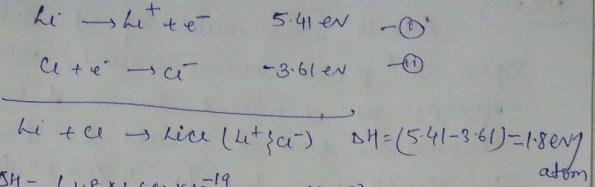Classification Of Elements And Periodicity In Properties - Class 11 Engineering Chemistry - Extra Questions
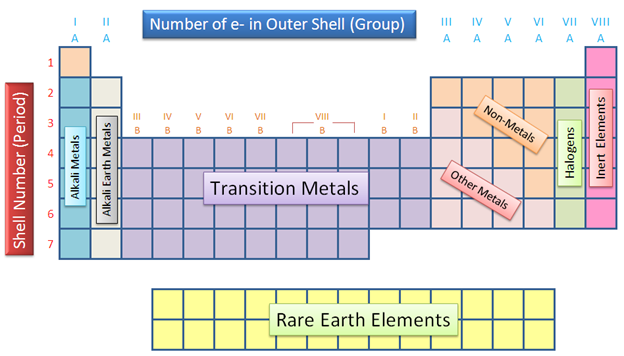
Write the number of vertical columns in the modern periodic table. What are these columns called?
How many vertical columns are there in the modern periodic table and what are they called?
Fill in the blanks in the following statements :
The horizontal rows in a periodic table are called .......
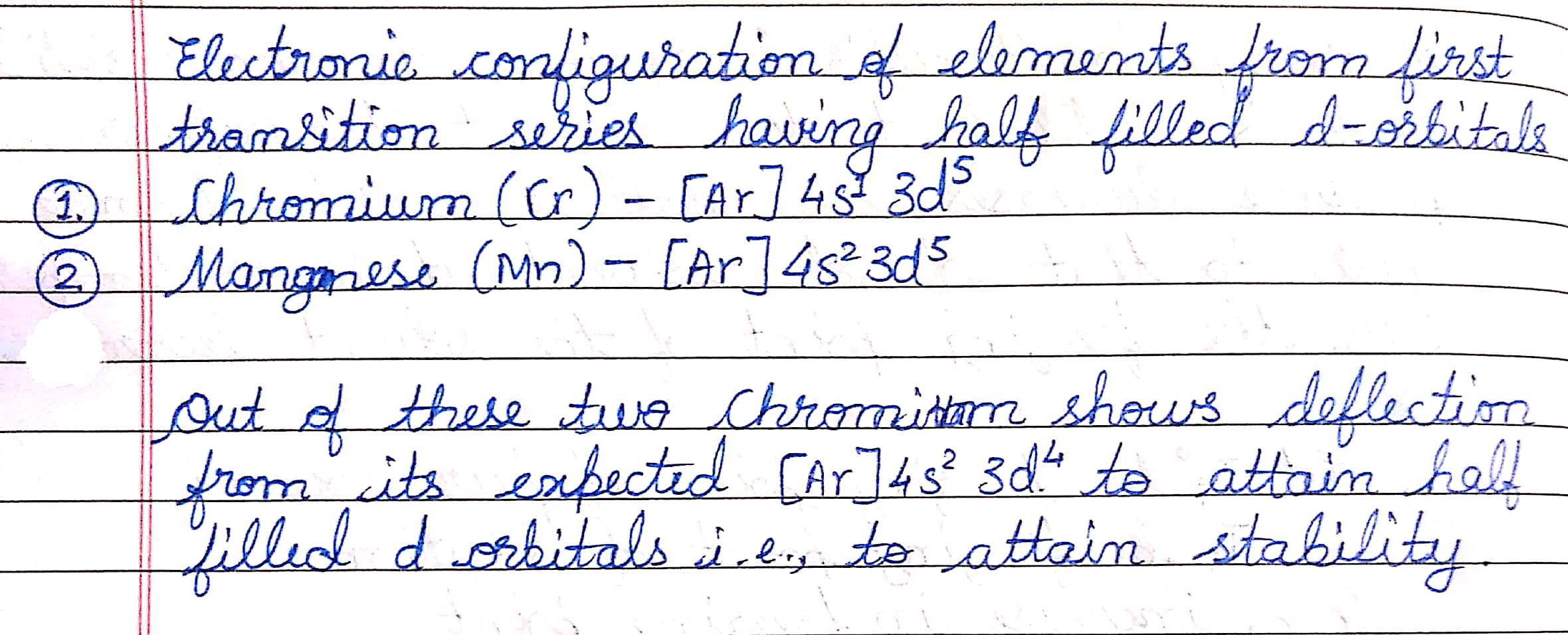
Write observed electronic configuration of elements from first transition series having half filled d-orbitals.
Which is the shortest period in the long form of the periodic table?
How many incomplete periods are there in the long form of the periodic table?
Which is the longest period in the long form of the periodic table?
Atomic size and ionic size decreases in I group elements of the modern periodic table if we move downwards.
If the statement is true enter 1 else 0
Column II give period to which element in column I, match them correctly.
What are periods in a periodic table?
What is meant by period number? What does it signify?
What are the difference between electron gain enthalphy and ionization energy towards 2 period elements and IIA and VA group elements.
Explain the following with suitable examples:
12−16 and 13−15 group compounds.
Match the correct ionization enthalpies and electron gain enthalpies of the following elements.
What is the group number, period and block of the element with atomic number 40?
In going across a period (R - L) in periodic table the atomic aiv of the atoms___________.
An elements ′X′ has mass numbers 35 and the number of neutrons, is 18. Identify the group number and period of ′X′
The correct order of increasing electron gain enthalpy with the negative sign for the elements O,S,F and Cl.
In which period of the periodic table,will an element, be found whose differentiating electron is a 4d electron?
Be and N has an extremely low value of EA (ie. less negative value ) against the trend. Explain
Explain the following:
Why noble gases have positive electron gain enthalpies?
Answer the following:
What is hydride gap?
Name the element of group 17, which has the following properties:
(a) highest electron affinity
How would the tendency to gain electrons change on moving from left to right in a period of the periodic table?
What does each group in the Periodic Table signify?
What do you understand by periods in the Periodic Table?
Define groups.
Give all characteristics of a group.
What are 'groups' and 'periods' in the periodic table ?
If two non-metallic elements X and Y have L and M shells as valence shells, respectively with the same no. of electrons. Then, between X and Y which one has a greater electron affinity?
State whether the statement is True or False:
Across a period there is an increase in electron affinity (EA) value and oxidizing capacity but both properties decrease down the group.
Total number of correct orders of electron affinity values among the following are:
(a) F>Cl
(b) O>O−
(c) Ne+ < F
(d)F>F−
Differentiating electron in inner transition elements enters the ________ orbital.(s/p/d/f)
Considering their electrons affinities, it is possible for alkali metals to form an anion like M− where M represents an alkali metal. If true enter 1, else enter 0.
The group of the element with electronic configurations: 1s22s22p63s23p64s2 isIf true enter 1, else enter 0.
The electron affinities of the alkaline earth metals positive. If true enter 1, else enter 0.
Define electronegativity? How does it differ from electron gain enthalpy ?
Why do elements in the same group have similar physical and chemical properties?
What are the factors that affect electron affinity?
Give two reasons, why the number of elements in first period is only 2?
True or False
Fifth period of the modern periodic table has 18 elements.
True or False
Elements of the same group have same number of electrons for bonding.
Fill in the blanks
_________ period is incomplete.
Electron affinity of chlorine is more than that of fluorine though electron affinity decreases on descending the group. Explain.
Fluorine has lesser electron affinity value than chlorine. However, fluorine shows the greater ease of formation of F− ion. than chlorine for Cl− ion. How do you justify this?
Two non-metallic elements X and Y have L and M shells as valence shells respectively with same no. of electrons. Compare the electron affinity values of X and Y.
Identify the wrong statements and correct them.
a. Sodium benzoate is used in food preservative
b. Nitric acid is not used as fertilizer in agriculture.
c. Sulphuric acid is called the king of chemicals.
d. The pH of acid is greater than 7.
Choose the correct answer from the four alternatives given in the brackets:
Number of periods in modern periodic table is _____ .
(7, 17, 8, 18)
Write down the characteristics and period number of an element having group number 17.
Why is the electron gain enthalpy of chlorine more negative than fluorine?
Write down the characteristics of the elements having an atomic number 17.
Define electron gain enthalpy. Explain, why electron gain enthalpies of some elements are positive? How does electron gain enthalpy vary in the group and in a period?
The amount of energy released when an atom in the gaseous state accepts an electron to form an anion. factor which affect electron affinity are: atomic size and Nuclear charge. As atomic radii increases, electron affinity increase. As nuclear charge increase, electron affinity increase. it decrease down a group and increases across a period. Explain.
Explain electron gain enthalpies of the noble gases are positive.
For the gaseous reaction, K+F→K++F−,ΔH was calculated to be 19 kcal under conditions where the cations and anions were prevented by electrostatic separation from combing with each other. The ionization potential of K is 4.3 eV. What is the electorn affinity of F?
How will the tendency to gain electrons change as we go from left to right across a period?
What is the value (including the sign) of the electron gain enthalpy or electron affinity of Na+ ion in electron -volts? The first ionization potential for Na is 118 k cal/mole.
Explain the appropriate reason for the following statement:
(a)Size of Mg2+ is less than Mg.
(b) Electron gain enthalpy of Cl is more than that of F.
Which of the following pairs of elements would have a more negative electron gain enthalpy? Explain
(a) N or O (b) F or Cl
Although electron gain enthalpy of fluorine is less negative as compared to chlorine, fluorine is a stronger oxdising agent than chlorine. Why?
Observed the Modern Periodic Table and explain the gradation in reactivity of Halogen family .
How would the tendency to gain electrons change on moving from left to right in a period of the periodic table?
Explain the various factors that affect electron affinity.
Arrange the following as property indicated against it:
(a) F,Cl,Br and I (increasing order of negative electron gain enthalpy)
(b) B,Al,Ga and In (decreasing order of atomic radius)
(c) C,N,0 and F (increasing order of second ionization enthalpy)
Six elements A,B, C, D, E and F have the following atomic numbers:
A = 12, B = 17, C = 18, D = 7, E = 9 and F = 11
(i) How many elements belong to the third period of the periodic table?
(ii) Identify the elements known as halogens.
Which of the following elements has most positive electron gain enthalpy?
Fluorine, Nitrogen, Neon
An element has its electronic configuration as 2,8,2. Now answer the following question:
What is the group of this element ?
Arrange the following in increasing order of the property indicated:
C. N, O and F (2nd ionisation enthalpy)
Which element has the highest negative electron gain enthalpy value?
An element has its electronic configuration as 2,8,2. Now answer the following question:
To which period does does this element belong ?
Arrange the following in increasing order of the property indicated:
F, Cl, Br and I (electron gain enthalpy)
Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionization enthalpy than nitrogen. Explain.
Explain the following:
Chlorine can be converted into chloride ion easily as compared to fluoride ion from fluorine.
Sulphur has more negative electron gain enthalpy than oxygen.
Explain the following:
Why are the electron gain enthalpies of halogens so high?
Explain the following:
Why are the electron gain enthalpies of halogens so high?
The atomic numbers of three elements A, B and C are given below:
Element Atomic number
A 5 B 7 C 10(i) Which element belongs to group 18? (ii) Which element belongs to group 15?
(iii) Which element belongs to group 13? (iv) To which period/periods do these elements belong?
(iii) Which element belongs to group 13?
(a) What is meant by (i) a group, and (ii) a period, in a periodic table
(b) How many periods and groups are there in the long from of periodic table
(c) Give two examples each of (i) group 1 elements (ii) group 17 elements (iii) group 18 elements.
Find the neutral atom in the periodic table which has the same number of electrons as K+ and Cl− What is this number ?
(a) What are the periods and group in a periodic table? Give two characteristics of each.
(b) In terms of electronic configurations explain the variation in the size of the atoms of the elements belonging to the same period and same group.
(c) Given alongside is a part of periodic table. As we move vertically downward from Li to Fr.
(i) What happens to the size of atoms? (ii) What happens to their metallic character?
(d) Name two properties of elements whose magnitudes change when going from top to bottom in a group of the periodic table. In what manner do they change? (e) Rewrite the following statement after correction, if necessary: Groups have elements with consecutive numbers.
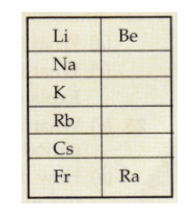
(d) Name two properties of elements whose magnitudes change when going from top to bottom in a group of the periodic table. In what manner do they change?
(a) What is a period in a periodic table? How do atomic structures (electrons arrangements) change in a period with increase in atomic numbers from left to right?
(b) How do the following change on going from left to right in a period of the periodic table?
(i) Chemical reactivity of elements. (ii) Nature of oxides of elements. Give examples in support of your answer.
(i) Chemical reactivity of elements.
(a) How does the electropositive character of elements changes on going down in a group of the periodic table ?
(b) State how valency of elements varies (i) in group and (ii) in a period of the periodic table
Name the element which is in:
(a) first group and third period.(b) seventeenth group and second period.
How does the number of valence elctrons vary on moving from left to right
(i) in the first period of the periodic table (ii) in the second period of the periodic table.
How does electropositive character of elements in a period vary from left to right?
Which group of elements could be placed in Mendeleev's Periodic Table without disturbing the original order? Give reason.
(i) Electropositive nature of the element(s) increases down the group and decreases across the period
(ii) Electronegativity of the element decreases down the group and increases across the period
(iii) Atomic size increases down the group and decreases across a period (left to right) (iv) Metallic character Increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to
(a) Name the most electropositive element among them
(b) Name the most electronegative element
(c) Name the element with smallest atomic size
(d) Name the element which is a metalloid
(e) Name the element which shows maximum valency.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to
(a) Name the most electropositive element among them
(b) Name the most electronegative element
(c) Name the element with smallest atomic size
(d) Name the element which is a metalloid
(e) Name the element which shows maximum valency.
What are horizontal rows and vertical columns in a periodic table known as ?
Correct the statement.
Elements in the same periods have equal valency.
What is a periodic table ? How many groups and periods does modern periodic table have ?
Name :
Which has higher E.A. fluorine or neon .
State the number of elements in periods 1, period 2 and period 3 of the periodic table.
Explain why the following is not correct :
Atoms of elements in the same group have the same number of electron(s).
What is meant in the periodic tables by:
A period.
What is meant in the periodic tables by:
A group
Write down the electronic configuration of the following elements from the given atomic numbers. Answer the following question with an explanation.
4Be, 6C, 8O, 5B, 13Al
Which is the most electropositive element among these?
Write down the word that will correctly complete the following sentences:
The horizontal rows in a periodic table are called ...........
State two characteristics of groups.
Define groups and periods.
Explain the periodicity of electron gain enthalpy in a group.
Give the characteristics of a period.
Define electron gain enthalpy. Explain the factors affecting it and it periodicity.
Anion has a larger size than the neutral atom.Explain.
Explain the reason that electron gain enthalpy of F is less than Cl .
Which element has highest electron gain enthalpy in periodic table ?
What is the relation between number of electrons present in the last ′s′ subshell and their group number ?
What is meant by group and period?
Electron gain enthalpy of fluorine less negative than that of chlorine. Explain.
What is the significance of the terms 'isolated gaseous atom' and 'ground state' while defining the ionization enthalpy and electron gain enthalpy?
Why are the electron gain enthalpies of Be and Mg are positive?
What is electron affinity and how it depends on atomic size, effective nuclear change and screening factor?
Be and N has extremely low value of EA against the trend. Explain.
The first IP of lithium is 5.41eV and electron affinity of Cl is −3.61eV. Calculate ΔH in kJ mol−1 for the reaction:
Li(g)+Cl(g)→Li+(g)+Cl−(g)
Give appropriate reasons
Fluorine has less gain enthalpy than chlorine yet it taken compunds. Why?
Define the term 'electron affinity'. State its unit.
Discuss the factors affecting electron gain enthalpy and the trend in its variation in the periodic table.
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions