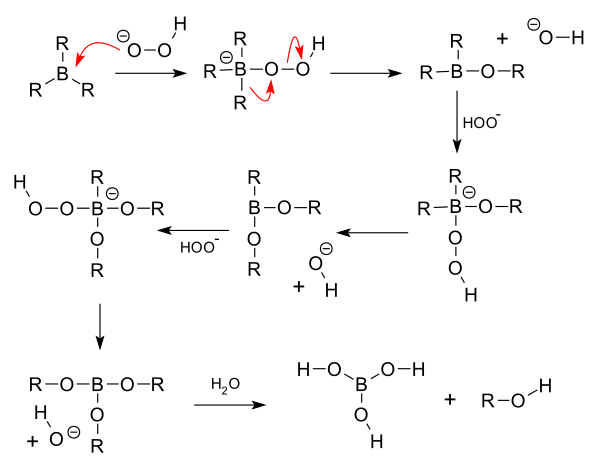
Trialkylborane on deomposition by $$CH_{3}COOH$$ forms ______.
Complete the following equations :
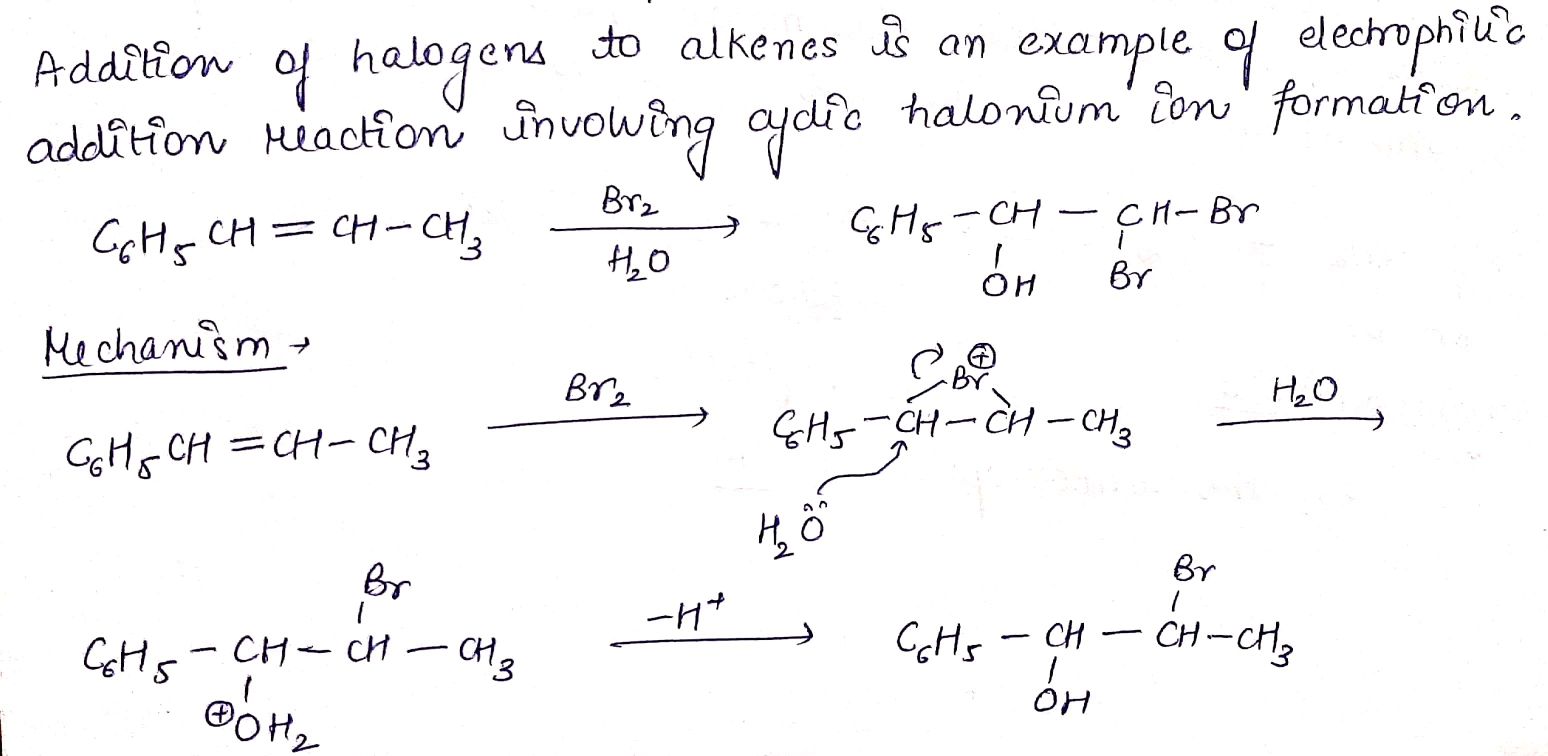
$$C_{6}H_{5}CH = CHCH_{3}\underset {H_{2}O}{\xrightarrow {Br_{2}}} (A)$$.
$$C_{6}H_{5}CH = CHCH_{3}\underset {H_{2}O}{\xrightarrow {Br_{2}}} (A)$$.
Complete the following equations:
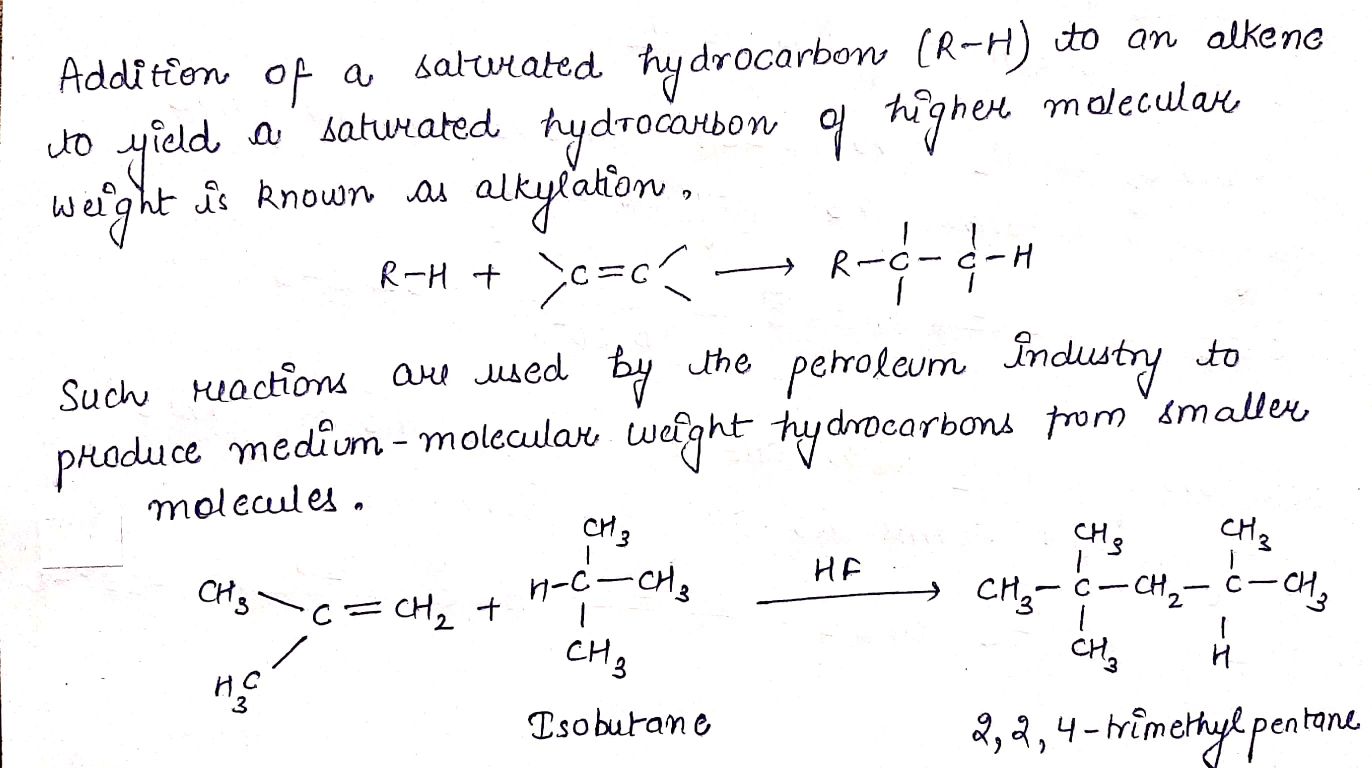
$$(CH_{3})_{2}C = CH_{2} + isobutane \underset {273\ K}{\xrightarrow {HF}} (A)$$.
What happens when :
Acetylene is passed through ammonical cuprous chloride solution?
What type of reactions usually occur in alkenes?
What happens when :
Acetylene is passed through ammoniacal solution of silver nitrate?
What happens when ethyl bromide is heated with zinc? Give equations only:
Fill in the blanks:
Electrolysis of an aqueous solution of sodium propionate will form ____
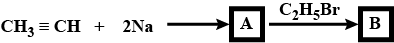
How will you synthesise?
$$But-2-yne$$ from propyne.
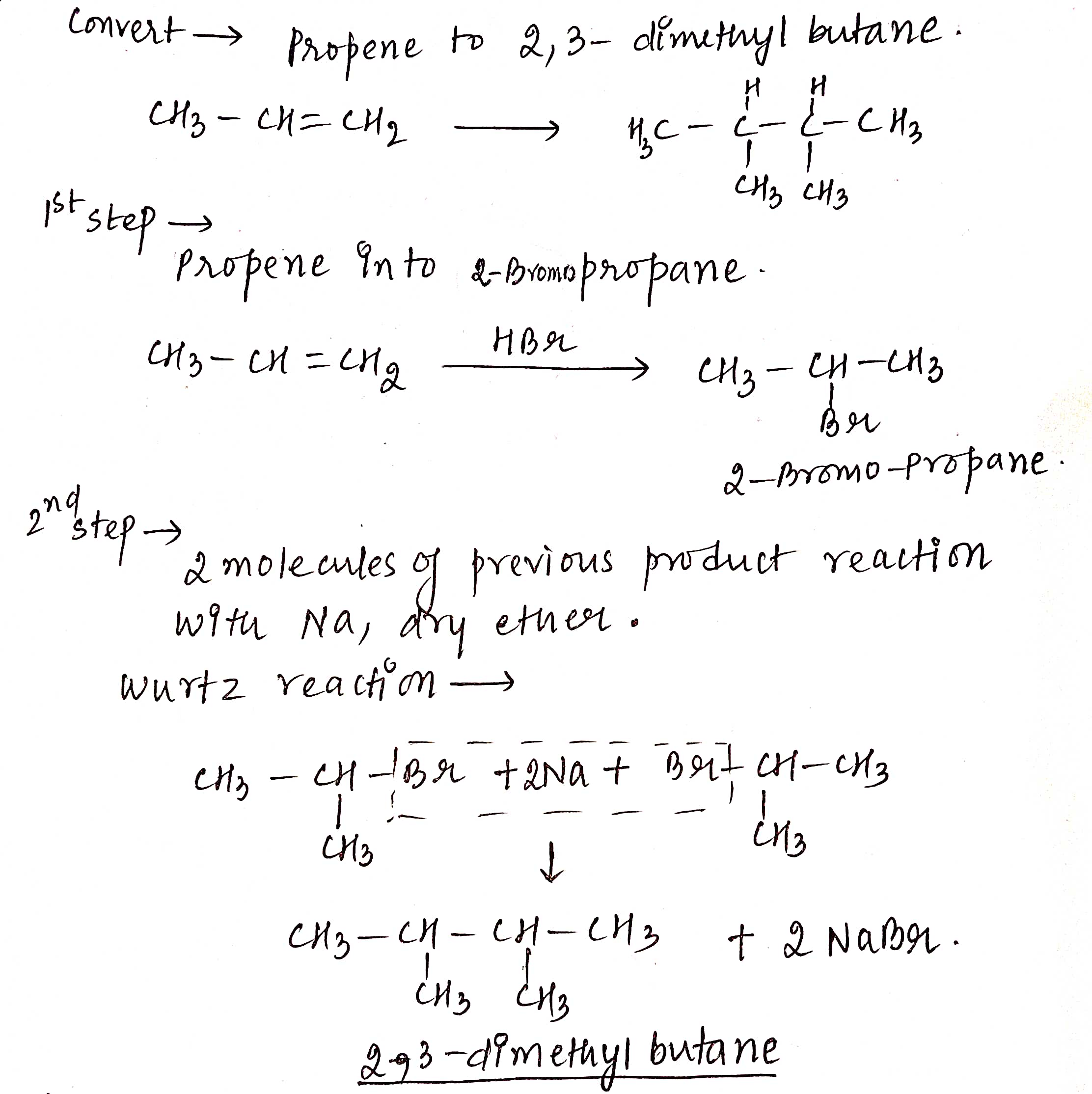
How will you synthesise $$2,3-$$Dimethylbutane from propene?
Provide the structures of compounds A, B, C, and D in the above questions.
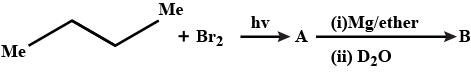
How will you bring about the following conversions in not more than two steps?
But$$-1-$$ene to but$$-2-$$ene
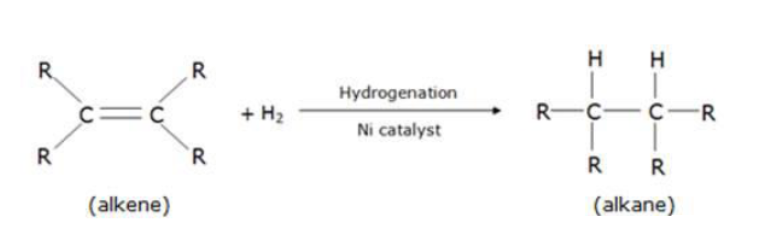
What are addition reactions? Which category of compounds undergoes addition reactions? Explain.
What is the function of conc. $$ H_{2}SO_{4} $$ in the formation of ethane from ethanol?
What are alkanes? Write general formula of alkanes and four general methods for preparation of alkanes.
Explain with chemical equation what happens when:
Hydrogen bromide is added to acetylene.
The boiling point of re-pentane is higher than neopentane. Explain the reason.
Why alkanes are chemically inert? Explain general reaction of alkanes.
Write the common name,IUPAC name and formula of isomers of butane, pentane and hexane.
Give reasons for the following:
The melting points alkanes with odd number of carbon atoms are lower than those with even number of carbon atoms
Pick out the suitable compounds from the box for the following reactions
$$CH_4,\ C_2H_4,\ C_3H_8,\ CH_3Cl$$
A) Addition Reaction
A) Addition Reaction
How can alkanes be prepared from alcohol
What effect does branching of an alkane have on its melting point?
Which of the following has the highest boiling point?
$$2-$$ methylpentane
$$2,3-$$ dimethylbutane
$$2,2-$$ dimethylbutane.
Discuss briefly the mechanism of halogenation of methane.
Arrange the following in order of increasing volatility : gasoline, kerosene and diesel.
Consider the electrophilic addition reaction of List 1 and match them with the properties of product from List 2
$$CH_3CH_2CH_2CH_2CH_2CHCl_2 +NaNH_2/NH_3 \rightarrow $$
How many $$sp$$ hybridized carbon atoms are present in the product.
For $$C_4H_8$$ (one double bond), write the structural formula and IUPAC name for all possible isomers.
Explain the mechanism of the following reaction:
$$CH_{3} - CH_{2} - OH\underset {443K}{\xrightarrow {H^{+}}} CH_{2} = CH_{2} + H_{2}^{-}O$$
(i) $$CH\equiv CH\xrightarrow {(A)} (B) \xrightarrow {(O)}CH_{3}COOH \xrightarrow {(C)}CH_{2}ClCOOH$$
Complete the given reactions:

Give reasons for the following:
Straight chain alkanes posses higher boiling points than the corresponding branched chain isomers.
Alkenes like ethene dissolve in conc. $${ H }_{ 2 }{ SO }_{ 4 }$$ but alkynes like ethyne do not dissolve in conc. $${ H }_{ 2 }{ SO }_{ 4 }$$, whereas but-2-yne dissolves. Explain.
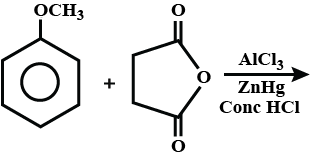
What is the product of the given reaction?
Propene $$(CH_3-CH=CH_2)$$ can be transformed to compound (a to j) listed in the left-hand column.
Write letter designating the reagent, you believe will achieve desired transformation. In the case of a multi step sequence, write the reagent in the order they are to be used.
| Desired product | No. of Steps | Write options | Reagent List | ||
| a. | $$CH_3CHBrCH_2Br$$ | one | A. | $$Hg(OAc)_2$$ in $$H_2O$$ | |
| b. | $$(CH_3)_2CHOH$$ | two | B. | $$B_2H_6$$ in THF | |
| c. | $$CH_3CH_2CH_2OH$$ | two | C. | $$NaBH_4$$ in alcohol | |
| d. | $$CH_3COCH_3$$ | three | D. | $$Br_2$$ in $$CH_2Cl_2$$ | |
| e. | $$CH_3CH_2CHO$$ | three | E. | $$H_2O_2$$ in aqueous base | |
| f. | $$CH_3CH(OH)CH_2Br$$ | one | F. | HOBr(NBS in aqueous acetone) | |
| g. | $$(CH_3)_2CHBr$$ | one | G. | HBr in $$CH_2Cl_2$$ | |
| h. | $$CH_3CH(OH)CH_2OH$$ | two | H. | $$OsO_4$$ in ether | |
| i. | $$CH_3-CH_2-CH_2-Cl$$ | three | I. | Thionyl chloride $$(SOCl_2)$$ | |
| j. | $$CH_3-C\equiv CH$$ | two | J. | $$NaHSO_3$$ in aqueous acetone | |
| K. | NaOH in alcohol and reflux | ||||
| L. | $$NaNH_2$$ (strong base) |
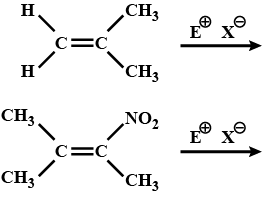
Among the given reaction which is faster & why?
(i) $$Me - \underset{Cl}{\underset{|}{C}}H - Me \xrightarrow{alc. KOH} CH_3-CH = CH_2$$
(ii) $$CH_3-CH_2-CH_2-Cl \xrightarrow{alc. KOH} CH_3-CH = CH_2$$
(i) $$Me - \underset{Cl}{\underset{|}{C}}H - Me \xrightarrow{alc. KOH} CH_3-CH = CH_2$$
(ii) $$CH_3-CH_2-CH_2-Cl \xrightarrow{alc. KOH} CH_3-CH = CH_2$$
Complete the reaction.
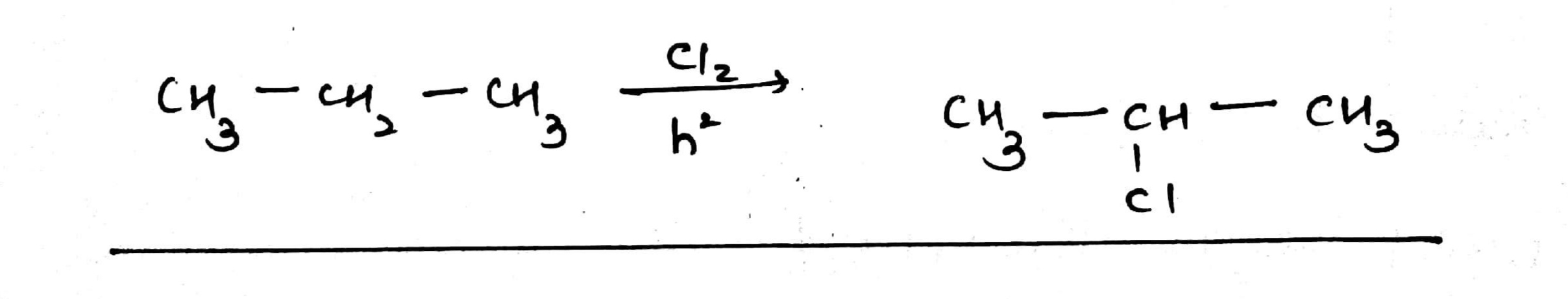
$$C{H_3} - C{H_2} - C{H_3}\,\xrightarrow[{{h \nu}}]{{c{l_2}}}$$
Fill in the blanks :
$$HC \equiv CH \underset {CCl_{4}}{\xrightarrow {Br_{2}}}$$ _______ $$\xrightarrow {Zn}$$ ______.
Fill in the blank:
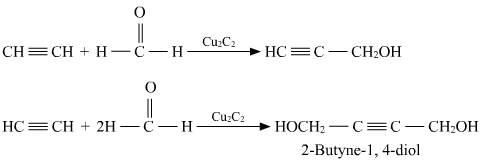
Acetylene and formaldehyde interact in the presence of copper acetylide as a catalyst to form the organic compound _____.
Fill in the blanks :
$$HC \equiv CH + CH_{3}OCH_{2} Cl\xrightarrow {AlCl_{3}}$$ _______.
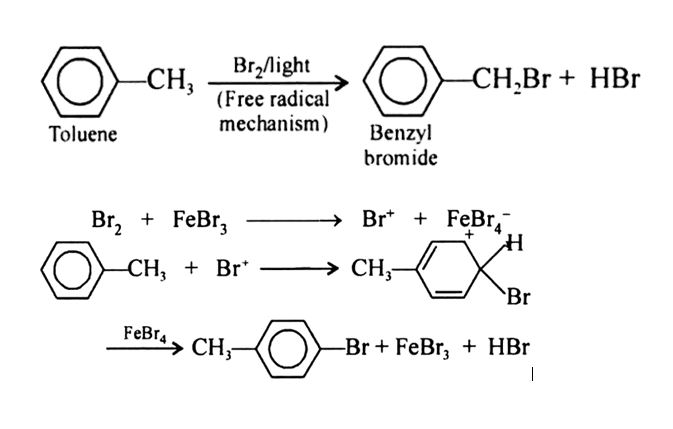
Why toluene reacts with bromine in presence of light gives benzyl bromide while in presence of $$ FeBr_3 $$ it gives p-bromotoluene?
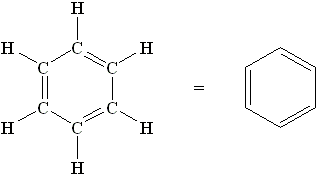
Molecules whose Lewis structures can be drawn as a ring with alternate single and double bonds is termed as a.....
How many alkenes can be hydrogenated to give an alkane with molecular formula $$C_{4}H_{10}$$ which give three monochloro derivative on monochlorination.
Synthesis the following:
Propene to 2, 3-dimethyl butane
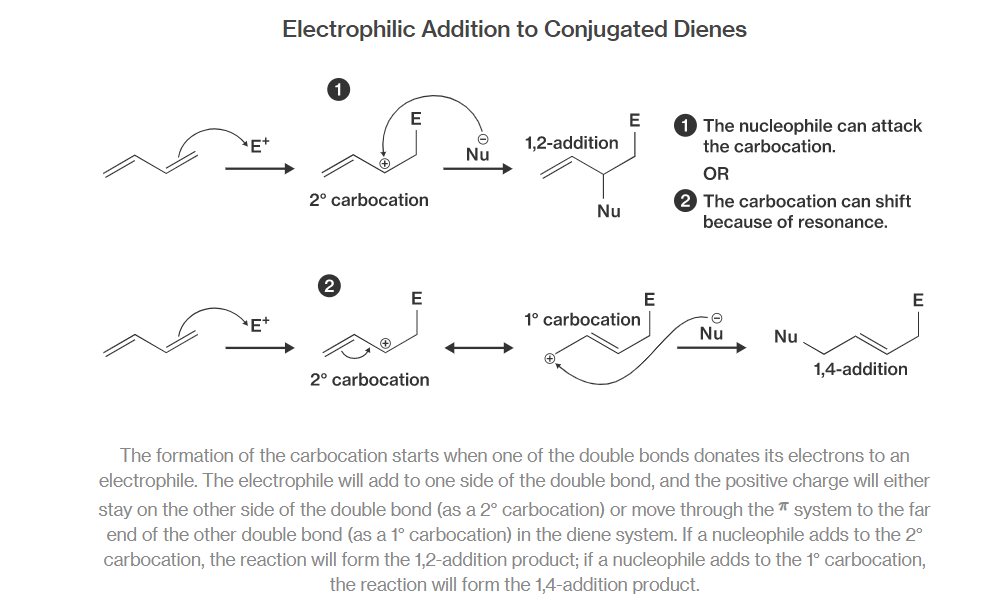
Why conjugated dienes undergo $$1,4-additions$$ ? Explain.
Complete the following reactions with appropriate structure of products?

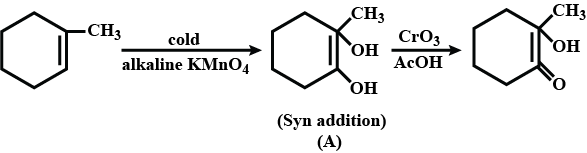
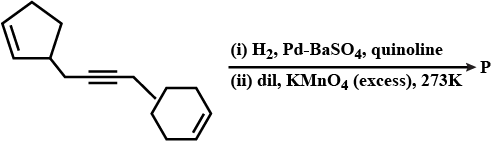
Total number of hydroxyl groups present in a molecular of the major product P is______.
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions