Some P-Block Elements - Class 11 Engineering Chemistry - Extra Questions
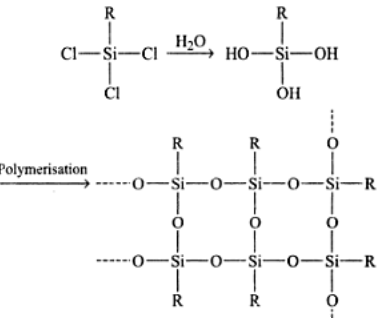
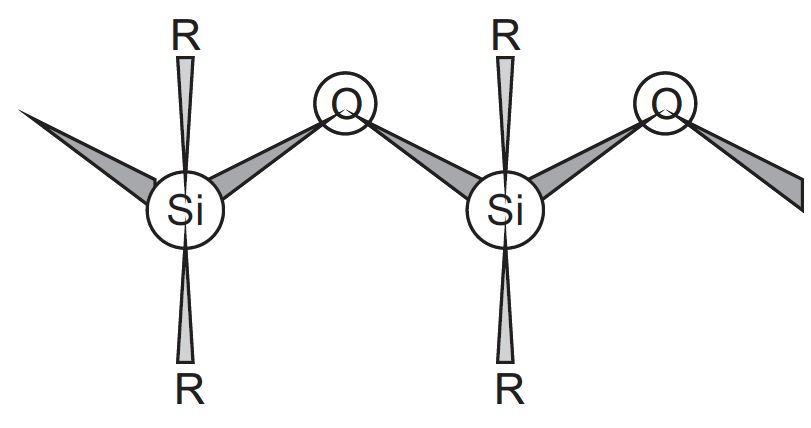
What are silicones? How are they prepared?
Write the formula of unit of Silicones.
If the starting material for the manufacture of silicones is $$RSiCl_3$$, write the structure of the product formed.
Answer the following:
Name one silicate and one phosphate ore of lithium.
Explain why the silicate oliving $$(FeMg)_2SiO_4$$ does not follow the law of constant composition.
Classify the following silicates:
Beryl
Classify the following silicates:
Feldspar
(a)What are silicones? State the uses of silicones.
(b)What are boranes? Give chemical equation for the preaparation of diborane
Classify the following silicates:
Zircon
Silicon has become a vital element in the modern electronics industry. Why?
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with —ic suffix.
B. If product is oxy acid with —ous suffix.
C. If product are two oxy acids one with —ic suffix and another one with —ous suffix.
D. If product is not oxy acid, neither with —ic suffix nor with —ous suffix.
$$\underline SiCl_4+H_2O \longrightarrow H_4SiO_4+HCl$$
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with ic suffix.
B. If product is oxy acid with ous suffix.
C. If product are two oxy acids one with ic suffix and otherone with ous suffix.
D. If product is not oxy acid, neither with ic suffix nor with ous suffix.
$$\underline SiH_4+H_2O \longrightarrow H_4SiO_4+H_2$$
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with —ic suffix.
B. If product is oxy acid with —ous suffix.
C. If product are two oxy acids one with —ic suffix and another one with —ous suffix.
D. If product is not oxy acid, neither with —ic suffix nor with —ous suffix.
$$H_6\underline {Si}O_7+H_2O \longrightarrow H_4SiO_4$$
How are linear silicones obtained?
What is general formula of silicones?
What are silicones?
$$HF$$ can be stored in a glass vessel.
If true enter 1, else enter 0.
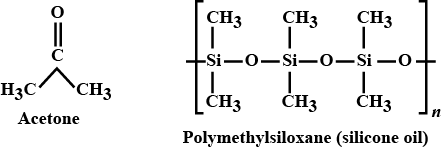
The organic solvent acetone has the molecular formula $$(CH_3)_2CO$$. The silicon analogue, a thermally stable lubricant, is a polymer, $$[(CH_3)_2SiO]_n$$. Account for the difference in structure.
The value of $$n$$ in the molecular formula, $$Be_nAl_2Si_6O_{18}$$ is :
Fill in the blanks:
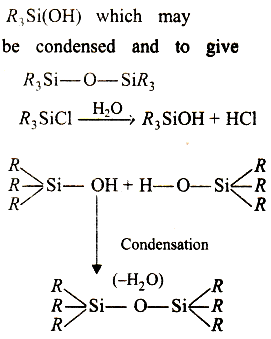
The hydrolysis of trialkyl chlorosilane, $$ R_3 SiCl $$, yields ______.
The hydrolysis of trialkyl chlorosilane, $$ R_3 SiCl $$, yields ______.
Write short notes on silicones.
Define:
Spodumene
Sillicon has same valence electrons like carbon but it do not show the property similar to carbon, why?
What are silicates?
Class 11 Engineering Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Organic Chemistry - Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- Some P-Block Elements Extra Questions
- States Of Matter Extra Questions
- Structure Of Atom Extra Questions
- Thermodynamics Extra Questions