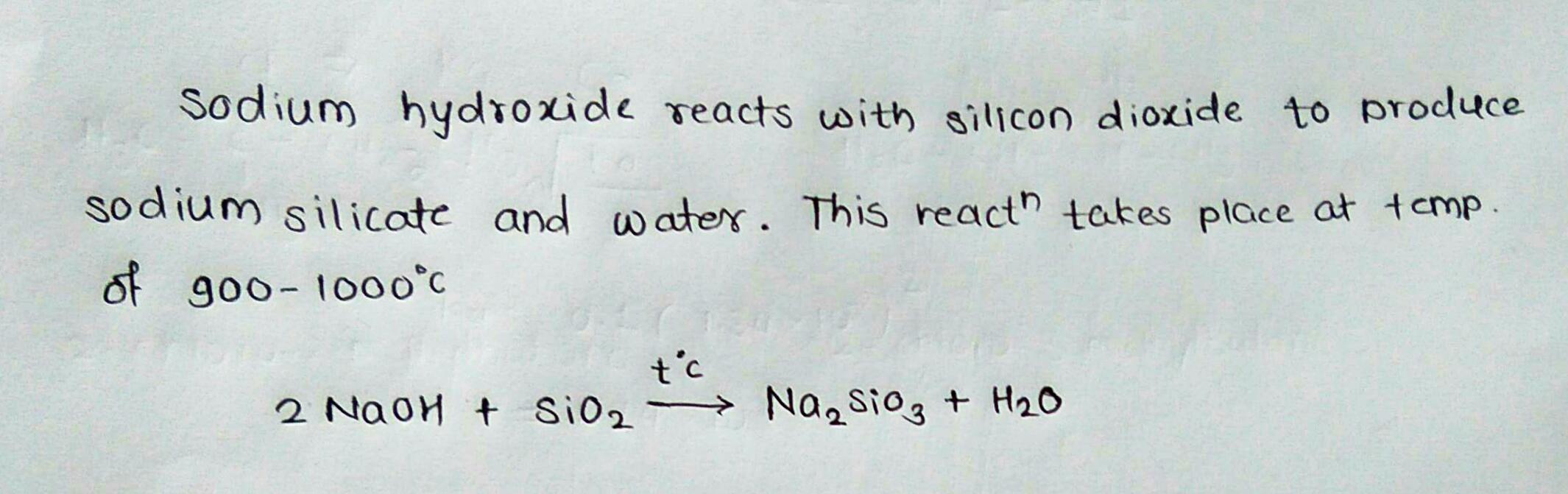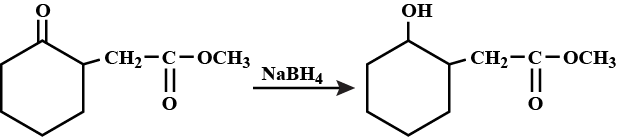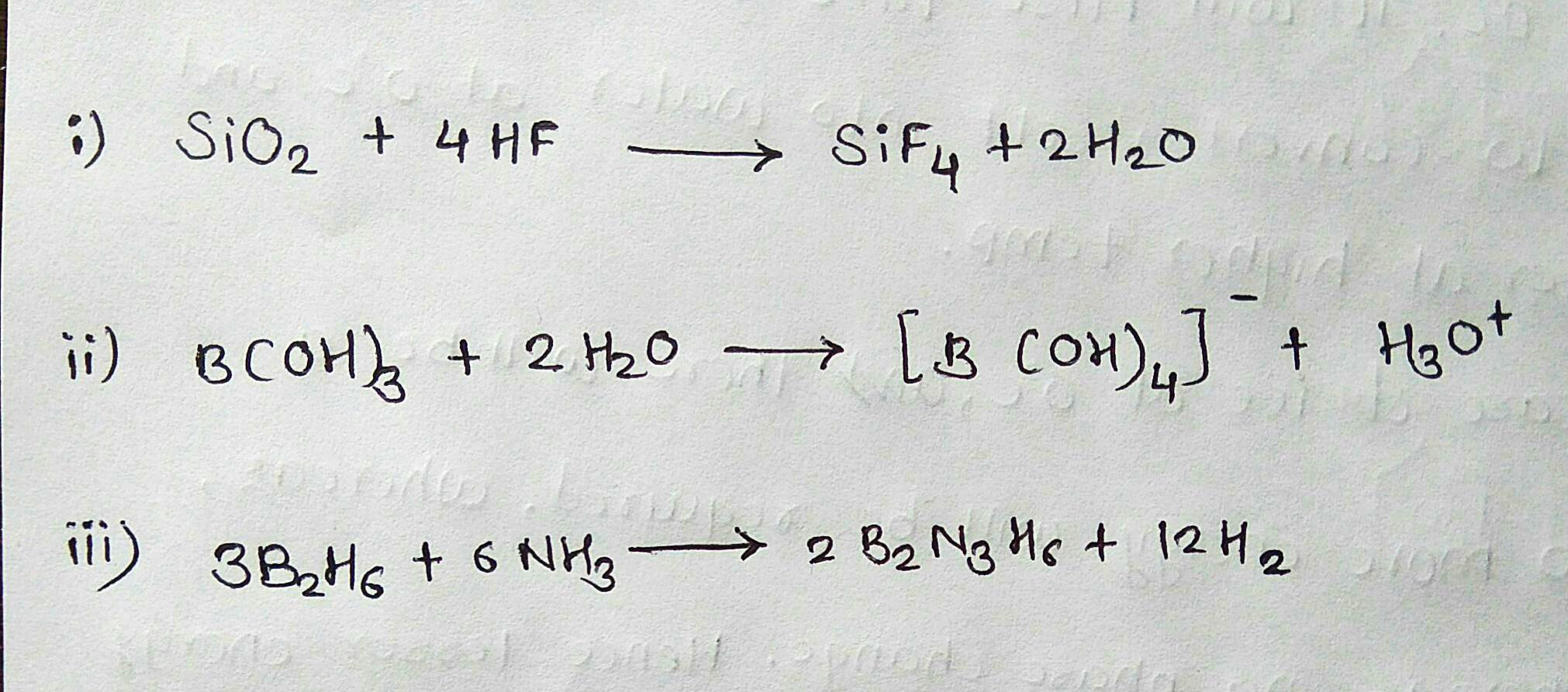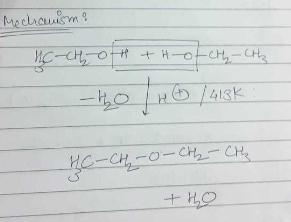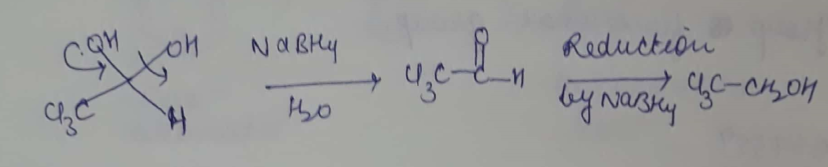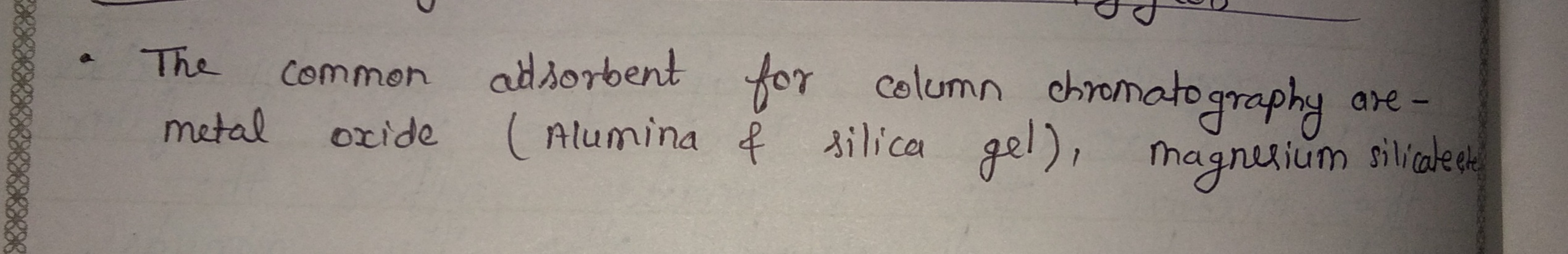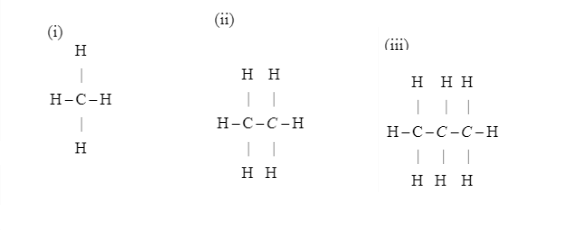Organic Chemistry - Some Basic Principles And Techniques - Class 11 Engineering Chemistry - Extra Questions
What would be the product of NaOH + SiO2 → ?
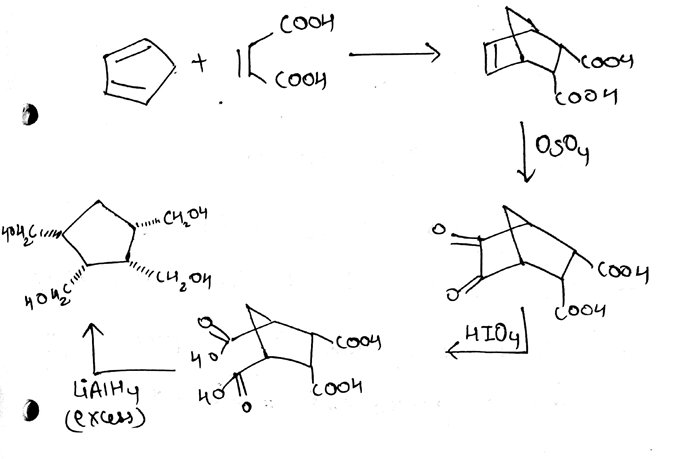
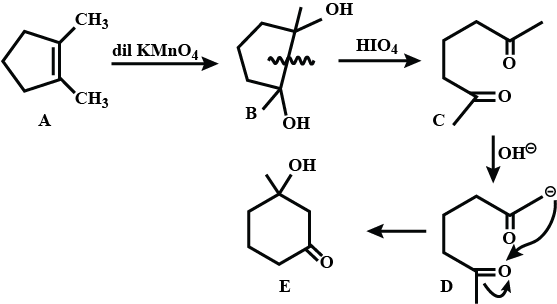
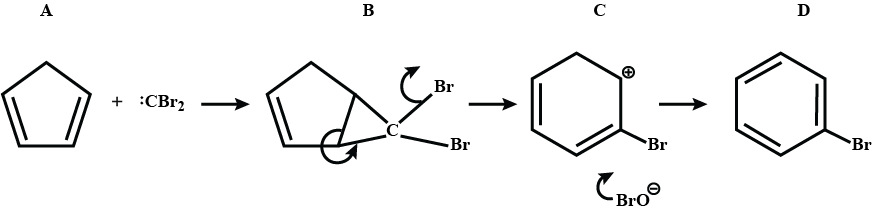
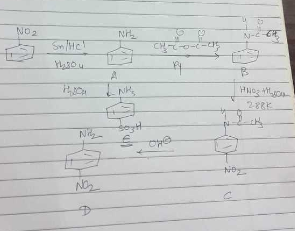
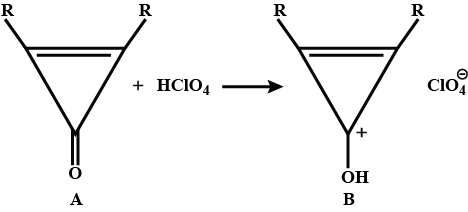
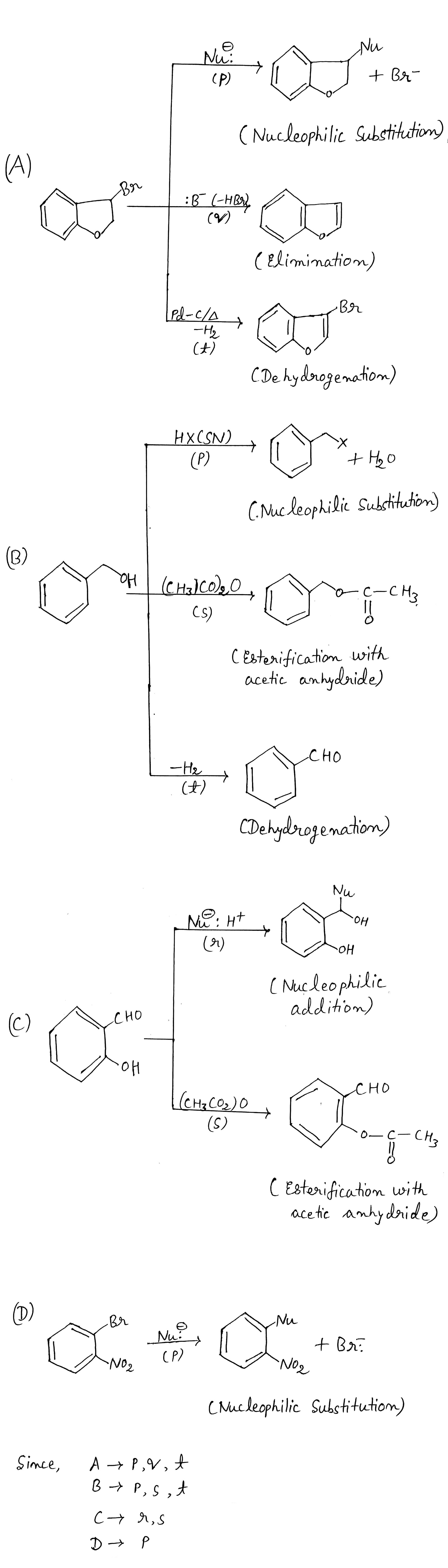
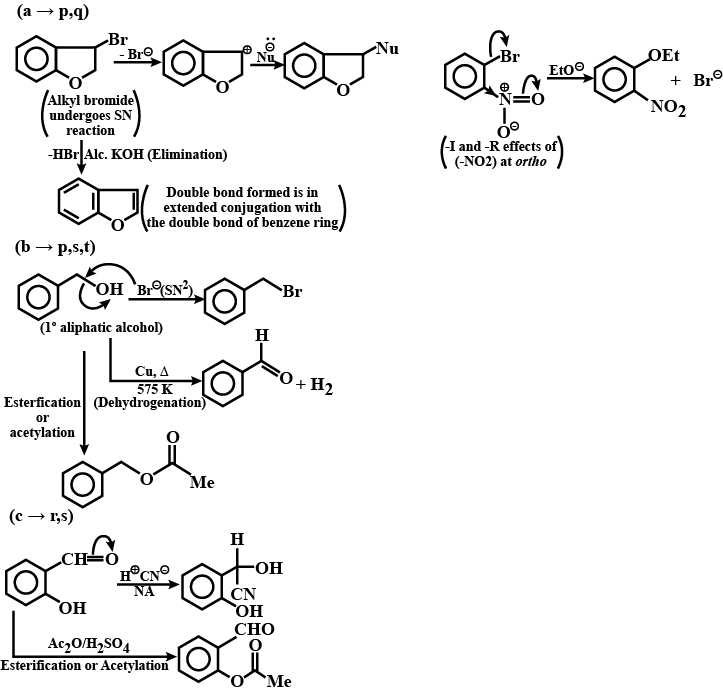
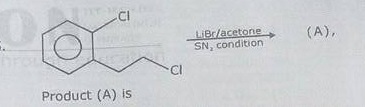
Identify A,B,C and D.
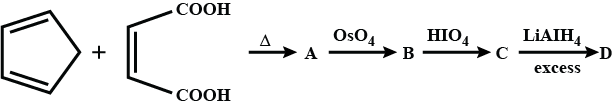
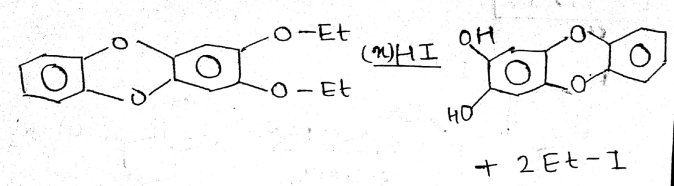
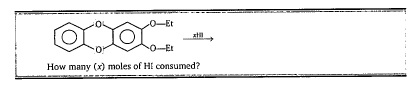
How many (x) moles of HI consumed?
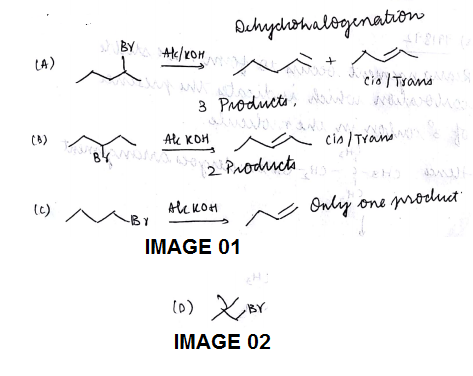
Match the column.
Column (I) - E2 reaction (elimination biomolecular)
Column (II) - No. of possible products. (including stereoisomerism)
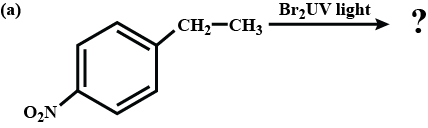
What is the product formed?
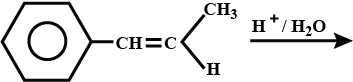
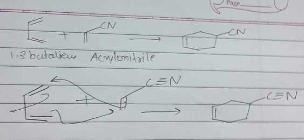
Find the product of the given reaction.
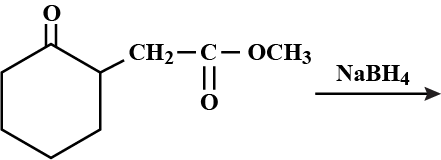
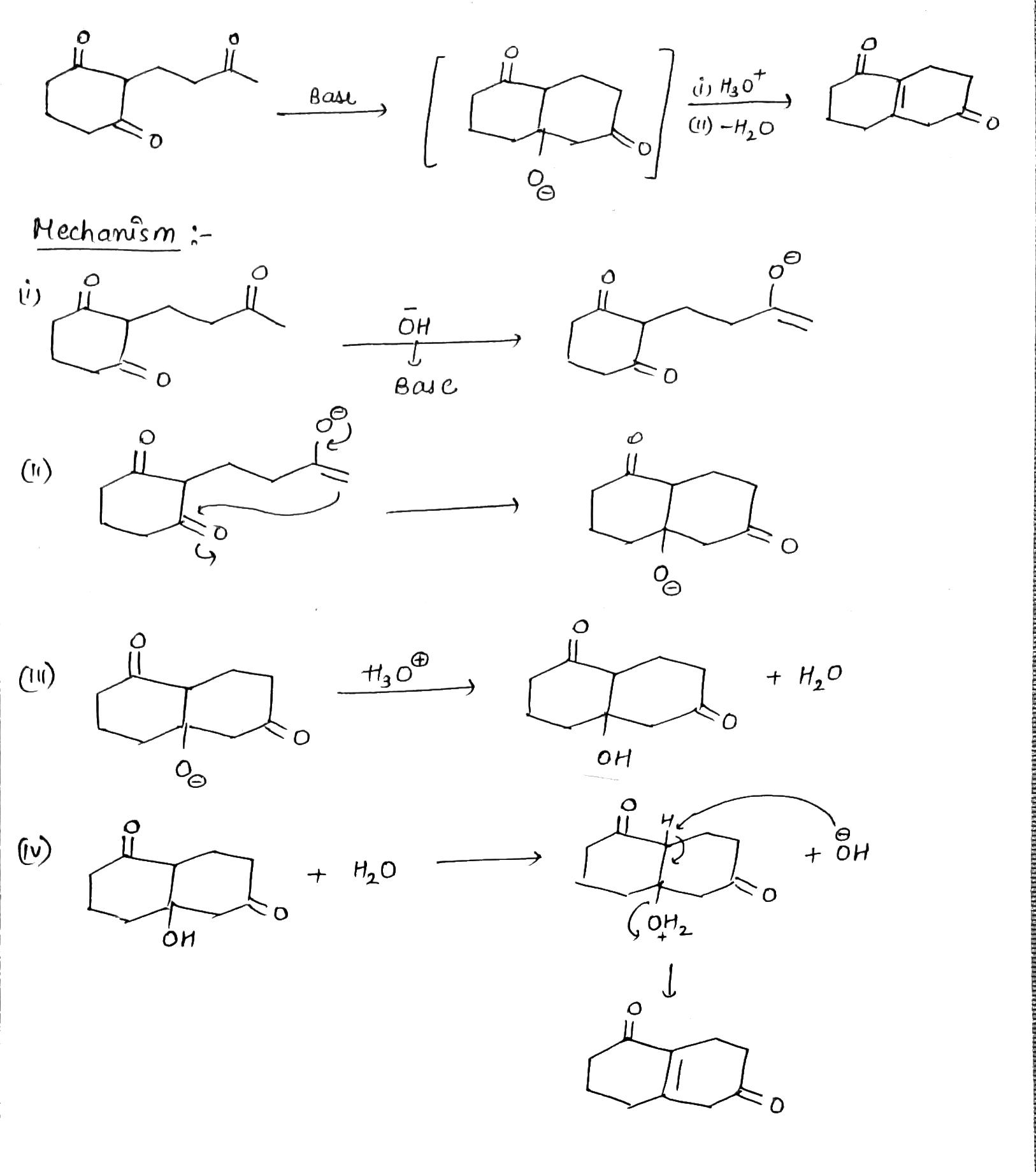
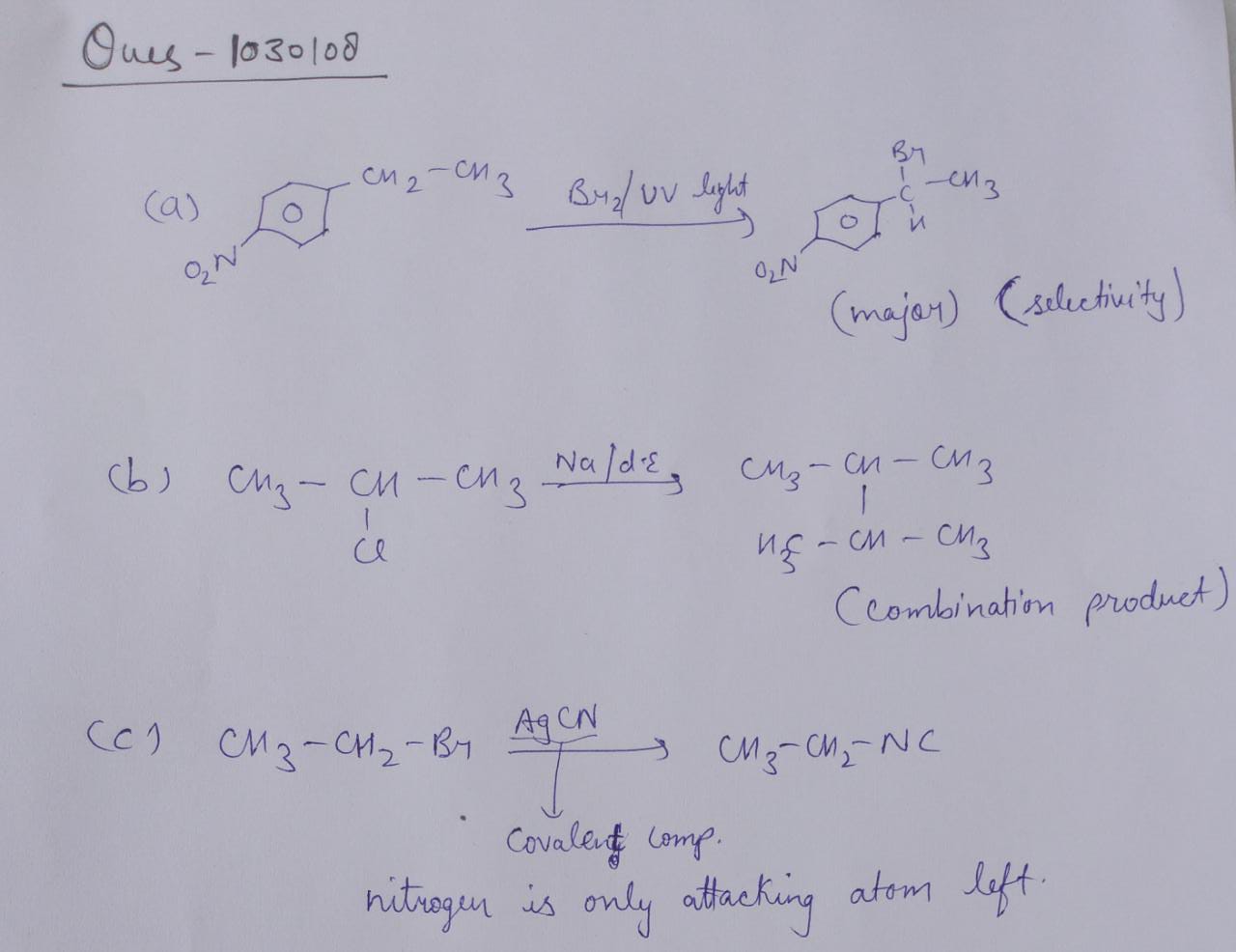
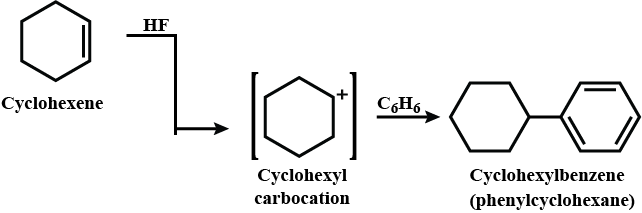
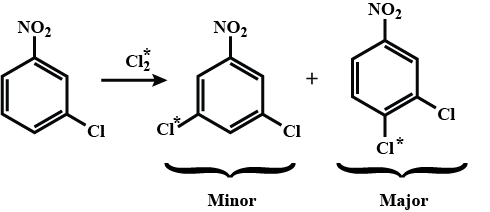
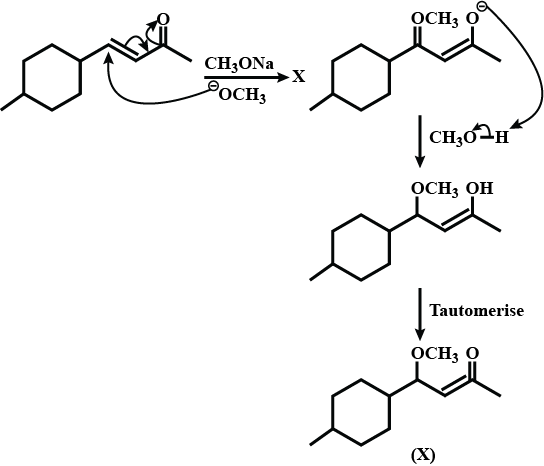
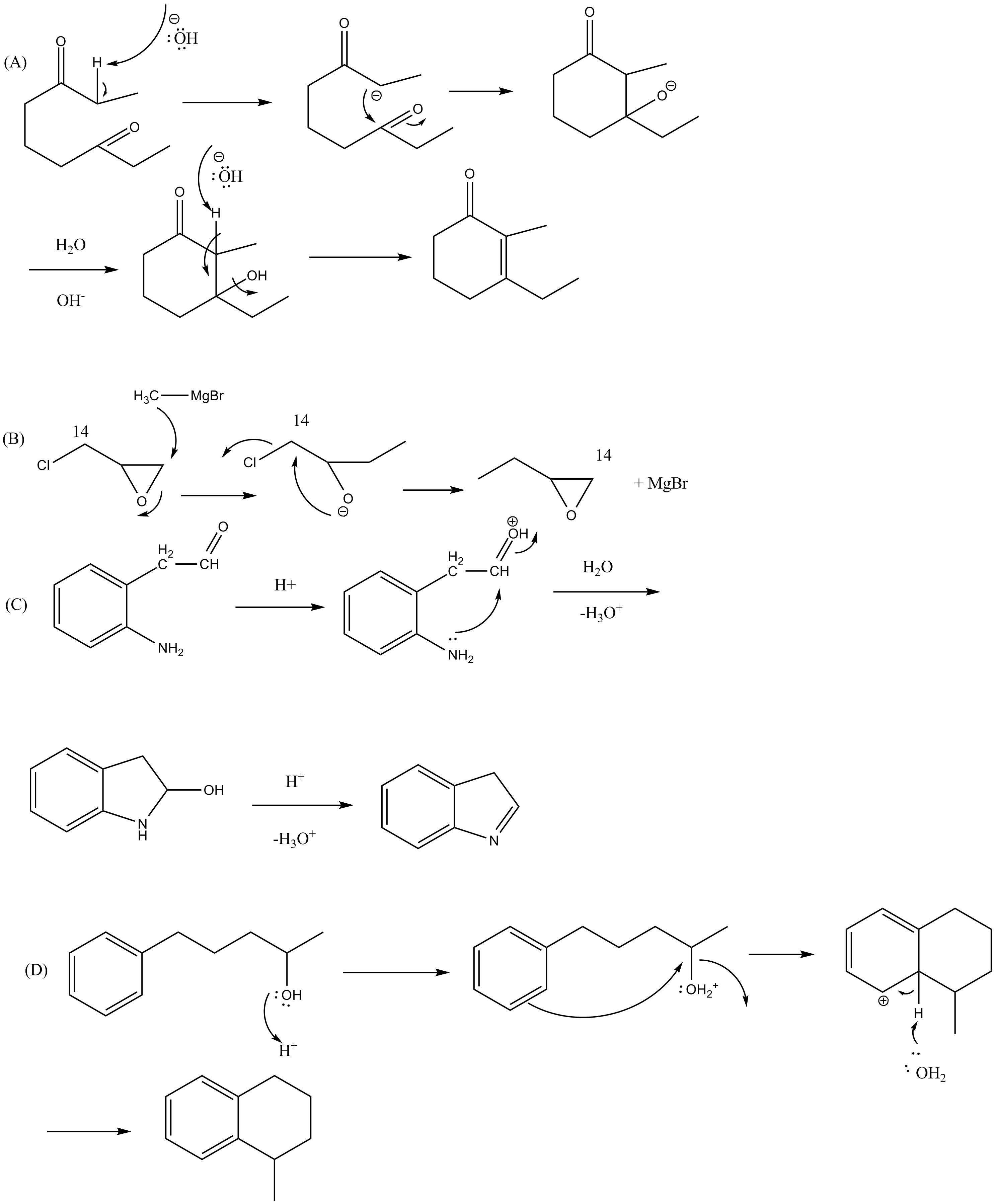
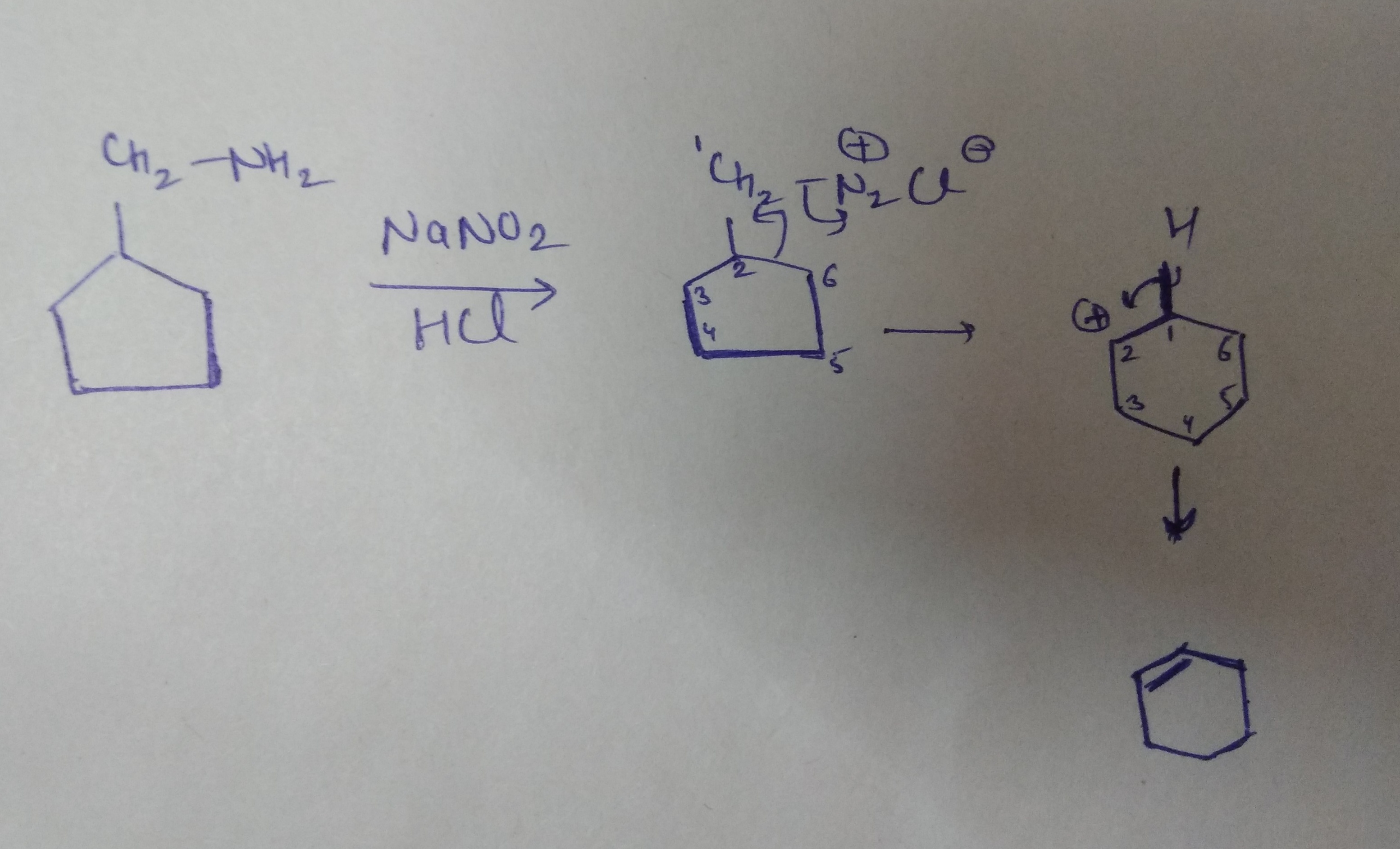
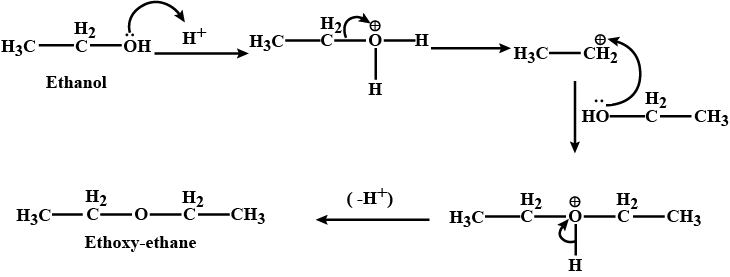
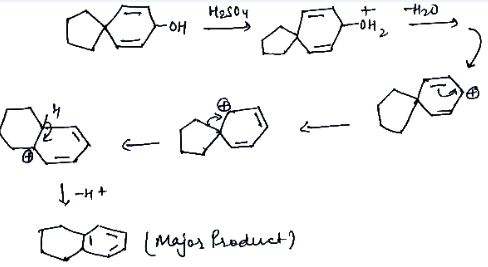
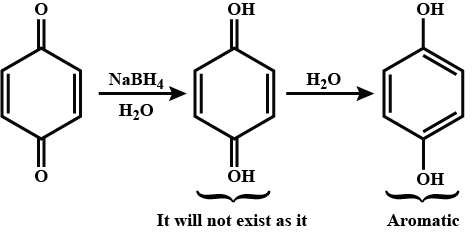
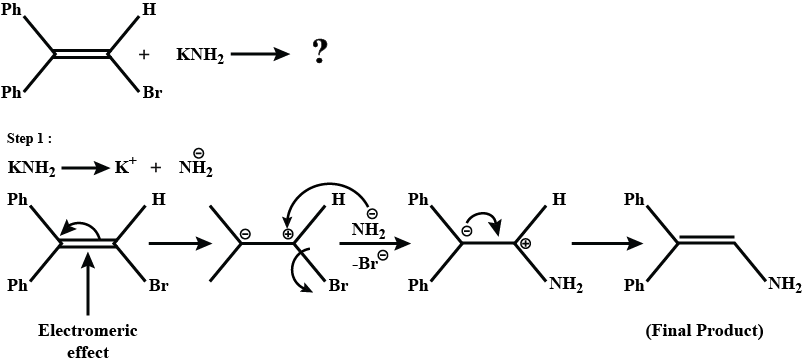
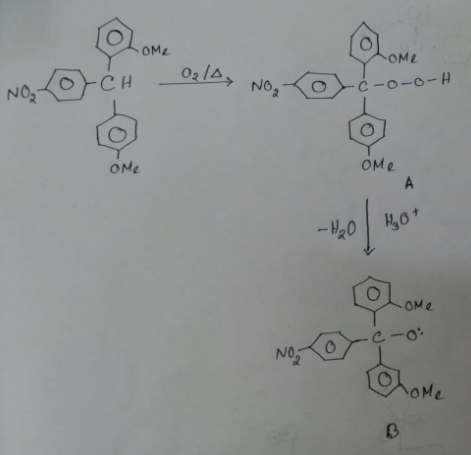
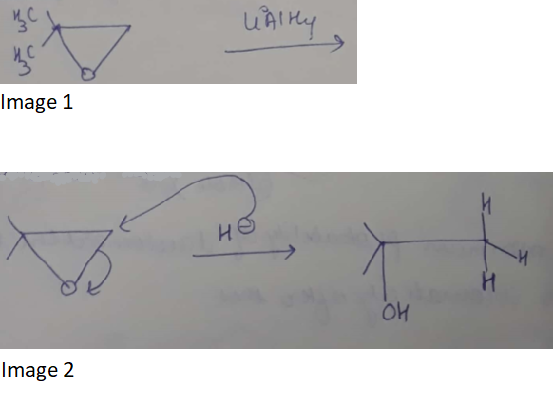
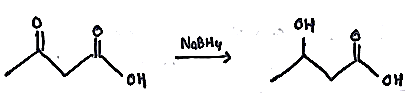
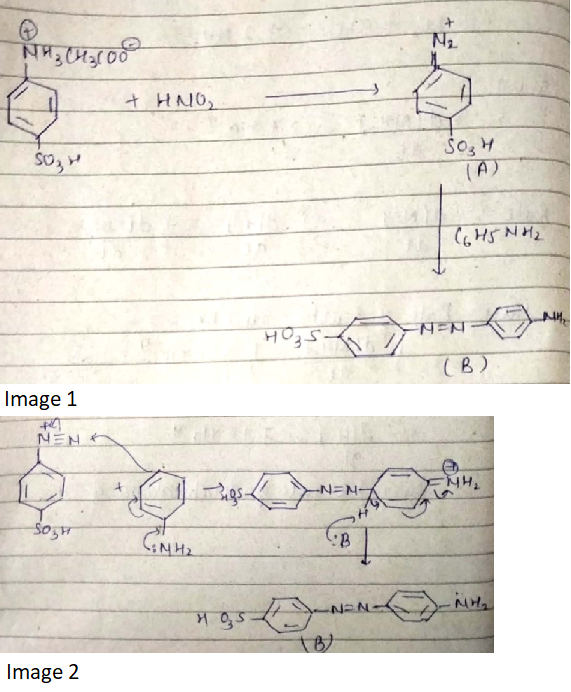
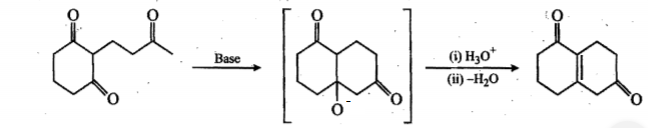
Provide a detailed reaction mechanism for the following.
Fill in the following blanks with suitable words:
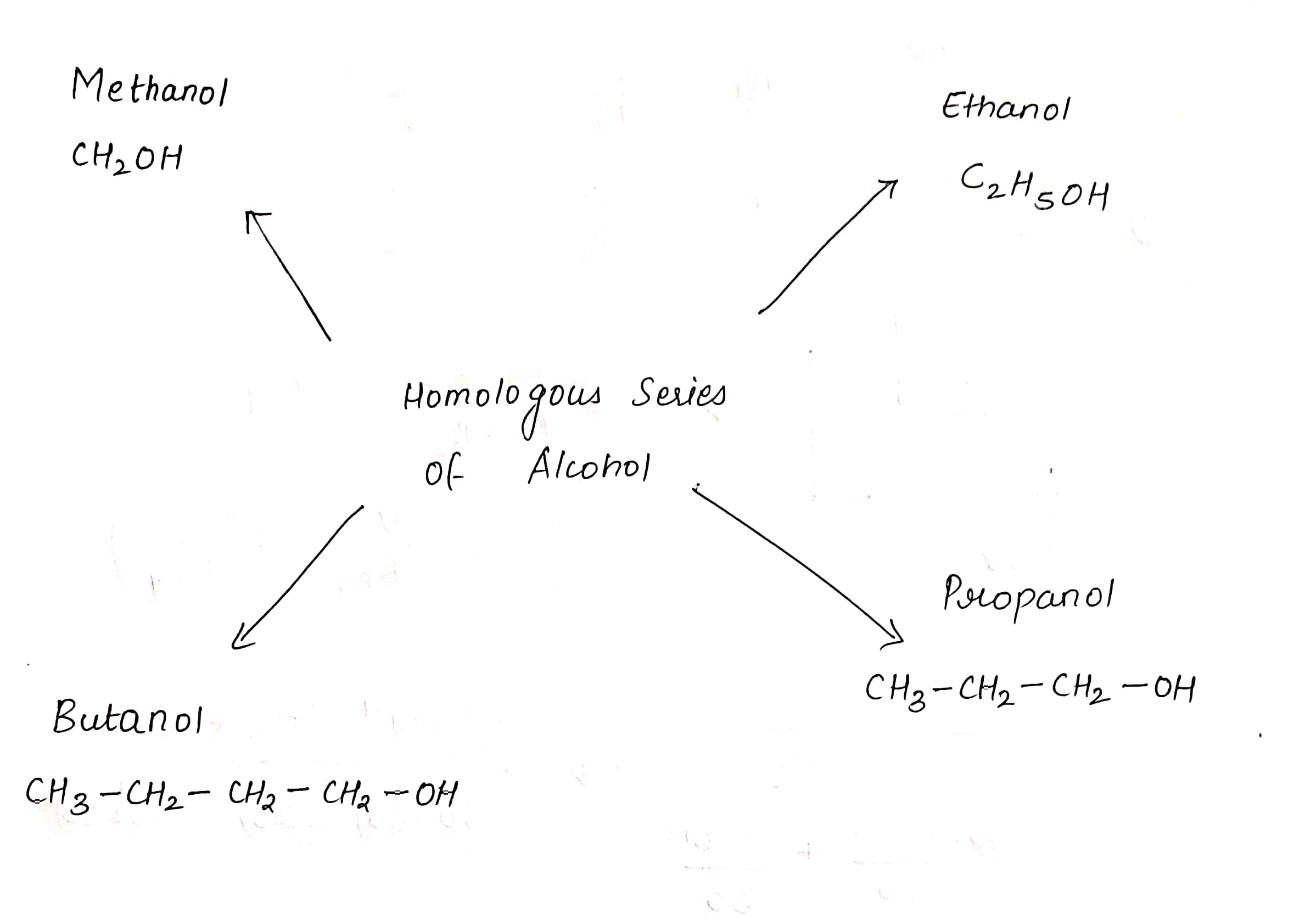
The next higher homologue of ethanol is ________.
The next higher homologue of ethane is _______.
Fill in the following blanks with suitable words:
The next homologue of C2H5OH is _______
Identify the unknown organic compounds (D) to (E) in the following series of chemical reactions.
(D)H2/Ni→Δ(E)
Write the next homologue of each of the following:
(i) C2H4
(ii) C4H6
What is the next homologue of C3H7OH called?
Which two of the following organic compounds belong to the same homologous series?C2H6,C2H6O,C2H4O2,CH4O
Fill in the blanks:
Propane and ethane are ________
The molecules of alkene family are represented by a general formula CnH2n. Answer the following:
Write the molecular formula of lower and higher homologous of an alkene which contains four carbon atoms.
The molecular formula of the first member of a certain group of organic compound is CH2O. Determine the name and molecular formula of the third member of this group if the member of this group are in series what is the general name of this group of organic compounds?
Find the final product.
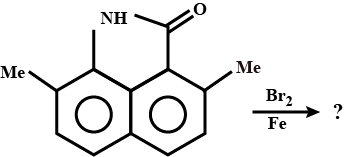
The product is?
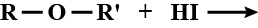
Complete the following table:
| Name | Molecular formula | Condensed structural formula | Number of carbon atoms | Number of−CH2−units |
| Methane | CH4 | CH4 | 1 | 1 |
| Ethane | C2H6 | CH3−CH3 | 2 | 2 |
| Propane | C3H8 | CH3−CH2−CH3 | 3 | 3 |
| Butane | C4H10 | CH3−CH2−CH2−CH3 | ||
| Pentane | C5H12 | CH3−CH2−CH2−CH2−CH3 | ||
| Hexane | C6H14 | CH3−CH2−CH2−CH2−CH2−CH3 |
Write the name and formula of the 2nd member of homologous series having general formula CnH2n.
In homologous series of aldehyde, the consecutive members of the series differ by the mass of:
Alkanes undergo only substitution reactions but alkenes and alkynes undergo both substitution and addition reactions. Why?
What is a homologous series? State any four characteristics of a homologous series?
Name the simplest hydrocarbon.
What will be the correct structural formula of product for the following reaction?

Explain the nature of C−X bond in both the compounds.
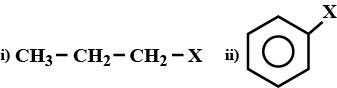
C3H6,C4H8 and C5H10 belong to the same homologous series.
Arrange these hydrocarbons in order of increasing boiling points.
What are homologous series of carbon compounds? Write the molecular formula of two consecutive members of homologous series of aldehydes. State which part of these compounds determines their (i) physical and (ii) chemical properties.
Complete the following reactions:
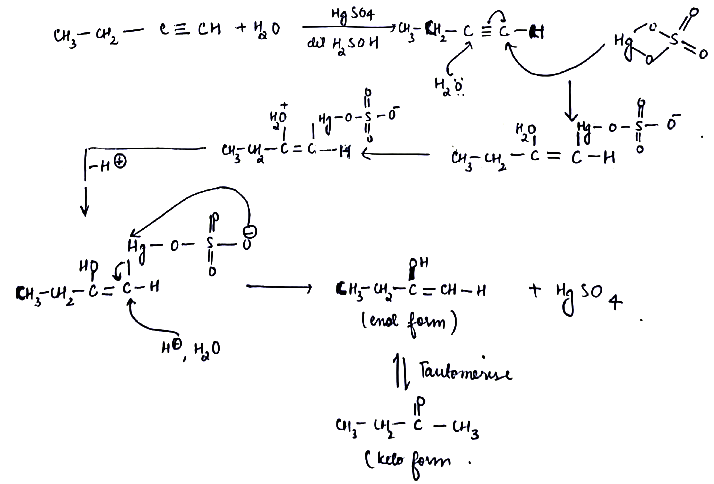
CH3−CH2−C≡CH+H201%HgSO4→40%H2SO4
Name the gas evolved in each of the following cases:
(i) Copper carbonate is heated strongly.
(ii) Action of dilute hydrochloric acid on sodium sulphite.
(iii) Nitrogen combines with hydrogen.
(iv) Action of dilute sulphuric acid on sodium carbonate.
(v) Addition of sodium to cold water.
What is a homologous series? Which two of the following organic compounds belong to the same homologous series?
C2H6,C2H6O,C2H6O2,CH4O
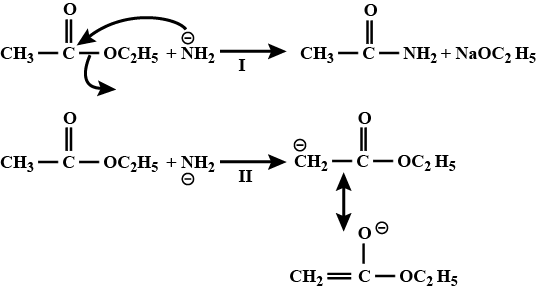
CH3−O||C−OC2H5+NaNH2→?
Write the major product in the following.
2CH3−C|HCl−CH3Na→dryether?
CH3−CH2−BrAgCN→ ?
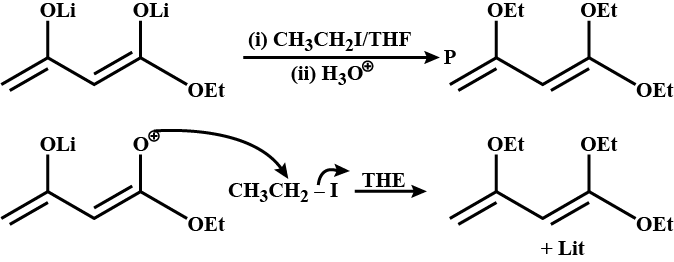
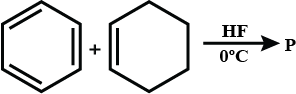
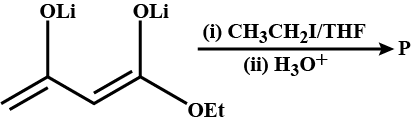
In the given reaction the product P is:
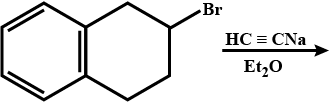
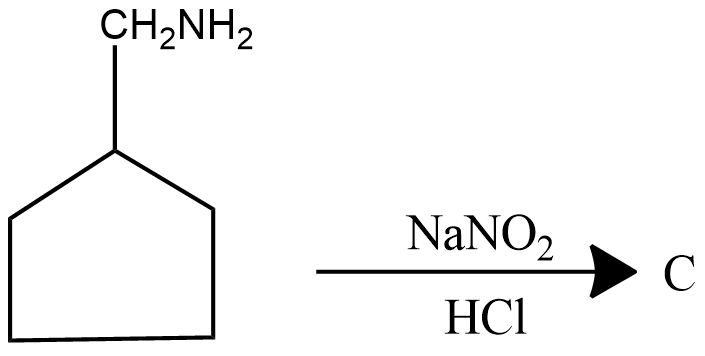
Identify the product C.
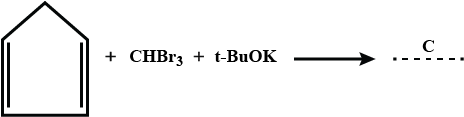
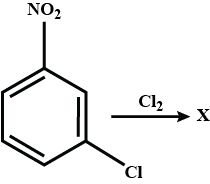
What will be the major product formed?
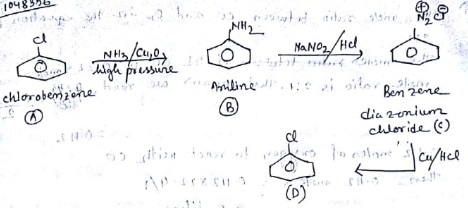
An aromatic organic compound [A] on heating with NH3 and Cu2O at high pressure gives [B]. The compound [B] on treatment with ice cold solution of NaNO2 and HCL gives [C].Which on heating with Cu/HCl gives compound [A] again. Identify the compounds [A], [B] and [C]. Write the name of the reaction for the conversion of [B] to [C] ?
Identify the major product P in the following reaction.
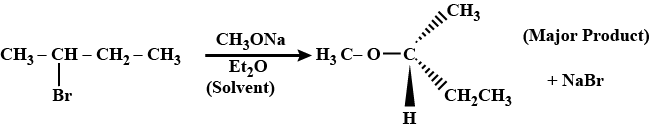
What is the major product obtained from the following reaction?CH3C|BrH−CH2−CH3CH3ONa→Et2O
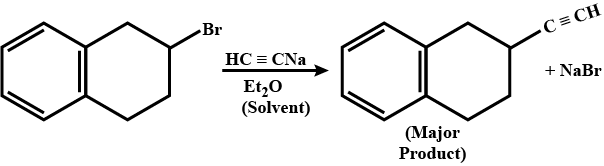
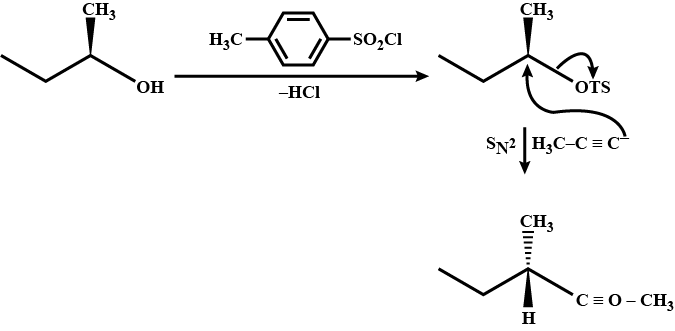
What are the products obtained from the following reaction?
N2H4+ClO−3→No+Cl−Balance and complete the reaction.
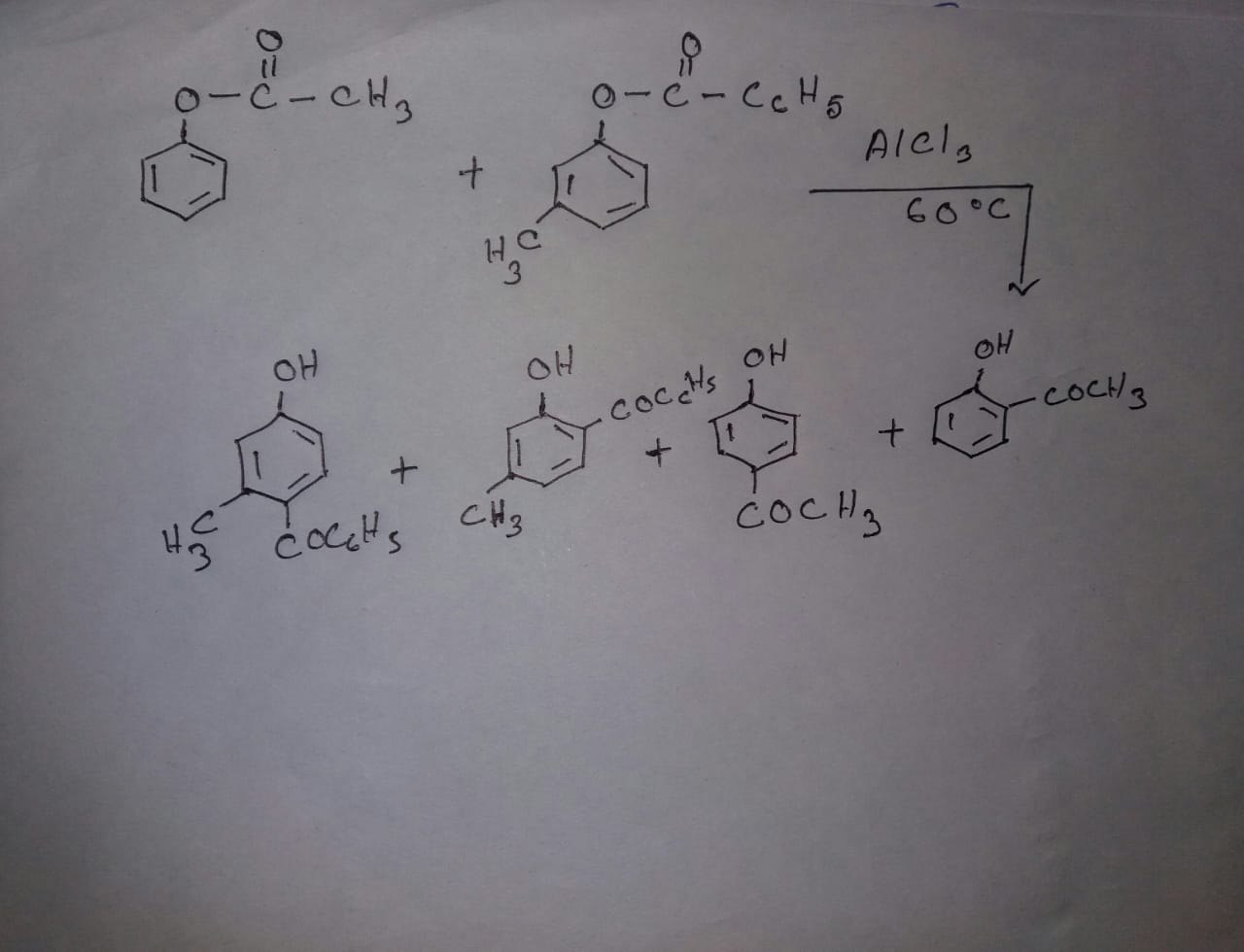
Write structures of the products A, B, C, D, and E in the following scheme.

In this product A is?
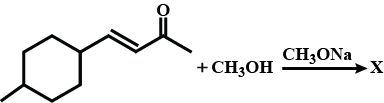
Find X.
The molecular formula of a compound is C10H18. Name the homologous series to which it belongs.
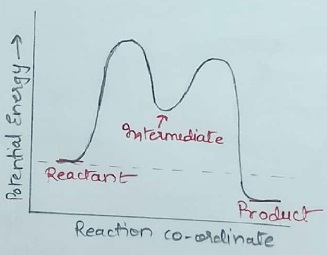
What is reaction intermediate?
Define homologous series.
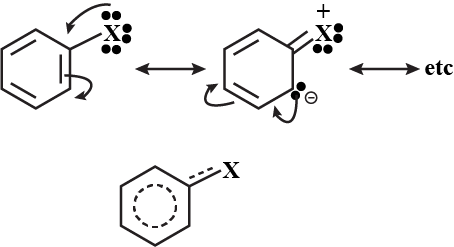
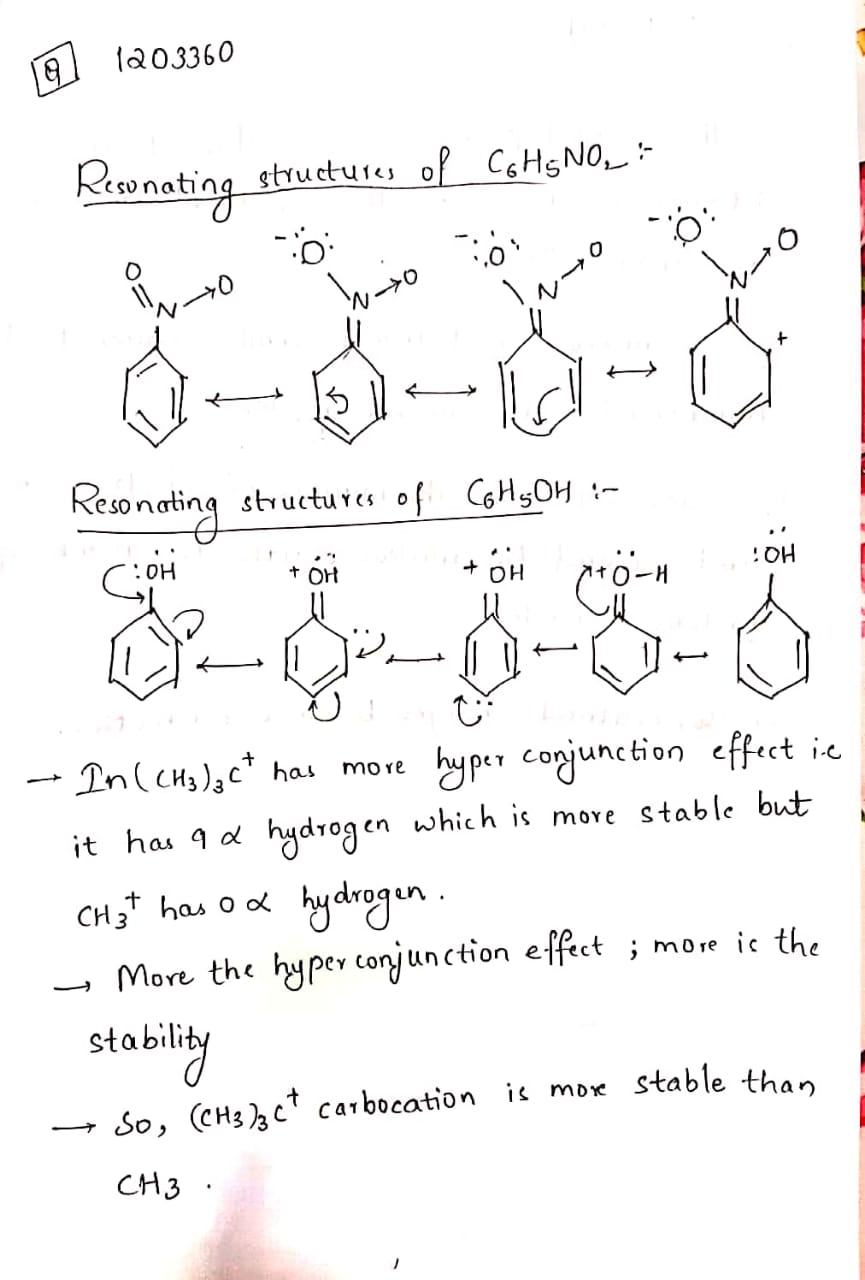
(I) Draw the resonating structure of C6H5NO2 and C6H5OH.
(II) Why (CH3)3C+ carbocation is more stable than CH3?
Write a balanced equation for
(i) Silicon dioxide is treated with hydrogen fluoride
(ii) Boric acid is added to water
(iii) Diborane reacts with NH3 followed by heating.
Write mechanisms for the following reaction
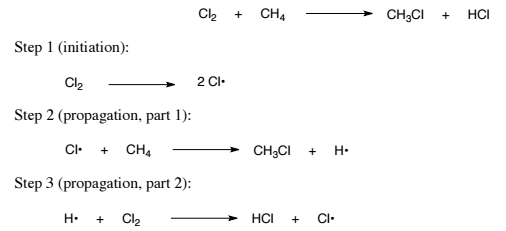
Phtochemical chlorination of methane
Explain the mechanism of the following reaction :

Write the structure of A,B,C,D,and E in following
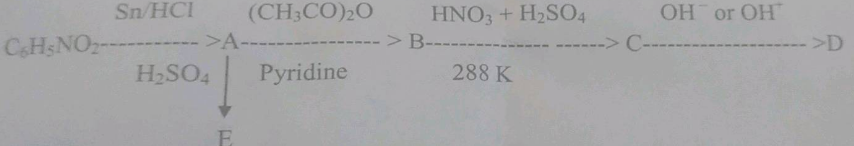
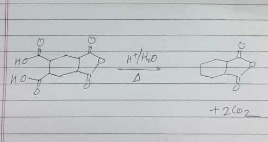
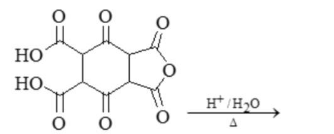
Define Decarboxylation. Methane is formed by decarboxylation
How many CO2 will releave in the following reaction
Write the molecular formula of first two members of homologous series having functional group −Cl.
What happens when Acrylonitrile reacts with 1, 3-butadiene
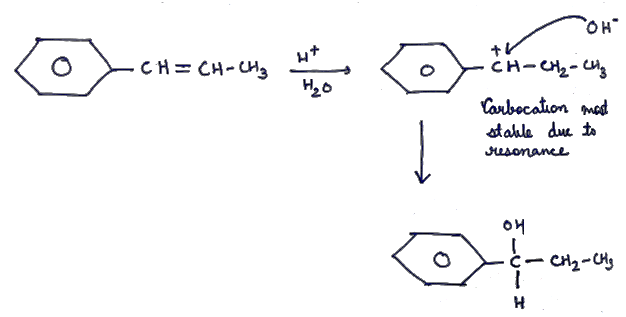
Explain the mechanism of the following reaction.
C2H5OHConc.H2SO4→443KCH2=CH2+H2O
Copy and complete the following table which relates to three homologous series of hydrocarbons:
General Formula CnH2n CnH2n−2 CnH2n+2 IUPAC name of the homologous series Characteristic bond type Single Bond IUPAC name of the first member of the series Type of reaction with chlorine Addition
What is the difference in the number of carbon and hydrogen atoms between two successive members of a homologous series? Also, give the difference in their atomic masses.
What is the difference between the mass of two successive members of a homologous series of hydrocarbons?
Give one example of homologous series, give two properties of it.
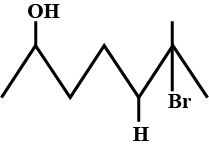
Name the following organic compounds :
The first homologue whose general formula is CnH2n.
Write names of first four homologous series of alcohols :
Match the reactions in List 1 with appropriate type of steps/reactive intermediates involved in these reactions as given in List 2.
Find product C in the given reaction.
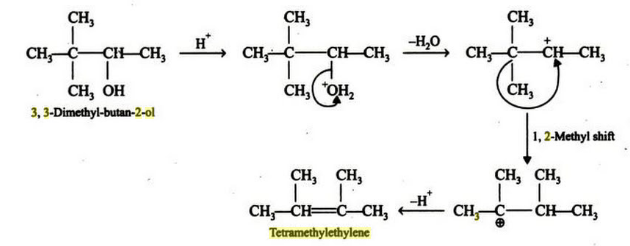
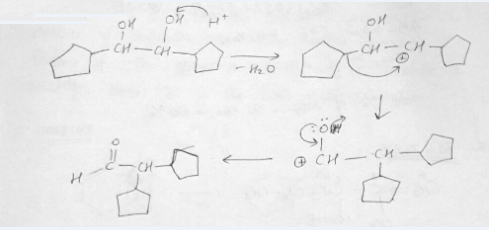
Write the mechanism of the following reaction:
3,3-Dimethylbutan- 2- ol loses a molecule of water in the presence of a concentrated sulphuric acid to give tetramethylethylene as a major product. Suggest a suitable mechanism.
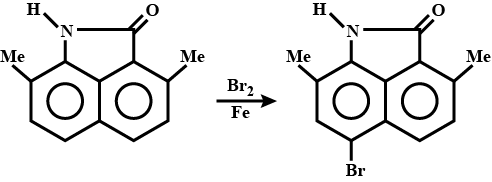
Identify the product.

Which of the following reactions will get affected by increase in pressure? Also mention whether the change will cause the reaction to go to the right or left direction.CH4(g)+2S2(g)⇌CS2(g)+2H2S(g)
CO2(g)+C(s)⇌2CO(g)
4NH3(g)+5O2(g)⇌4NO(g)+6H2O(g)
C2H4(g)+H2(g)⇌C2H6(g)
COCl2(g)⇌CO(g)+Cl2(g)
CaCO3(s)⇌CaO(s)+CO2(g)
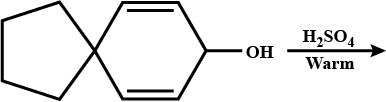
What is the major product of the following reaction?
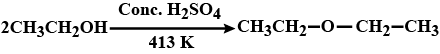
What is the Major product in the following reaction?
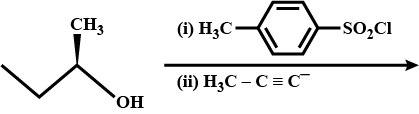
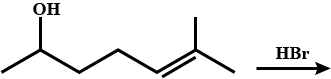
What is the major product in the above reaction?
What is the major product of the following reaction?
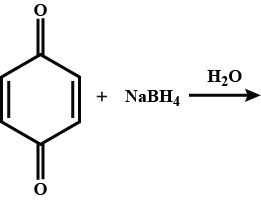
Trichloroethanol may be prepared by the direct reduction of chloral hydrate in water with sodium borohydride. Suggest a mechanism for this reaction. (Warning! Sodium borohydride does not displace hydroxide from carbon atoms!)

Identify the product B.
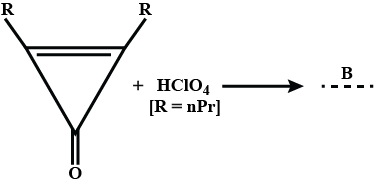
Visualize the formation of four particles in the given reaction.
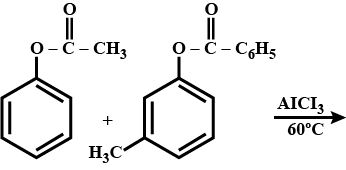
.
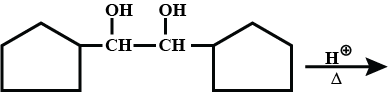
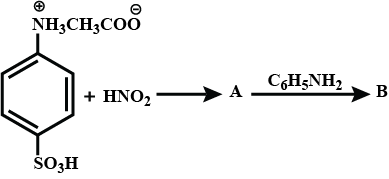
Find A and B?
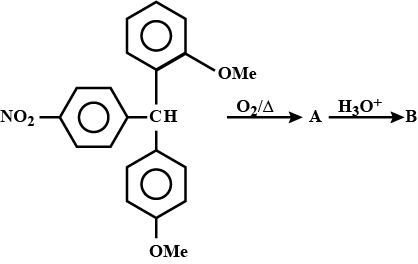
A,A is:
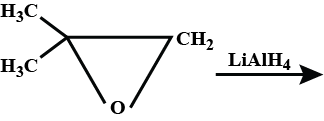
What is the product formed?
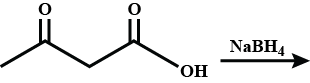
Product A and B formed in the following reactions are respectively.
(a) What is a homologous series? Explain with an example.
(b) State two characteristics of a homologous series.
(c) The molecular formula of an organic compound is C18H36. Name its homologous series.
(d) Select the hydrocarbons which belong to the same homologous series. Give the name of each series.
CH4,C2H2,C3H4,C2H6,C4H10,C3H4,C3H6.
(e) What is meant by 'heteroatom'? Give examples. Write the names and formulae of two organic compounds containing different heteroatoms.
Match each of the compounds given in Column I with the reaction(s), that they can undergo with one or or more statement(s), given in Column II.
2,2−Dimethyloxirane can be cleaved by acid (H⊕). Write the mechanism.
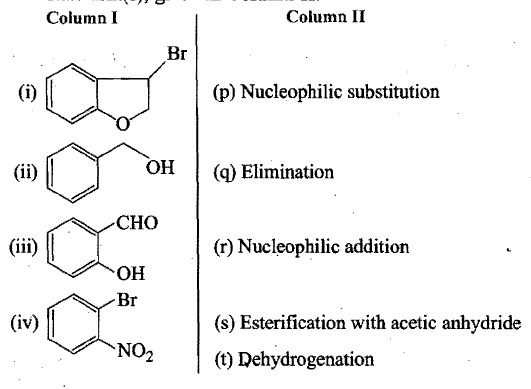
Match each of the compounds given in Column I with the reaction(s) they can undergo given in Column II.
What is the suitable adsorbent in the process of column chromography?
How do the boiling point and melting point change in the homologous series of alcohols?
What is the difference in the molecular formula of any two adjacent homologues:
in terms of molecular mass.
Give the names and the structural formula of the first three members of the homologous series of alkanes.
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions