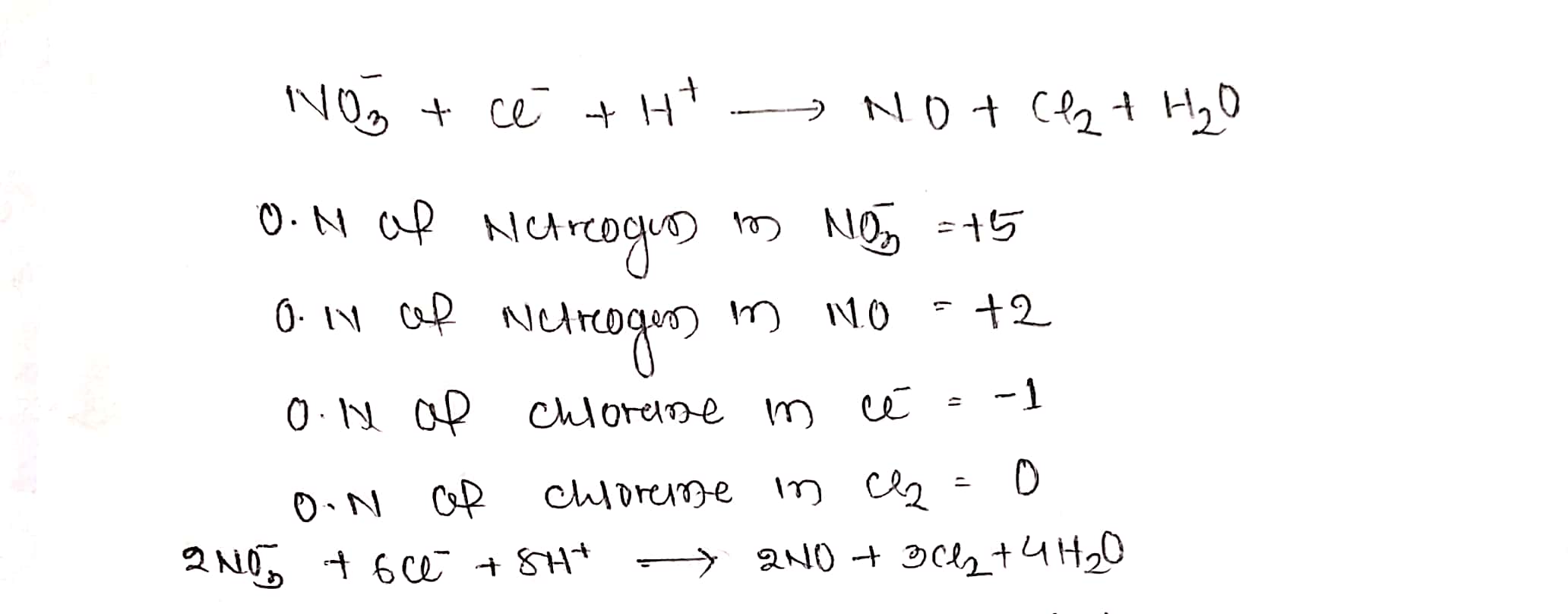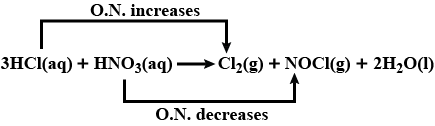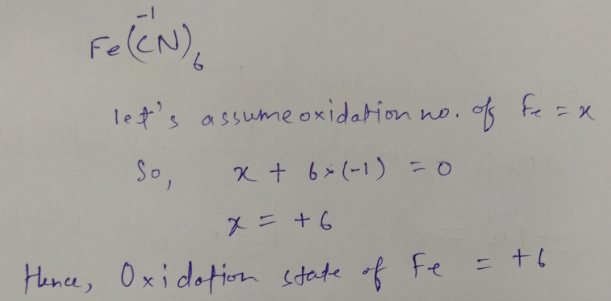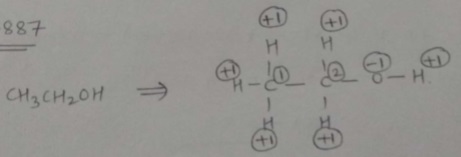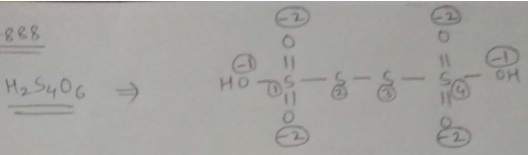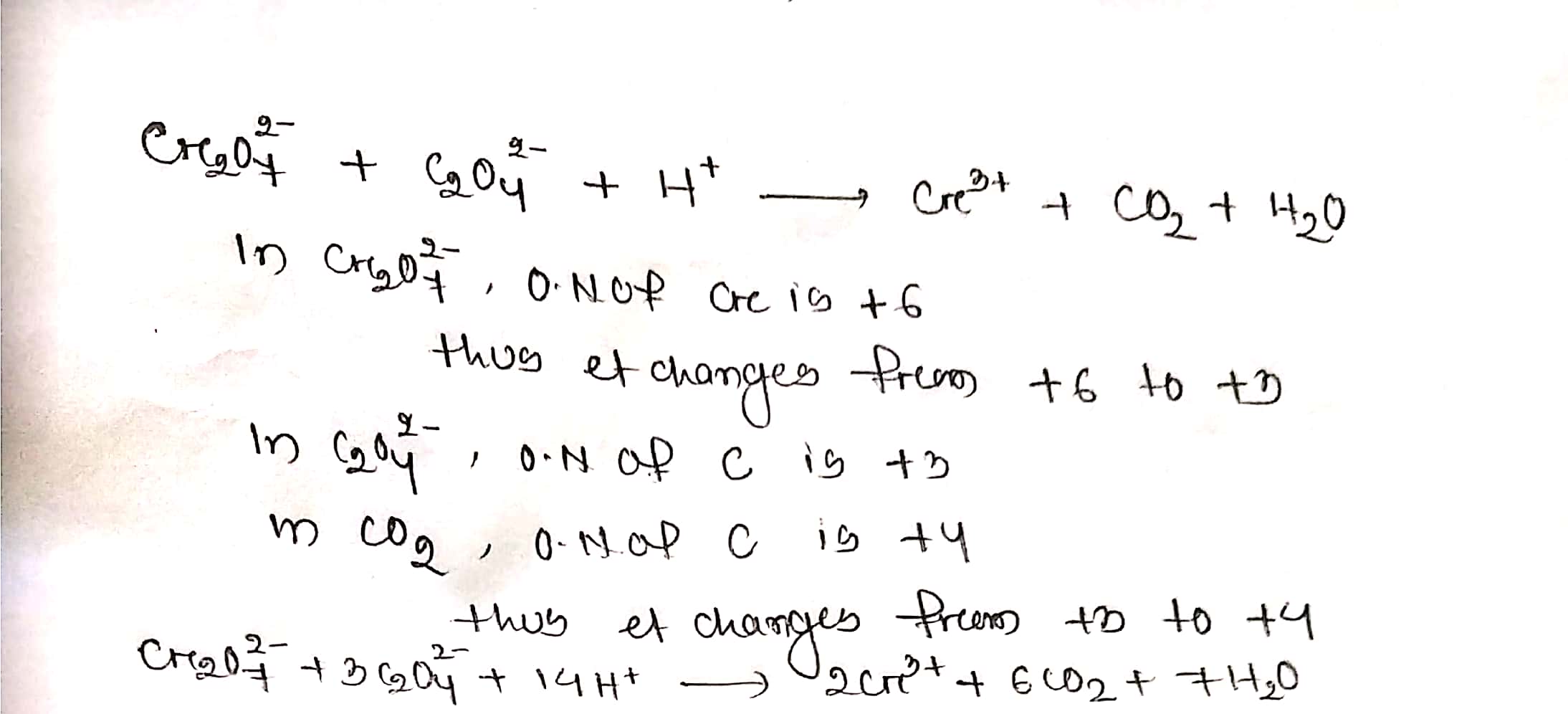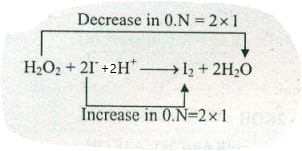Redox Reactions - Class 11 Engineering Chemistry - Extra Questions
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation
$$KMnO_{4}+H_{2}S+ H_{2}SO_{4}\to KHSO_{4}+MnSO_{4}+S+H_{2}O$$
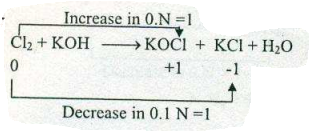
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation: $$ Cl_{2}+KOH \to KOCl + KCl +H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation
$$ NO_{3}^{-} +Cl^{-}+H^{+}\to NO+Cl_{2}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ Fe_{2}(SO_{4})_{3}+Fe\to FeSO_{4}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ Cu(NH_{3})_{4}Cl_{2}+ KCN+H_{2}O\to K_{2}Cu(CN)_{3}+NH_{3}+KCNO+NH_{4}Cl+KCl $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ Ag +KCN +H_{2}O+O_{2}\to KAg(CN)_{2}+KOH$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$Cr_{2}O_{7}^{2-}+NO_{2}^{-}+H^{+}\to Cr^{3+} + NO_{3}^{-}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ S_{2}O_{3}^{2-}+ Sb_{2}O_{5}+H^{+}+H_{2}O\to SbO+H_{2}SO_{3}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$Zn+NO_{3}^{-}+H^{+}\to Zn^{2+}+NO_{2}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ AsO_{3}^{3-}+ IO_{3}^{-} \to AsO_{4}^{3-} +I^{-}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ MnO_{4}^{-}+CN^{-}+H_{2}O \to MnO_{2}+CNO^{-}+OH^{-}$$
Both $${VO}_{2}^{+}$$ and $${CO}^{2+}$$ are known as vanadyl ion
(a) Determine the oxidation number of vanadium in each
(b) Which one of them is oxovanadium(iv) ion and which are in dioxovanadium (v) ion
Write the balanced chemical equation for the following reaction and identify the type of reaction :
Ethene is burnt in presence of oxygen to form carbon dioxide , water and releases heat and light.
Calculate the oxidation number of sulphur atom in $$\mathrm{Na}_{2} \mathrm{SO}_{4} $$.
Calculate the oxidation number of each sulphur atom in $$ \mathrm{Na}_{2} \mathrm{S}_{2} \mathrm{O}_{3}$$.
Identify the oxidising and reducing agents in
$$ 3 H C l(a q)+H N O_{3}(a q) \rightarrow C l_{2}(g)+N O C l(g)+2 H_{2} O(l) $$
Calculate the oxidation number of phosphorus in $$ P O_{4}^{3-} $$.
Calculate the oxidation number of sulphur atom in $$ \mathrm{Na}_{2} \mathrm{SO}_{3} $$.
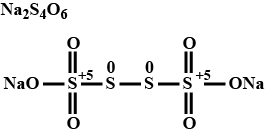
Calculate the oxidation number of each sulphur atom in $$ \mathrm{Na}_{2} \mathrm{S}_{4} \mathrm{O}_{6} $$.
Calculate the oxidation number of phosphorus in the following species.
$$HPO_{3}^{2-}$$
Calculate oxidation number of $$S$$ in $$H_2S_2O_7$$.
Calculate oxidation number of $$Fe$$ in $$Fe(CO)_5$$.
Calculate oxidation number of $$P$$ in $$H_3PO_3$$.
Calculate oxidation number of the following compounds:
$$Fe$$ in $$FeSO_4$$
Calculate oxidation number of the following compounds:
$$P$$ in $$NaH_2PO_2$$
Complete the table
| Compound | Oxidation state of $$Fe$$ | Symbol of $$Fe$$ ions |
| $$FeCl_2$$ | ||
| $$FeCl_3$$ |
Calculate oxidation number of the following compounds:
$$C$$ in $$C_{12}H_{22}O_{11}$$
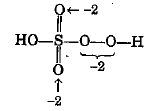
Oxidation number of sulphur in $${ H }_{ 2 }{ SO }_{ 5 }$$ is $$+6$$. If true enter 1 else 0.
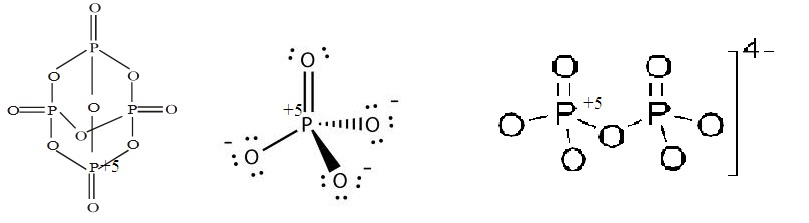
The oxidation number of phosphorus in $$PO^{3-}_4, P_4O_{10}$$ and $$P_2O_7^{4-}$$ respectively, is :
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ K_{2}Cr_{2}O_{7}+ HCl \to KCl+CrCl_{3}+H_{2}O+Cl_{2}$$
The oxidation state of chromium in the final product formed in the reaction between KI and acidified potassium dichromate solution is :
$$1$$ mole of $${ N }_{ 2 }{ H }_{ 4 }$$ loses $$10$$ mol of electrons to form a new compound $$Y$$. Assuming that all the nitrogen appear in the new compound, what is the oxidation state of nitrogen in $$Y$$? (no change in the oxidation state $$H$$).
Consider the elements $$Cs,\ Ne,\ I$$ and $$F$$.Identify the element that exhibits only negative oxidation state.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ N_{2}O_{4}+BrO_{3}^{-} +H_{2}O\to NO_{3}^{-}+Br^{-}+H^{+}$$
What is the oxidation state of Fe in $$ Fe(CN)_6 $$ ?
What is oxidation number of carbon in $$CN^-$$?(Give the absolute value)
Calculate the oxidation number of $$Mn$$ in the product obtained on an alkaline oxidative fusion of $$MnO_2$$.(Give the absolute value of an answer)
Calculate the oxidation number of $$Mn$$ in the product formed on strongly heating $$Mn_2O_7$$.(write absolute value of an answer)
The change in oxidation number of the underlined element in the reaction given below is__________.
$$NaH\underline { C } O_{ 3 }+HCl\rightarrow NaCl+H_{ 2 }O+CO_{ 2 }$$
The compound $${ \left( { NH }_{ 3 } \right) }_{ 3 }Cr{ O }_{ 4 }$$ has peroxy linkage if oxidation number of $$Cr$$ is $$+4$$.
If true enter 1, else enter 0.
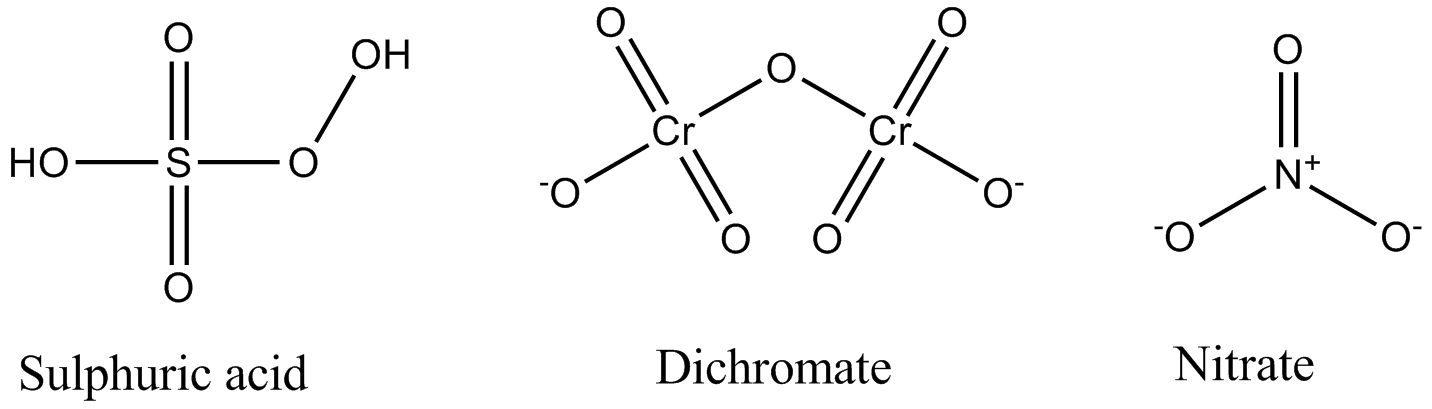
Calculate the oxidation number of sulphur, chromium and nitrogen in $$H_2SO_5\,,Cr_2O^{2-}_7$$ and $$NO_3^-$$. Suggest structure of these compounds. Count for the fallacy.
Which reaction is this?$$CaO + H_{2}O \rightarrow Ca(OH)_{2}$$
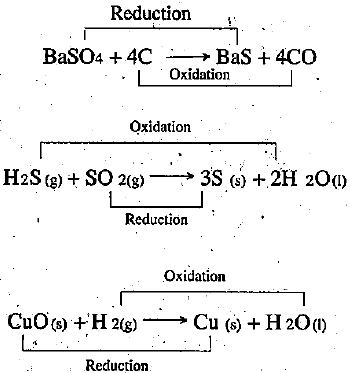
What are redox reactions? Identify the substances that are oxidised and the substances that are reduced in the following reactions?
A. $$2H_2S_{(g)} + SO_{2(g)} \rightarrow 3S_{(s)} + 2H_2O_{(1)} $$
B. $$CuO_{(s)} + H_{2(g)}\rightarrow Cu_{(s)} +H_2O$$
Find the oxidation number of $$S$$ in $${SO_4}^{2-}$$ ion?
What is the oxidation number of $$Cr$$ in $$K_2Cr_2O_7$$?
Arrange the following in the order of increasing oxidation number of iodine: $$I_2, HI, HIO_4, ICl$$.
Find the oxidation number of I in $$KIO_3$$.
Find the oxidation number of Xe in $$BaXeO_6$$.
Calculate the oxidation state of the following.
$$C$$ in diamond
Find out oxidation states of phosporus in $${PO_4}^{3-}, P_4O_{10}$$ and $${P_2O_7}^{4-}$$.
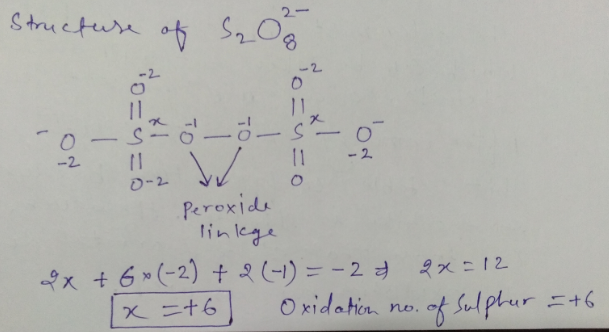
Oxidation number of Sulphur in $$ S_2 O_8^{-2}$$ ?
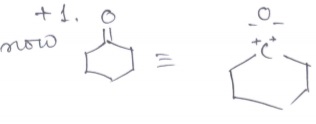
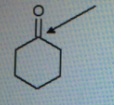
Find out the oxidation state of shown carbonyl carbon.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation
$$ Cr(OH)_{3}+IO_{3}^-+OH^{-}\to I^{-}+CrO_{4}^{2-}+ H_{2}O$$
(i) Calculate oxidation number of $$Cl$$ in $$Cl_{2}O_{7}$$.
(ii) $$2FeCl_{3} + H_{2}S \rightarrow 2FeCl_{2} + 2HCl + S$$
Write the name of oxidising agent and reducing agent in above reaction:
What is the oxidation number of oxygen in oxide, peroxide and superoxide?
The $$Mn^{3+}$$ ion is unstable in solution and undergoes disproportional reaction to give $$Mn^{2+}, MnO_2$$ and $$H^+$$. Write a balanced ionic equation for the reaction.
Balance the following redox reaction.$$MnO^{\ominus}_4(aq)+I^{\ominus}(aq)\rightarrow MnO_2(s)+I_2(s)$$.What is the coefficient of $$I^-$$?
What is the oxidation number of nitrogen in $$NH_2OH$$?
Find oxidation number each carbon atom separately in $$CH_{3}CH_{2}OH$$.
What is the oxidation number of C in HNC?
Find oxidation number of Sulphur in $$H_{2}S_{4}O_{6}$$.
Find oxidation number of Carbon in $$C_{3}O_{2}$$.
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$K_2Cr_2O_7$$
$$NaHCO_3$$
Identify the substance oxidized,substance reduced ,oxidizing agent and reducing agent :
$$MnO_2+4HCl\rightarrow MnCl_2+2H_2O+Cl_2$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ P+HNO_{3}\to HPO_{3}+NO+H_{2}O$$
Assign oxidation number to atoms of only those elements which undergo O. No. change in the following redox reactions and then balance the equation
$$ Cr_{2}O_{7}^{2-}+C_{2}O_{4}^{2-}+H^{+}\to Cr^{3+}+CO_{2}+H_{2}O$$
$$ 12 .53 \mathrm{cm}^{3} $$ of $$ 0.051 \mathrm{M} \mathrm{SeO}_{2} $$ reactsexactly with $$ 25 \cdot 5 \mathrm{cm}^{3} $$ of $$ 0 \cdot 1 \mathrm{M} $$ CrSO $$ _{4} $$ which is oxidised to $$ \mathrm{Cr}_{2}\left(\mathrm{SO}_{4}\right)_{3} . $$ To what oxidation state is the selenium converted during the reaction?
Match the reaction in List 1 with the colour at the final stage in List 2.
A volume of $$12.5$$ mL,$$0.05$$ M $$SeO_{2}$$ reacts with $$25$$ mL of $$ CrSO_{4}$$ which is oxidised to $$0.1$$M $$Cr^{3-}$$.
The oxidation state at which selenium is converted by the reaction is_________.
$$1$$ mol of $${ N }_{ 2 }{ H }_{ 4 }$$ loses $$10$$ mol of electrons to form a new compound $$Y$$. Assuming that all the nitrogen appear in the new compound, what is the oxidation state of nitrogen in $$Y$$ (no change in the oxidation state of $$H$$)?
Match the different compounds of $$Mn$$ (in List I) with oxidation number (in List II).
State True or False.
$$H{NO}_{3}$$ is added in $$II$$ group. filtrate during basic radical analysis to convert $${Fe}^{2+}$$ to $${Fe}^{3+}$$ as well as to oxidise $${H}_{2}S$$ in solution.
Enter 1 if true else 0.
Match the half-reactions (in List I) with change in oxidation number (in List II).
Match list 1 containing compounds with list 2 containing oxidation states of nitrogen and select answer using the codes given below the lists.
Code $$\colon\,\,(a)\,\,(b)\,\,(c)\,\,(d)$$
$$(A)\,\,\,\,\,\,\,\,\,\,3\,\,\,\,\,\,4\,\,\,\,\,\,2\,\,\,\,\,\,1$$
$$(B)\,\,\,\,\,\,\,\,\,\,4\,\,\,\,\,\,3\,\,\,\,\,\,2\,\,\,\,\,\,1$$
$$(C)\,\,\,\,\,\,\,\,\,\,3\,\,\,\,\,\,4\,\,\,\,\,\,1\,\,\,\,\,\,2$$
$$(D)\,\,\,\,\,\,\,\,\,\,4\,\,\,\,\,\,3\,\,\,\,\,\,1\,\,\,\,\,\,2$$
Suggest a list of the substances where carbon can exhibit oxidation states from -4 to +4 and nitrogen from -3 to +5.
Write Nearst equation and explain the terms involved in it. What part of the equation represent the correction factor for nonstandard state conditions?
Balance equation with oxidation number of method.
$${P}_{4} + {OH}^{-}\rightarrow {PH}_{3} + {HPO}_{2}^{-}$$
In Column I, some reactions are given and in Column II, the type of some reactions on the basis of oxidation and reduction processes are given. Match the reactions with their correct type.
Select the type of redox reaction from the following on the basis of type of redox changes (a) intermolecular redox; (b) intramolecular redox; (c) auto redox. If none, write none.
(a) $${C}_{6}{H}_{5}CHO\xrightarrow [ ]{ NaOH } {C}_{6}{H}_{5}{CH}_{2}OH+{C}_{6}{H}_{5}COONa$$
(ii) $${Cr}_{2}{O}_{7}^{2-}+2{OH}^{-}\longrightarrow 2Cr{O}_{4}^{2-}+{H}_{2}O$$
(iii) $$2{Mn}_{2}{O}_{7}\longrightarrow 4Mn{O}_{2}+3{O}_{2}$$
(iv) $${NO}_{3}^{-}+{H}_{2}S+{H}_{2}O+{H}^{+}\longrightarrow {NH}_{4}^{+}+H{SO}_{4}^{-}$$
(v) $$Fe+{N}_{2}{H}_{4}\longrightarrow {NH}_{3}+Fe{(OH)}_{2}$$
(vi) $$2KOH+{Br}_{2}\longrightarrow KBr+KBrO$$
(vii) $$2{Cu}^{+}\longrightarrow Cu+{Cu}^{2+}$$
(viii) $$Ag{({NH}_{3})}_{2}^{+}\xrightarrow [ ]{ 2{ H }^{ + } } {Ag}^{+}+2{NH}_{4}^{+}$$
(ix) $$5KI+KI{O}_{3}+6HCl\longrightarrow 3{I}_{2}+6KCl+3{H}_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$H_{2}O_{2}+ 2I^{-}+2H^{+}\to I_{2}+2H_{2}O$$
Match the reactions in column I with nature of reaction / type of the products in column II.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation.
$$ C_{2}H_{5}OH + Cr_{2}O_{7}^{2-}+ H^{+} \to Cr^{3+}+C_{2}H_{4}O + H_{2}O $$
Calculate the oxidation number of Mn in the product of alkaline oxidative fusion of $$MnO_2$$.
$$25 \,mL$$ of $$0.017M \,H_2SO_3$$ in strongly acidic medium required $$16.9 \,mL$$ of $$0.01 \,MKMnO_4$$ and in neutral medium required $$28.6 \,mL$$ of $$0.01M \,KMnO_4$$ for complete conversion of $$SO_3^{2-}$$ to $$SO_4^{2-}$$. Assign the oxidation no. of Mn in the product formed in each case.
On adding dilute $$HCl$$ to $$CuO$$ powder, the solution formed is blue-green. Predict the new compound formed which imparts a blue-green color to the solution.
Classify the given chemical reactions in the table below.
a) $$Mg + O_2 \rightarrow 2MgO$$
b) $$H_2 + I_2 \rightarrow 2HI$$
c) $$2H_2O \rightarrow 2H_2 + O_2$$
d) $$NaCl + AgNO_3 \rightarrow AgCl + NaNO_3$$
e) $$ZnSO_4 + BaCl_2 \rightarrow BaSO_4 + ZnCl_2$$
f) $$Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2$$
g) $$FeSO_4 + Zn \rightarrow ZnSO_4 + Fe$$
h) $$CaCO_3 \rightarrow CaO + CO_2$$
What happens when:
(a) copper powder is heated in china dish?
(b) hydrogen gas is passed over hot copper (II) oxide?
Find out the oxidation number of chlorine in the following compounds and arrange
them in increasing order of oxidation number of chlorine.
$$NaClO_{4}, NaClO_{3}, NaClO, KClO_{2}, Cl_{2}O, ClO_{3}, Cl_{2}O, NaCl, Cl_{2}, ClO_{2}$$
Which oxidation state is not present in any of the above compounds?
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions