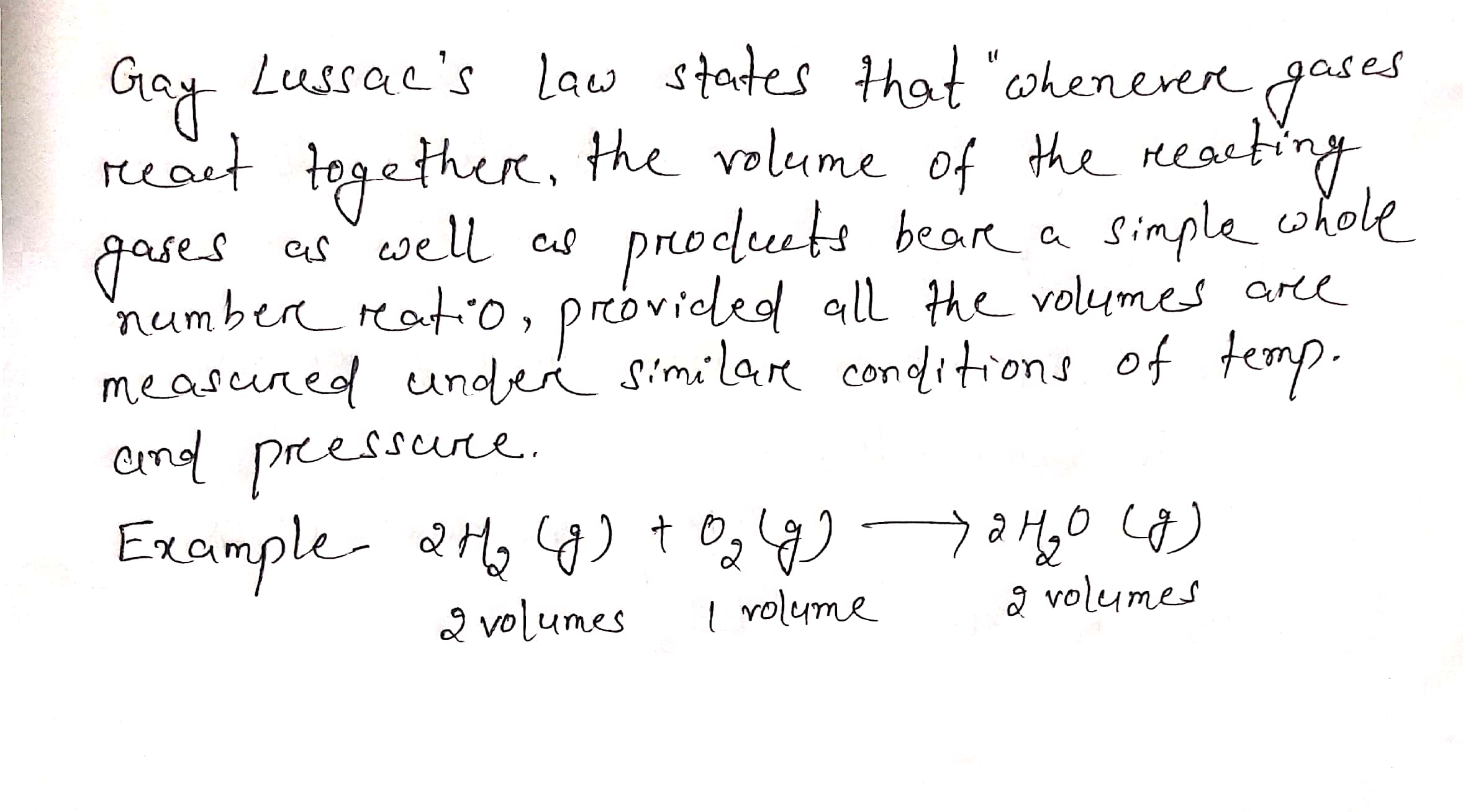Some Basic Concepts Of Chemistry - Class 11 Engineering Chemistry - Extra Questions
Define a) Atomic mass unit
b)Mole
Define mole.
How many atoms of hydrogen are there in 36 g of $$NH4^+$$?
If $$100$$ grams of calcium carbonate (whether in the form of marble or chalk ) are decomposed completely, then $$56$$ grams of calcium oxide and $$44$$ grams of carbon dioxide are obtained. Which law of chemical combination is illustrated by this statement?
Explain Gay Lussac's Law of combining volume with example .
If $$100$$ grams of pure water taken from different sources is decomposed by passing electricity. $$11$$ grams of hydrogen and $$89$$ grams of oxygen are obtained. Which chemical law is illustrated by this statement?
$$1\ g$$ of $$N_2$$ and $$1\ g$$ of $$O_2$$ are put in a two-litre flask at $$27^oC$$. Calculate partial pressure of each gas, the total pressure and the composition of the mixture in mole percentage.
What is meant by a mole of a substance?
Give short answers:
What is alchemy?
Experiment and _____________ are the two important basics of chemistry.
Define :
Relative atomic mass
Sodium chloride reacts with silver nitrate to produce silver chloride and sodium nitrate. State the law that satisfies this equation.
Which isotope is used as a reference on the atomic scale? What is one amu or one 'u' ?
Number of moles present in 28g of nitrogen atoms are
What is the SI definition of mole?
In the estimation of sulphur by Carius method, 0.468 g of an organic sulphur compound afforded 0.668 g of barium sulphate. Find out the percentage of sulphur in the given compound.
At STP, number of molecules in $$5.6 l$$ of sulphur trioxide gas is _________.
Calculate the number of molecules of ammonia present in $$5.6\ dm^3$$ of its volume.
Calculate the mass of the following
i) 0.5 moles of $$N_2$$ gas
ii) 0.5 moles of N atom
Calculate the number of moles for the following:
i) 52 g of He
ii) number of He atom
How is $$1$$ mole NaOH different from $$1$$ molar NaOH.
Convert the following into moles: i) 12g of $${ O }_{ 2 }$$ gas
ii) 20g of water
iii) 22g of $${ CO }_{ 2 }$$
iv) 52g of helium
ii) 20g of water
iii) 22g of $${ CO }_{ 2 }$$
iv) 52g of helium
Calculate the percentage composition of each element in Potassium chlorate, $$KClO_3.$$
Urea is a very important nitrogenous fertilizer. Its formula is $$CON, H_4.$$ Calculate the percentage of carbon in urea. $$(C = 12, O = 16, N = 14 \,and \,H = 1)$$
Fill in the blank.
Isotopes are the atoms of an element having the same _________ number but a different __________ number.
$$ 0 \cdot 5 \mathrm{g} $$ mixture of $$ \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7} $$ and $$ \mathrm{KMnO}_{4} $$ wastreated with excess KI in acidic medium. Iodine liberated required $$ 100 \mathrm{cm}^{3} $$ of $$ 0 \cdot 15 \mathrm{N} $$ sodium thiosulphate solution for titration. Find the percent amount of each in the mixture (At. wts. K $$ =39, \mathrm{Cr}=52, \mathrm{Mn}=55, \mathrm{Na}=23 \mathrm{S}=32) $$
18.4g mixture of $$CaCO_3$$ and $$MgCO_3$$, on strongly heating gives 8.8g of $$CO_2$$ gas. Calculate mole percentage of $$MgCO_3$$ in the mixture.
If the water molecules in 1.0 g of water were distributes uniformly over the surface of earth, If there are $$6.5\times10^{-x}$$such molecules would there be in $$1.0\, cm^2$$ of earth's surface , then what is the value of x?
The melting points of four solids A, B, C and D are $$78{\,^o}C,\,213{\,^o}C,100{\,^o}C$$ and $$154{\,^o}C.$$ Arrange them in increasing order of their interparticle forces of attraction.
Two small iron particles, each of mass $$280 mg $$ , are placed at a distance $$10 cm $$ apart. If $$0.01 \% $$ of the electrons of one particle are transferred to the other, find the electric force between them. Atomic weight of iron is $$56 g mol^{-1} $$ and there are $$26$$ electrons in each atom of iron.
On passing $$25 \ mL$$ of a gaseous mixture of $$N_2$$ and $$NO$$ over heated copper, $$20 \ mL$$ of the gas remained. Calculate the percentage of each in the mixture.
Excess of KI and dil $$ H_2SO_4 $$ were mixed in 50 mL $$ H_2O_2 $$ The liberated 12 required 20 mL of 0.1 N $$ Na_2S_2O_3 $$ Find out the strength of $$ H_2O_2 $$ in g/litre.
$$40 \ mL$$ of a mixture of hydrogen, $$CH_4$$ and $$N_2$$ was exploded with $$10 \ mL$$ of oxygen. On cooling, the gases occupied $$36.5 \ mL$$. After treatment with $$KOH$$, the volume reduced by $$3 \ mL$$ and again on treatment with alkaline pyrogallol, the volume further decreased by $$1.5 \ mL$$. Determine the composition of the mixture.
(i) A solution of a mixture of $$KCl$$ and $$KOH$$ was neutralised with $$120mL$$ of $$0.12N$$ $$HCl$$. Calculate the amount of $$KOH$$ in the mixture.
(ii) After titration, the resultant solution was made acidic with $$H{NO}_{3}$$. Then excess of $$Ag{NO}_{3}$$ solution was added to precipitate the $$AgCl$$ which weighed $$3.7g$$ after drying. Calculate percentage of $$KOH$$ in the original mixture.
$$38 \ mL$$ of a mixture of $$CO$$ and $$H_2$$ was exploded with $$31 \ mL$$ of $$O_2$$. The volume after the explosion was $$29 \ mL$$ which reduced to $$12 \ mL$$ when shaken with $$KOH$$. Find the percentage of $$CO$$ and $$H_2$$ in the mixture.
Match the column $$I$$ with column $$II$$:
$$ 2.5\,g $$ of a mixture of $$ BaO $$ and $$ CaO $$ when treated with an excessof $$ H_2SO_4 $$ , produced $$ 4.713\,g $$ of the mixed sulphates . Find the percentage of $$ BaO $$ present in the mixture .
$$4.00g$$ of mixture of $$NaCl$$ and $${Na}_{2}{CO}_{3}$$ was dissolved in water and the volume made up to $$250mL$$; $$25mL$$ of this solution required $$50mL$$ of $$N/10$$ $$HCl$$ for complete neutraliztion. Calculate percentage composition of the original mixture.
A mixture of $$0.535$$ g of ethanol and acetaldehyde when heated with Fehling’s solution gave $$1.2$$ g of a red precipitate. What is the percentage of acetaldehyde in the mixture? (Cu = $$63.5$$)
A mixture of $$KMn{O}_{4}$$ and $${ K }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }$$ weighing $$0.24g$$ on being treated with $$KI$$ in acid solution liberates just sufficient $${I}_{2}$$ to react with $$60mL$$ of $$0.1N$$ $${Na}_{2}{S}_{2}{O}_{3}$$ solution. Calculate percentage of $$Cr$$ and $$Mn$$ in the mixture.
A $$20.0 \,cm^{3}$$ mixture of $$CO, CH_{4}$$ and $$He$$ gases is exploded by an electric discharge at room temperature with excess of oxygen. The volume contraction is found to be $$13.0 \,cm^{3}.$$ A further contraction of $$14.0\,cm^{3}$$ occurs when the residual gas is treated with $$KOH$$ solution. Find out the composition of the gases mixture.
A mixture of $$ \mathrm{FeO} $$ and $$ \mathrm{Fe}_{3} \mathrm{O}_{4} $$ was heated in air to a constant mass. It was found to gain $$ 10 \% $$ in its mass. Calculate the percentage composition of the original mixture.
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions