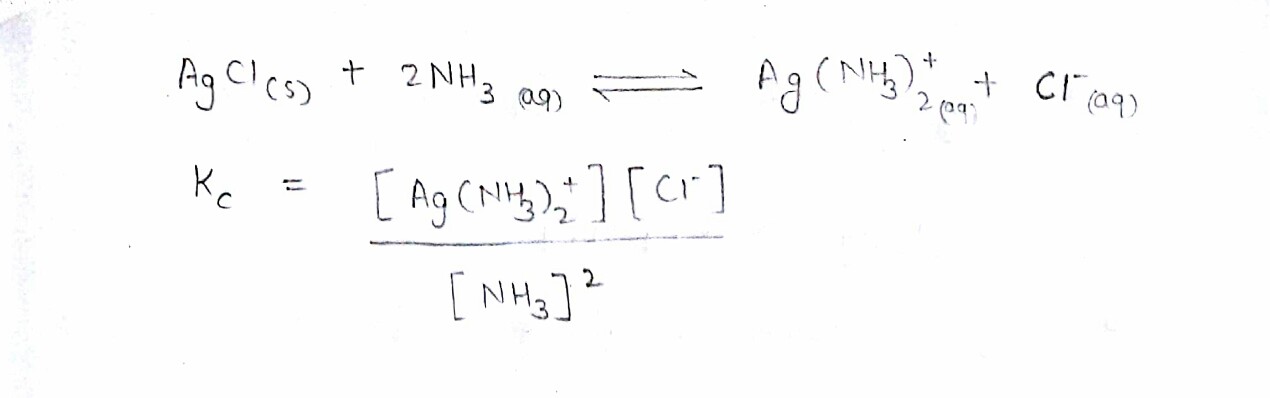Equilibrium - Class 11 Engineering Chemistry - Extra Questions
An endothermic reaction which proceeds with decrease in volume will give maximum yield of the products at high $$P$$ and high $$T$$ .
If true enter 1, if false enter 0.
An exothermic reaction which proceeds with decrease in volume will give maximum yield at high $$P$$ and low $$T$$.
If true enter 1, if false enter 0.
The solubility of a solute decrease with temperature when dissolution is exothermic.
If true enter 1, if false enter 0.
Addition of an inert gas at constant volume to the equilibrium mixture does not influence the equilibrium.
If true enter 1, if false enter 0.
For a reversible system at constant temperature, the value of $${K}_{c}$$ increases if the concentrations are changed at equilibrium.
If true enter 1, if false enter 0.
The degree of dissociation of $$PC{l}_{5}$$ of decreases with increase in pressure.
If true enter 1, if false enter 0.
When a liquid and its vapours are at equilibrium and the pressure is suddenly decreased, cooling occurs.
If true enter 1, if false enter 0.
Solubility of $$NaOH$$ increases with increase in temperature.
If true enter 1, if false enter 0.
The equilibrium constant does not depend on the initial concentration of reactants but depends on concentration of various species at equilibrium .
If true enter 1, if false enter 0.
Le Chatelier's principle finds extensive application in predicting the conditions for getting maximum yield of products in reactions of industrial importance.
If true enter 1, if false enter 0.
High pressure is favourable for those reversible reactions in which there is decrease in the number of molecules.
If true enter 1, if false enter 0.
The equilibrium constants for the reaction, $${ N }_{ 2 }(g)+{ O }_{ 2 }(g)\leftrightharpoons 2NO(g)$$ at $${ 1727 }^{ o }C$$ and $${ 2227 }^{ o }C$$ are $$4.08\times { 10 }^{ -4 }$$ and $$3.6\times {10}^{-3}$$ respectively. Calculate the enthalpy change for the reaction. (Given $$R=1.987\ cal\ mol^{-1} K^{-1}$$)
The value of equilibrium constant of a reaction depends upon the initial values of concentration of reactants.
If true enter 1, else enter 0.
The reaction : $$CaC{ O }_{ 3 }\left( g \right) \rightleftharpoons CaO\left( s \right) +C{ O }_{ 2 }\left( g \right)$$, would proceed to completion if the reaction vessel is connected to bottle containing $$KOH$$ solution or carried out in open vessel.
If true enter 1, if false enter 0.
The dissociation of $$CaC{O}_{3}$$ is suppressed at high pressure.
If true enter 1, if false enter 0.
In an equilibrium reaction, the concentration of reactants decreases initially and then becomes constant.
If true enter 1, if false enter 0.
In a reversible reaction, some amount of heat energy is liberated in the forward reaction. Name the reaction. What change in temperature favours the forward reactions?
What is the effect of pressure on the equilibrium of the nitrogen and oxygen to give nitric oxide?
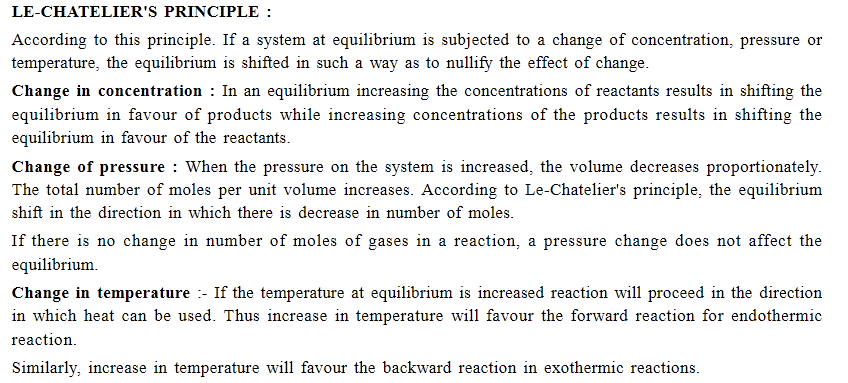
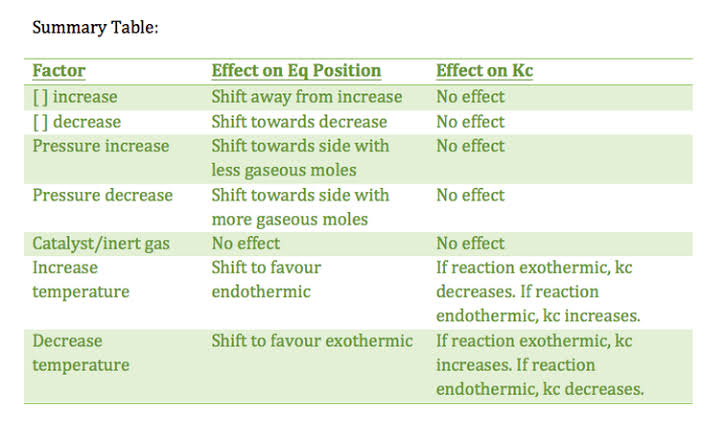
State the Le Chatelier's principle?
State Le Chatelier's principle.
State Le-chatlier's principle.
Which measurable property becomes constant in water $$\rightleftharpoons $$ water vapour equilibrium at constant temperature?
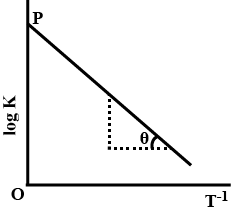
Variation of equilibrium constant $$K$$ with temperature $$T$$ is given by van't Hoff equation:
$$\log { K } =\log { A } -\cfrac { \Delta { H }^{ o } }{ 2.303RT } $$
A graph between $$\log { K } $$ and $${T}^{-1}$$ was a straight line as shown in the figure and having $$\theta =\tan ^{ -1 }{ (-0.5) } $$ and $$OP=10$$. Calculate:
(a) $$\Delta { H }^{ o }$$ (Standard heat of reaction) when $$T=298K$$
(b) $$A$$ (pre-exponential factor)
(c) Equilibrium constant $$K$$ at $$298K$$
(d) $$K$$ at $$798K$$, if $$\Delta { H }^{ o }$$ is independent of temperature
Match the reaction equilibrium from List I with the factors which can affect it from List II.
Explain the effect of change in concentrations on the following equillibria:
a) $$PCl_{ 5(g) }\rightleftharpoons PCl_{ 3(g) }{ Cl }_{ 2(g) }$$
b) $$2SO_{ 2(g) }+{ O }_{ 2(g) }\rightleftharpoons 2SO_{ 3(g) }$$
The $$K_p$$ for the reaction; $$H_2+I_2\rightleftharpoons 2HI$$, at $$460^{o}C$$ is $$49$$. If the initial pressure of $$H_2$$ and $$I_2$$ is $$0.5\ atm$$ respectively, determine the partial pressure of each gas at equilibrium.
Why does a gas fizz when a soda water bottle is opened?
(a) What would be the effect of an increase in pressure on following equilibria:
(i) $$CO(g) + 2H_2(g)\rightleftharpoons CH_3OH(g)$$
(ii) $$2HBr(g)\rightleftharpoons H_2(g)+Br_2(g)$$
(iii)$$CO_2(g) + C(s)\rightleftharpoons 2CO(g)$$
(iv)$$COCI_2(g)\leftrightarrow CO(g)+CI_2(g)$$
(b)In above cases if temperature is increased then what should be the direction o f reaction
In a gaseous reaction, $$A_{(g)} + B_{(g)} \rightleftharpoons C_{(g)}$$. Predict the effect of addition of inert gas if addition is made at (a) constant volume, (b) constant pressure.
How do the following help in bringing about a chemical change?
Pressure
What is the effect of increasing pressure on the equilibrium?
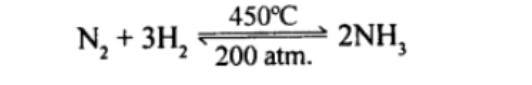
$$N_2 + 3H_2 \overset{\rightharpoonup}{\leftharpoondown} \ 2NH_3 \ ?$$
Name the factors that affect equilibrium position of a reversible reaction.
Under what conditions the synthesis of $$SO_3$$ is more ? Explain.
What will be the effect on equilibrium when $$\Delta \ n_g$$ is negative and pressure is decreased?
Which of the following reactions will get affected by increasing the pressure ? Also, mention whether change will cause the reaction to go into forward or backward direction.
(i) $$COCl_2(g) \rightarrow CO(g) + Cl_2(g)$$
(ii) $$CH_4(g) + 2H_2S(g) \rightarrow CS_2(g) + 4H_2(g)$$
(iii) $$CO_2(g) + C(s) \rightarrow 2CO(g)$$
(iv) $$2H_2(g) + CO(g) \rightarrow CH_3OH(g)$$
(v) $$CaCO_3(s) \rightarrow CaO(s) + CO_2(g)$$
(vi) $$4NH_3(g) + SO_2(g) \rightarrow 4NO(g) + 6H_2O(g)$$
For the system, $$N_2 + O_2 \rightarrow 2NO $$, -44 kcal, at equilibrium, what will be the effect of the following:
(i) Increasing the temperature
(ii) Decreasing the pressure
(iii) Increasing the concentration of NO
(iv) Presence of catalyst
Describe the factors which affect chemical equilibrium?
Write the examples of the reaction in which
(a) product increases on increasing the pressure.
(b) product increases on increasing the temperature.
The equilibrium for the synthesis of ammonia is
$$N_2 + 3H_2 \rightleftharpoons NH_3 + x kJ$$,
What is the effect of pressure, temperature and concentration on this equilibrium?
What will be the effect of temperature and pressure on the following equilibrium?
$$N_2(g) + 3H_2(g) \rightarrow 2NH_3(g)$$
What is the effect of reducing the volume on the system described below?
$$H_{2}(g)+I_{2}(g)\rightleftharpoons 2HI(g)+$$ Heat
The following circumstances influence the reaction
Increase the temperature.
$$H_{2}(g)+I_{2}(g)\rightleftharpoons 2HI(g)+$$ Heat
The following circumstances influence the reaction
Increase the pressure
At normal temperature and pressure, 5.29mL hydrogen reacts with 0.040 gm iodine at $$444^0C$$ and gives 6.35 mL hydrogen iodide. Calculate equilibrium constant for the synthesis of HI at this temperature.
A process is given below. What happens to the process if it subjected to a change given in the brackets?
$$N_2(g)+O_2(g) \rightleftharpoons 2NO(g)-180.7\ kJ$$ ( Pressure is increased and temperature is decreased).
The following reaction has attained equilibrium
$$CO(g) +2H_2(g) \rightleftharpoons CH_3OH(g), \Delta H^o=-92.0\ kJ\ mol^{-1}$$
What will happen if Volume of the reaction vessel is suddenly reduced to half?
$$H_{2}(g)+I_{2}(g)\rightleftharpoons 2HI(g)+$$ Heat
The following circumstances influence the reaction
Increase the concentration of $$H_{2}$$.
The following reaction has attained equilibrium
$$CO(g) +2H_2(g) \rightleftharpoons CH_3OH(g), \Delta H^o=-92.0\ kJ\ mol^{-1}$$
What will happen if the partial pressure of hydrogen is suddenly doubled?
Hydrogen gas is obtained from natural gas by partial oxidation with steam according to the following endothermic reaction
$$CH_4(g)+H_2O(g) \rightleftharpoons CO(g)+3H_2(g)$$
How will the value of $$K_p$$ and composition of the equilibrium mixture be affected by increasing the pressure?
Hydrogen gas is obtained from natural gas by partial oxidation with steam according to the following endothermic reaction
$$CH_4(g)+H_2O(g) \rightleftharpoons CO(g)+3H_2(g)$$
How will the value of $$K_p$$ and composition of the equilibrium mixture be affected by increasing the temperature?
The following system is in equilibrium
$$SO_2Cl_2+Heat \rightleftharpoons SO_2Cl_2$$, What will happen to the temperature of the system if some $$Cl_2$$ is added into at constant volume? Give reason.
List I and List II contain four entries each. Entries of List I are to be matched with entries of List II. One or more than one entries of List I may match with the same entry of List II.
List I and List II contains four entries each. Entries of List I are to be matched some entries of List II. One or more than one entries of List I may have the match with the same entry of List II.
Column I and Column II contains four entries each.Entries of Column I are to be matched some entries of Column II.One or more than one entries of Column I may have the matching with the same entries of Column II.
List I and List II contains four entries each. Entries of List I are to be matched some entries of List II. One or more than one entries of List I may have the match with the same entry of List II.
List I and List II contains four entries each. Entries of List I are to be matched some entries of List II. One or more than one entries of List I may have the match with the same entry of List II.
Statement: The equilibrium mole obtained during the experiment for the given reaction in 1 L vessel is shown below the reactants and products.
$$2A\left(g\right) + B\left(g\right) \rightleftharpoons 3C\left(g\right) + 3D$$
At equilibrium: 8 mol 16 mol 4 mol 4 mol
If the reaction in the forward direction is to be made, the initial concentration of reactants and products should be such that the reaction quotient should be less than four.
If the given statement is true enter 1 if false enter 0.
If the reaction in the forward direction is to be made, the initial concentration of reactants and products should be such that the reaction quotient should be less than four.
A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased.
How do rates of evaporation and condensation change initially?
A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased.
What is the initial effect of the change on vapour pressure?
A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased.
What happens when equilibrium is restored finally and what will be the final vapour pressure?
Match the column. The codes for the lists have choices $$(A),\,(B),\,(C)\; and\;(D)$$ out of which only ONE is correct.
At 473 K, equilibrium constant $$\displaystyle { K }_{ c }$$ for decomposition of phosphorus pentachloride, $$\displaystyle { PCl }_{ 5 }$$ is $$\displaystyle 8.3\times { 10 }^{ -3 }$$. If decomposition is depicted as,
$$\displaystyle { PCl }_{ 5 }\left( g \right) \rightleftharpoons { PCl }_{ 3 }\left( g \right) +{ Cl }_{ 2 }\left( g \right) $$ $$\displaystyle { \Delta }_{ r }{ H }^{ \ominus }=124.0kJ{ mol }^{ -1 }$$
(a) Write an expression for $$\displaystyle { K }_{ c }$$ for the reaction.
(b) What is the value of $$\displaystyle { K }_{ c }$$ for the reverse reaction at the same temperature?
(c) What would be the effect on $$\displaystyle { K }_{ c }$$ if (i) more $$\displaystyle { PCl }_{ 5 }$$ is added (ii) pressure is increased (iii) the temperature is increased ?
Dihydrogen gas is obtained from natural gas by partial oxidation with steam as per following endothermic reaction:
$$\displaystyle { CH }_{ 4 }\left( g \right) +{ H }_{ 2 }O\left( g \right) \rightleftharpoons CO\left( g \right) +3{ H }_{ 2 }\left( g \right) $$
(a) Write as expression for $$\displaystyle { K }_{ p }$$ for the above reaction.
(b) How will the values of $$\displaystyle { K }_{ p }$$ and composition of equilibrium mixture be affected by
(i) increasing the pressure,
(ii) Increasing the temperature and
(iii) Using a catalyst?
$$\displaystyle { CH }_{ 4 }\left( g \right) +{ H }_{ 2 }O\left( g \right) \rightleftharpoons CO\left( g \right) +3{ H }_{ 2 }\left( g \right) $$
(a) Write as expression for $$\displaystyle { K }_{ p }$$ for the above reaction.
(b) How will the values of $$\displaystyle { K }_{ p }$$ and composition of equilibrium mixture be affected by
(i) increasing the pressure,
(ii) Increasing the temperature and
(iii) Using a catalyst?
Which of the following reactions will get affected by increasing the pressure? Also, mention whether change will cause the reaction to go into forward or backward direction.
(i) $$\displaystyle { COCl }_{ 2 }\left( g \right) \rightleftharpoons CO\left( g \right) +{ Cl }_{ 2 }\left( g \right) $$
(ii) $$\displaystyle { CH }_{ 4 }\left( g \right) +2{ S }_{ 2 }\left( g \right) \rightleftharpoons { CS }_{ 2 }\left( g \right) +2{ H }_{ 2 }S\left( g \right) $$
(iii) $$\displaystyle { CO }_{ 2 }\left( g \right) +C\left( s \right) \rightleftharpoons 2CO\left( g \right) $$
(iv) $$\displaystyle 2{ H }_{ 2 }\left( g \right) +CO\left( g \right) \rightleftharpoons { CH }_{ 3 }OH\left( g \right) $$
(v) $$\displaystyle { CaCO }_{ 3 }\left( s \right) \rightleftharpoons CaO\left( s \right) +{ CO }_{ 2 }\left( g \right) $$
(vi) $$\displaystyle 4{ NH }_{ 3 }\left( g \right) +5{ O }_{ 2 }\left( g \right) \rightleftharpoons 4NO\left( g \right) +6{ H }_{ 2 }O\left( g \right) $$
Does the number of moles of reaction products increase, decrease or remain same when each of the following equilibria is subjected to a decrease in pressure by increasing the volume?
(a) $$\displaystyle { PCl }_{ 5 }\left( g \right) \rightleftharpoons { PCl }_{ 3 }\left( g \right) { Cl }_{ 2 }\left( g \right) $$
(b) $$\displaystyle CaO\left( s \right) +{ CO }_{ 2 }\left( g \right) \rightleftharpoons { CaCO }_{ 3 }\left( s \right) $$
(c) $$\displaystyle 3Fe\left( s \right) +4{ H }_{ 2 }O\left( g \right) \rightleftharpoons { Fe }_{ 3 }{ O }_{ 4 }\left( s \right) +4{ H }_{ 2 }\left( g \right) $$
State Le-chatelier's principle and apply it to the following equilibrium.
$$N_{2_{(g)}} + 3H_{2_{(g)}} \rightleftharpoons 2NH_{3_{(g)}}; \triangle H = -92.0\ KJ$$
State Le Chatelier's principle and apply it to the following equilibrium.
$$2SO_{2_{(g)}} + O_{2_{(g)}} \rightleftharpoons 2SO_{3_{(g)}}; \triangle H = -189 k.J$$
When $$3.06\ g$$ of $${ NH }_{ 4 }HS(s)$$ is introduced into a $$two\ litre$$ evacuated flask at $${27}^{o}C$$, $$30$$% of the solid decomposes into gaseous ammonia and hydrogen sulphide.
(i) Calculate $${K}_{c}$$ and $${K}_{p}$$ for the reaction at $${27}^{o}C$$.
(ii) What would happen to the equilibrium when more $${ NH }_{ 4 }HS(s)$$ is introduced into the flask?
For the reaction, $$S{ O }_{ 2 }(g)+\cfrac { 1 }{ 2 } { O }_{ 2 }(g)\rightleftharpoons { SO }_{ 3 }(g)$$, $${K}_{p}$$ is $$32\ atm^{ -1/2 }$$ at $$800K$$ and $$\Delta H$$ for the reaction is $$-187.9kJ$$ $${mol}^{-1}$$. Calculate its value at $$900K$$, if it is assumed that $$\Delta H$$ remains constant over this range of temperature.
Write the effect of temperature pressure and concentration on the following reaction $$2SO_{2}+O_{2} \rightleftharpoons 2So_{3};\Delta H=-198\ kJ/mol$$
In a gaseous reaction; $$A_{(g)} + B_{(g)} \rightleftharpoons C_{(g)} + D_{(g)}$$, the increase in temperature causes the change in the concentrations of $$A, B, C$$ and $$D$$. The concentrations of $$C$$ and $$D$$ also change on addition of some amount of $$A$$. Does the value of $$K$$ change in either of the two situations?
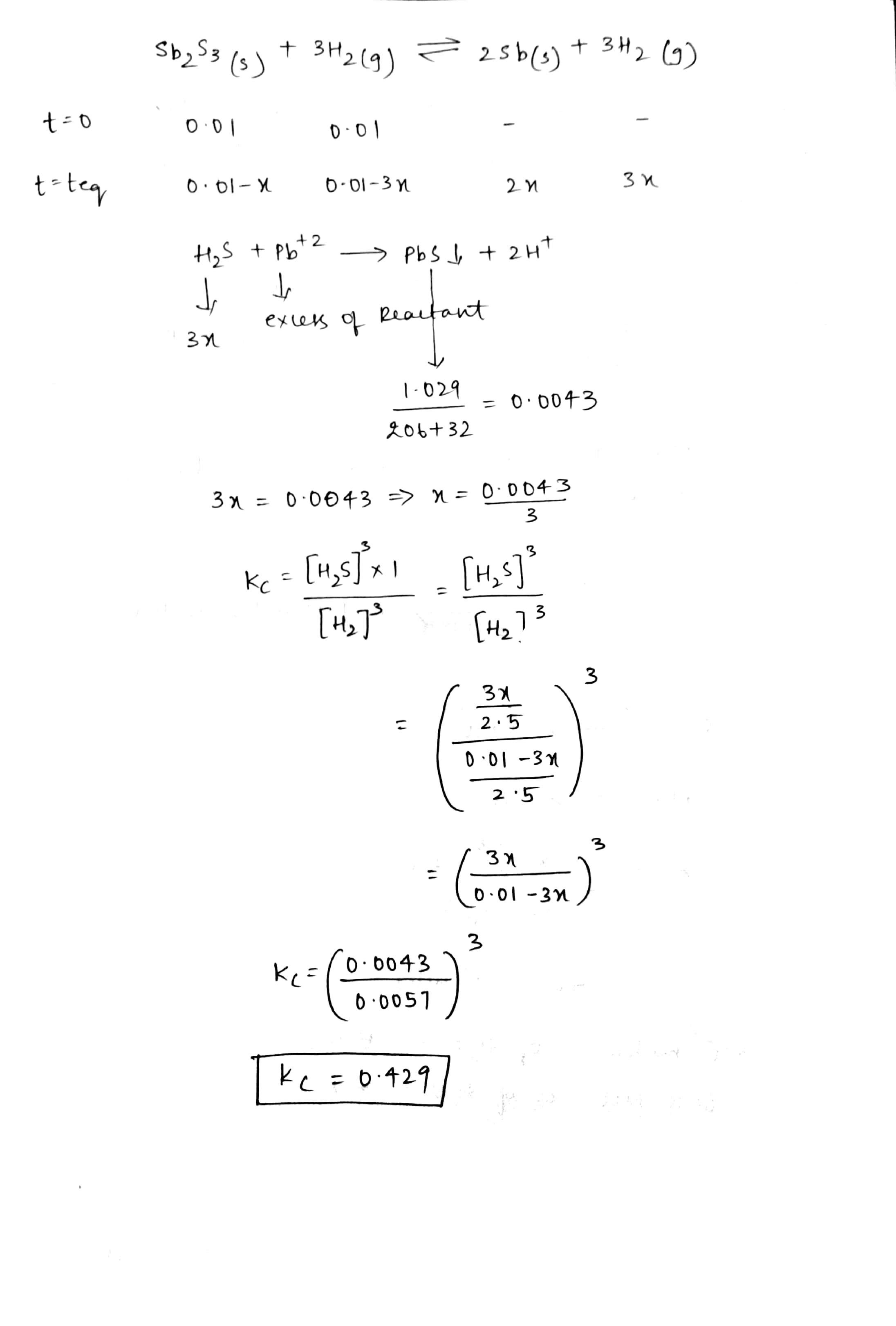
The reaction:
$$Sb_2S_3(s)+3H_2(g)\rightleftharpoons 2Sb(s)+3H_2S(g)$$
It was studied by analysing the equilibrium mixture for the amount of $$H_2S$$ produced. A vessel whose volume was $$2.5$$ litre was filled with $$0.01$$ mole of $$Sb_2S_3$$ and $$0.01$$ mole of $$H_2S$$. After the mixture came to equilibrium in the closed vessel at $$440^o$$C, the gaseous mixture was removed and the $$H_2S$$ was dissolved in water. Sufficient $$Pb^{2+}$$ ions were added to react completely with the $$H_2S$$ to precipitate $$PbS$$. If $$1.029$$g of $$PbS$$ was obtained, what is the value of $$K_c$$ at $$440^o$$C?
$$Sb_2S_3(s)+3H_2(g)\rightleftharpoons 2Sb(s)+3H_2S(g)$$
A tenfold increase in pressure on the reaction, $$N_2(g)+3H_2\rightleftharpoons 2NH_3(g)$$ at equilibrium results in .......... in $$K_p$$.
What is the equilibrium constant for this dissolving process?
Derive the best conditions for dissociation of $$NH_{3}.$$ Given $$2NH_{3} \rightleftharpoons N_{2} + 3H_{2}; \triangle H = +91.94\ kJ$$.
The chemical equation of one of the different stages of manufacturing sulphuric acid by the contact process is given below. Find out the influence of the following factors in the reaction given below.
$$2SO_{2}(g)+O_{2}(g)\rightleftharpoons 2SO_{3}(g)+heat$$
Increase the amount of oxygen.
Following data is given for the reaction: $$CaCO_{3}(s)\rightarrow CaO(s)+CO_{2}(g)$$
$$\Delta _{f}H^{\circleddash}[CaO(s)]=-635.1kJ$$ $$mol^{-1}$$
$$\Delta _{f}H^{\circleddash}[CO_{2}(g)]=-393.5kJ$$ $$mol^{-1}$$
$$\Delta _{f}H^{\circleddash}[CaCO_{3}(s)]=-1206.9kJ$$ $$mol^{-1}$$
Predict the effect of temperature on the equilibrium constant of the above reaction.
The chemical equation of one of the different stages of manufacturing sulphuric acid by contact process is given below. Find out the influence of the following factors in the reaction given below.
$$2SO_{2}(g)+O_{2}(g)\rightleftharpoons 2SO_{3}(g)+heat$$
Pressure is increased
For an exothermic reaction, what happens to the equilibrium constant if temperature is increased?
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions