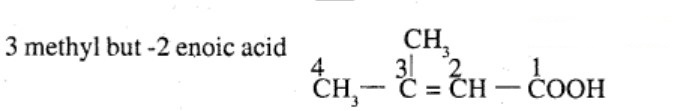
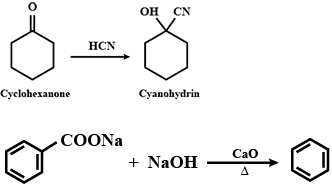
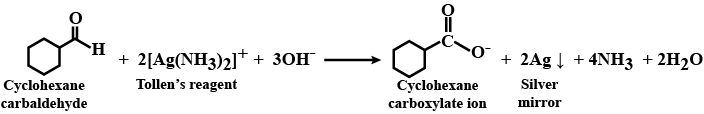
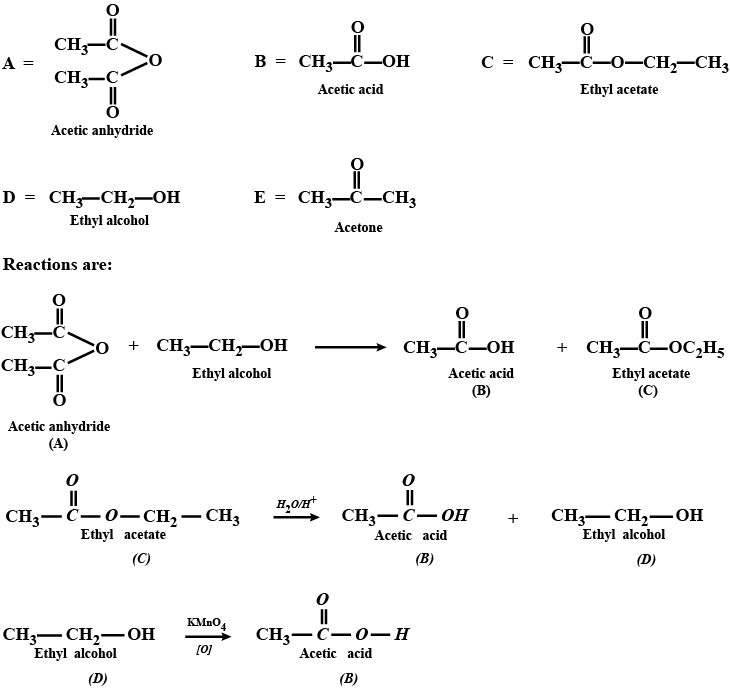
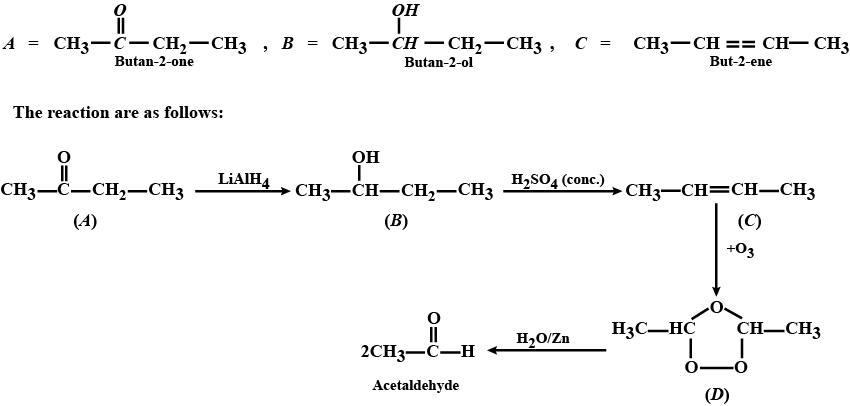
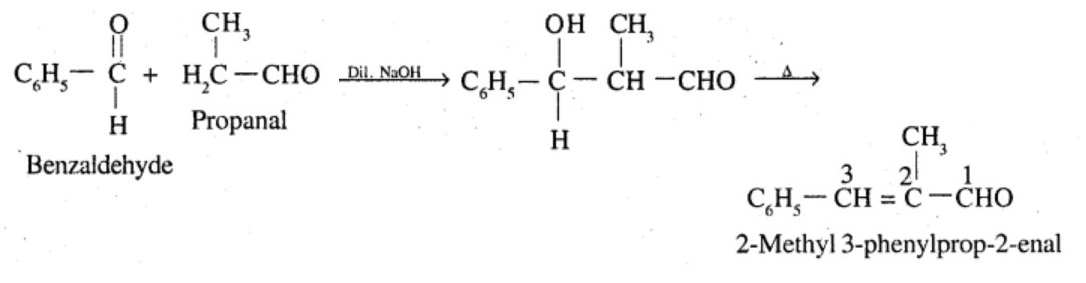
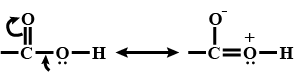
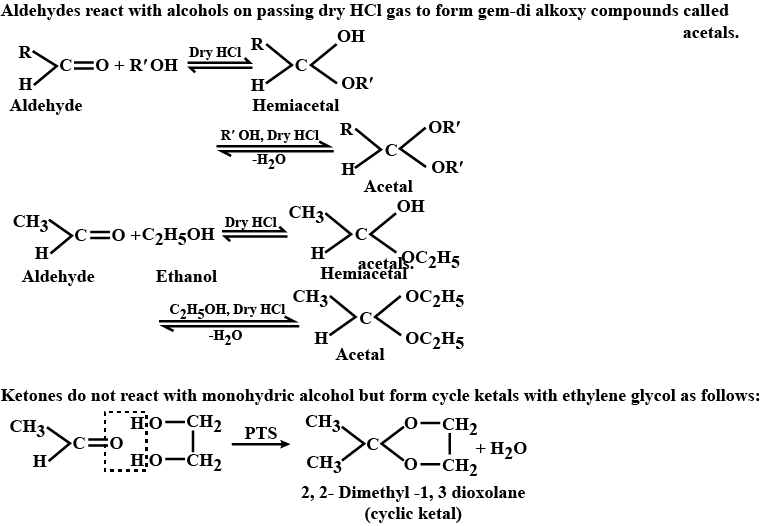
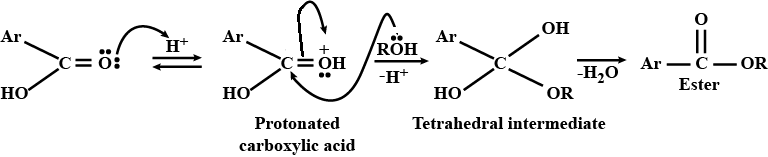
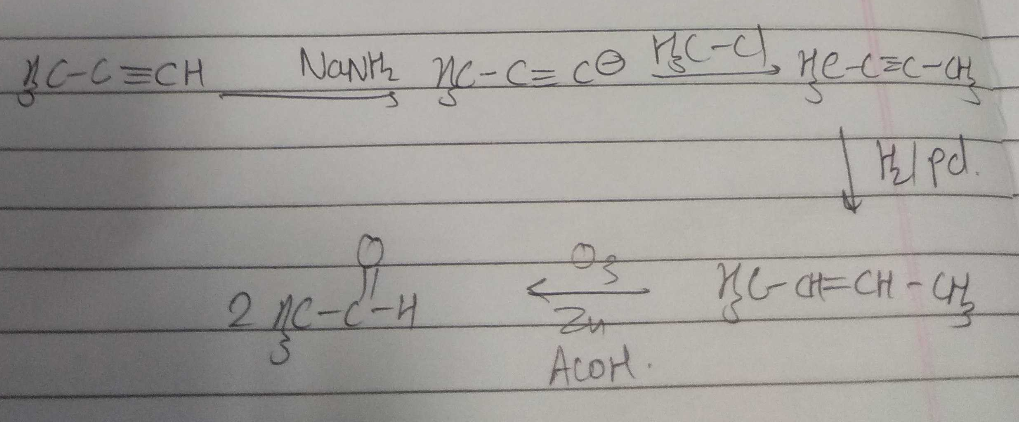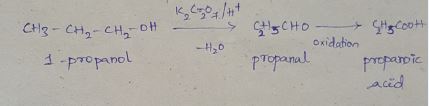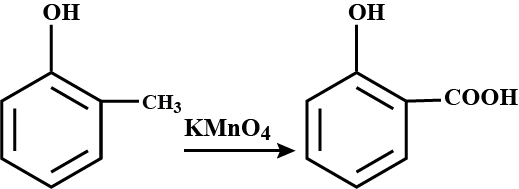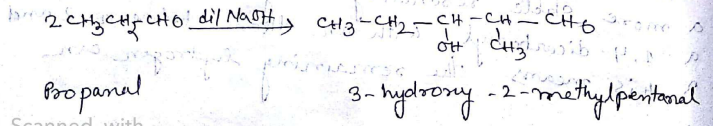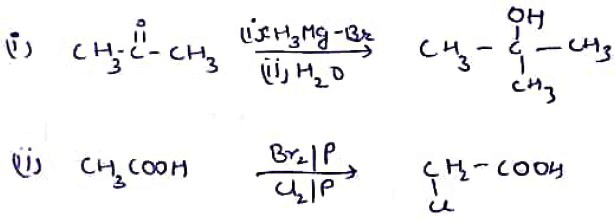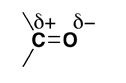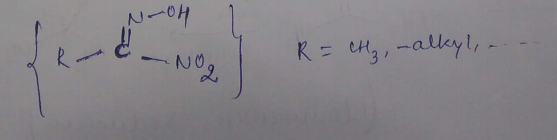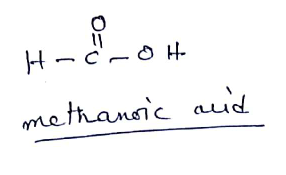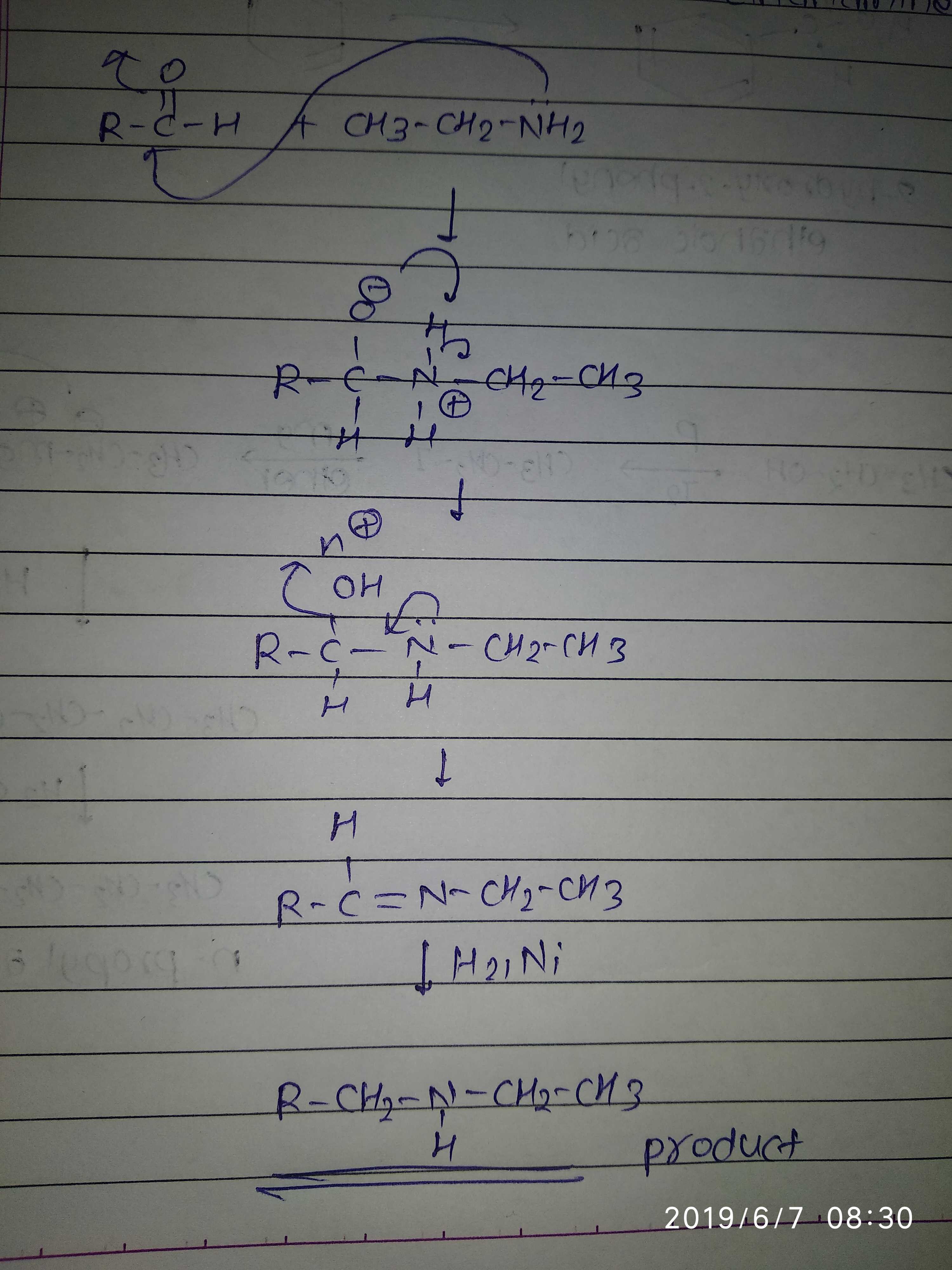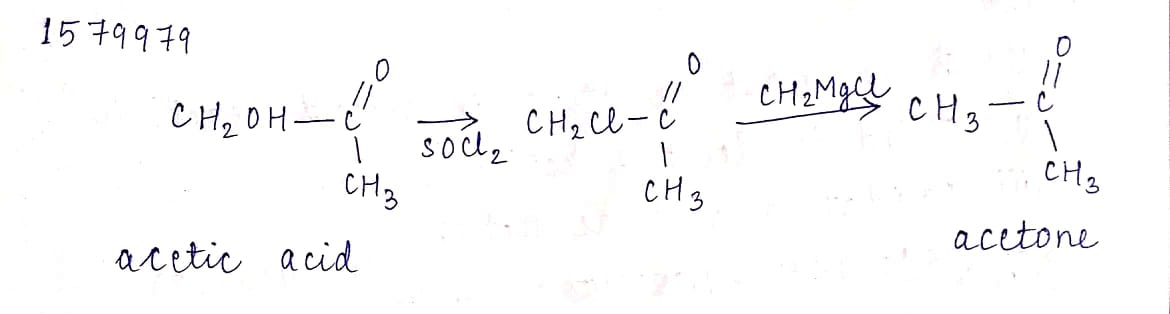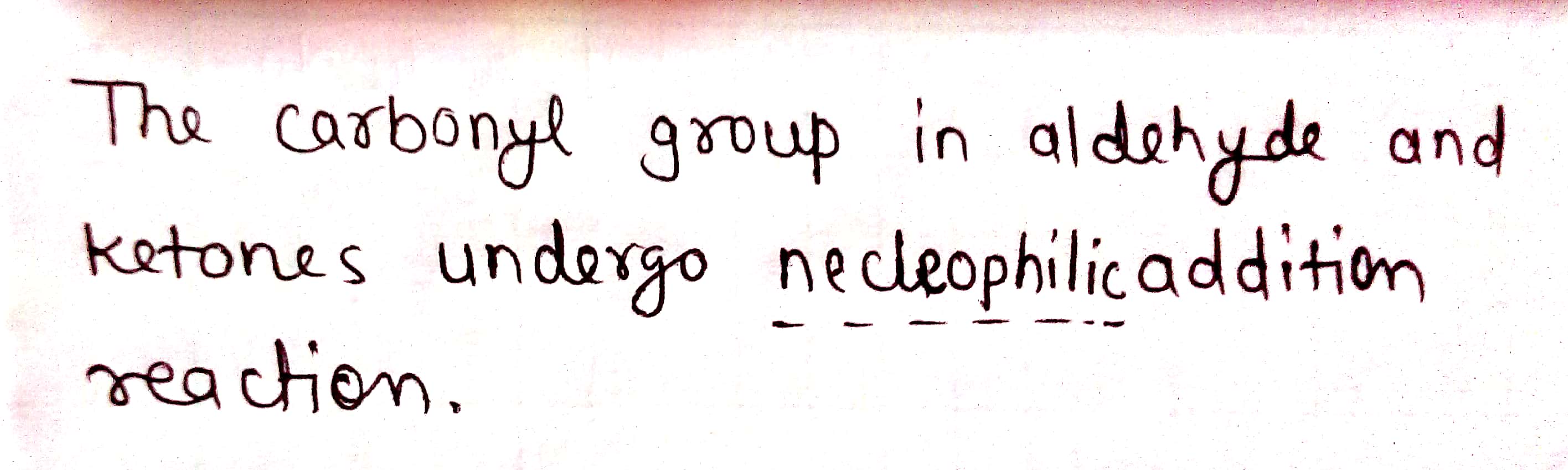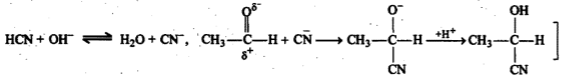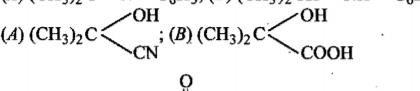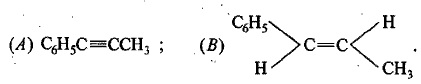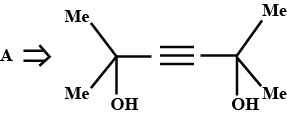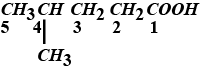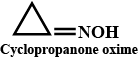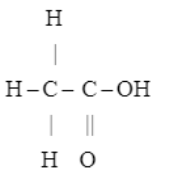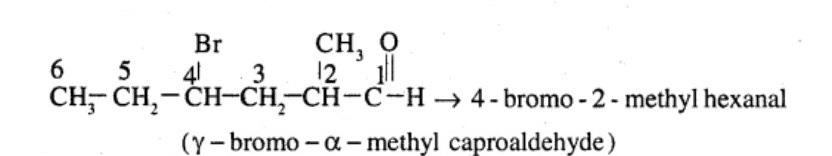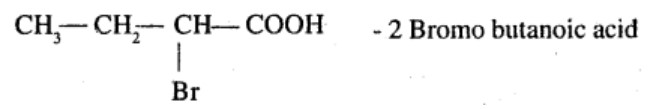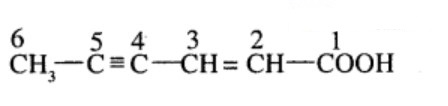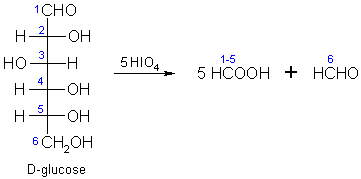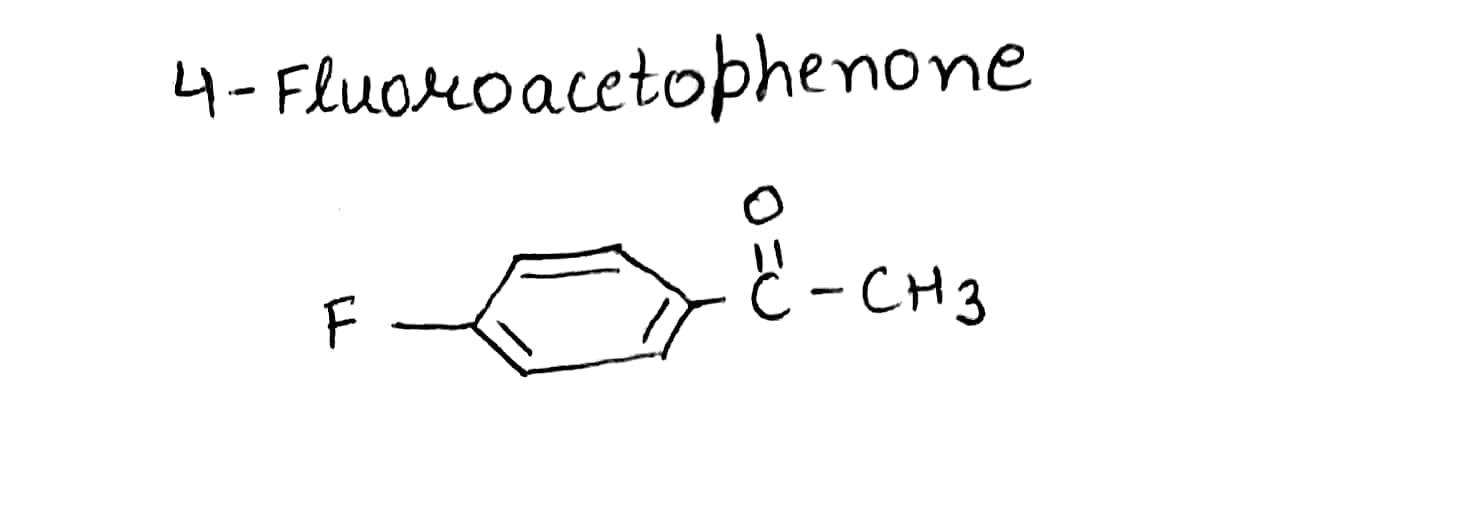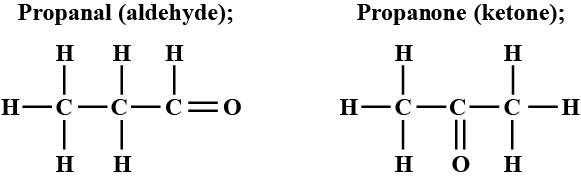Aldehydes,Ketones And Carboxylic Acids - Class 12 Engineering Chemistry - Extra Questions
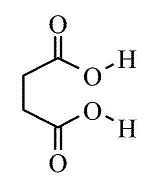
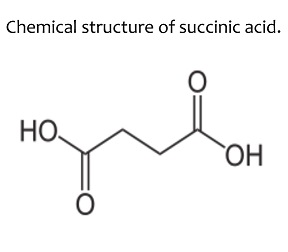
The IUPAC name of succinic acid is Butane-1,4-dioic acid.
If true enter 1, if false enter 0.
Write chemical equations for the following conversions:
(i) Nitrobenzene to benzoic acid.
(ii) Benzyl chloride to 2-phenylethanamine.
(iii) Aniline to benzyl alcohol.
Give the structural formulae and IUPAC names of the following compounds:
(a) Formaldehyde
(b) Acetone.
Why are aldehydes and ketones polar compounds?
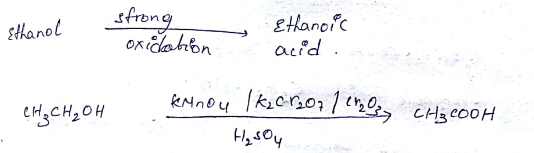
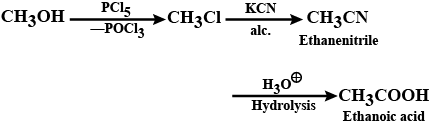
Ethanol can be oxidised to ethanoic acid. Write the equation involve in the reaction.
figure
Draw the structure of the simplest ketone.
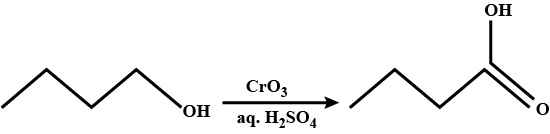
Write the structure of the principal organic product formed in the reaction of 1-propanol with each of the following reagents: (i) Potassium dichromate $$(K_{2}Cr_{2}O_{7})$$ in adqueous sulfuric acid, heat
What is the IUPAC name of succinic acid?
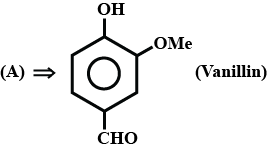
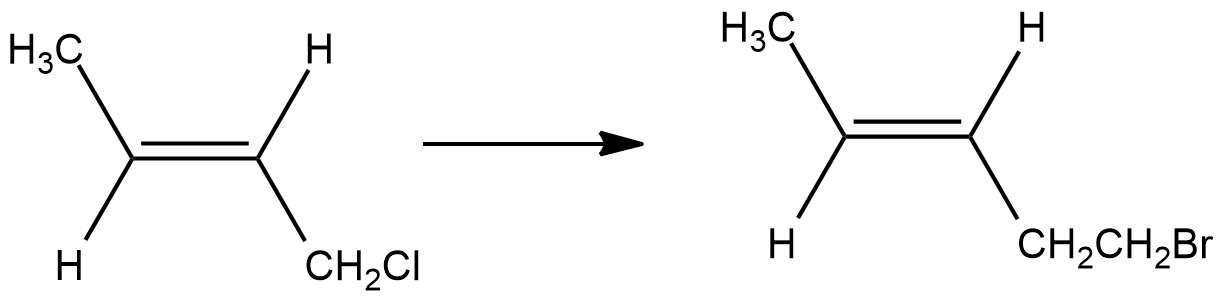
How can the second compound be obtained from the first compound?
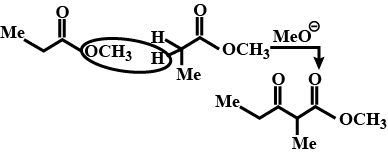
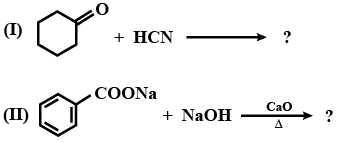
Write the product of this reaction ?
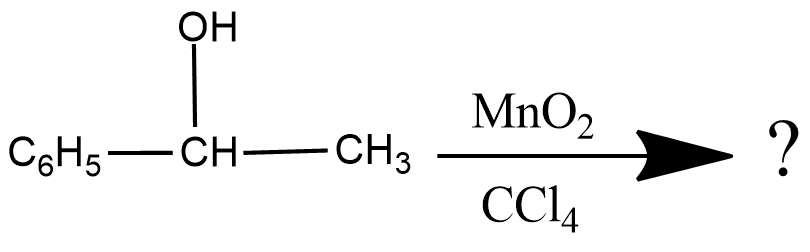
Write the product of this reaction :
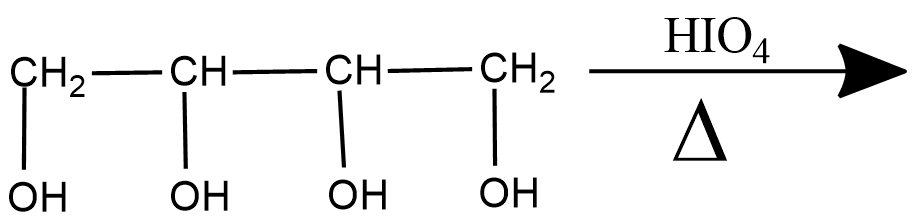
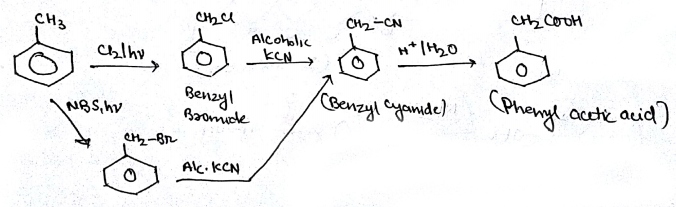
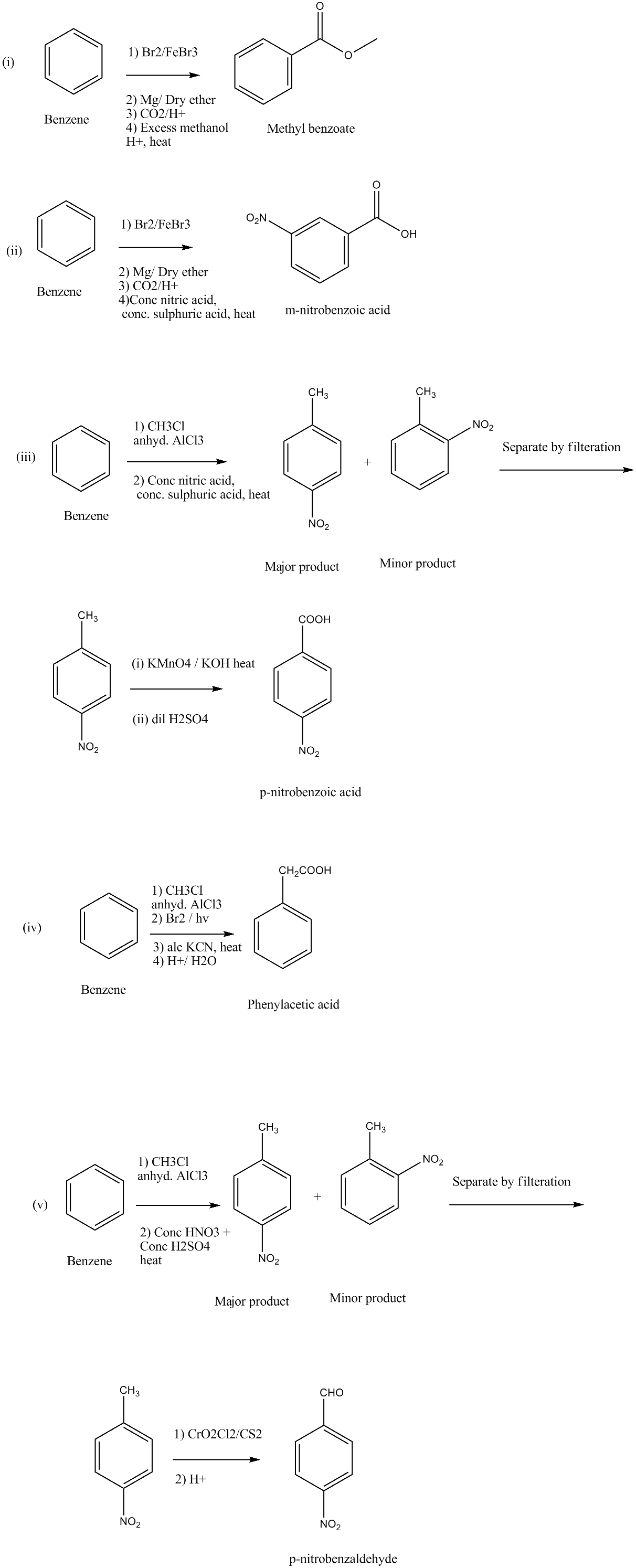
Bring about the following transformation:$$Toluene \longrightarrow C_6H_5CH_2COOH$$
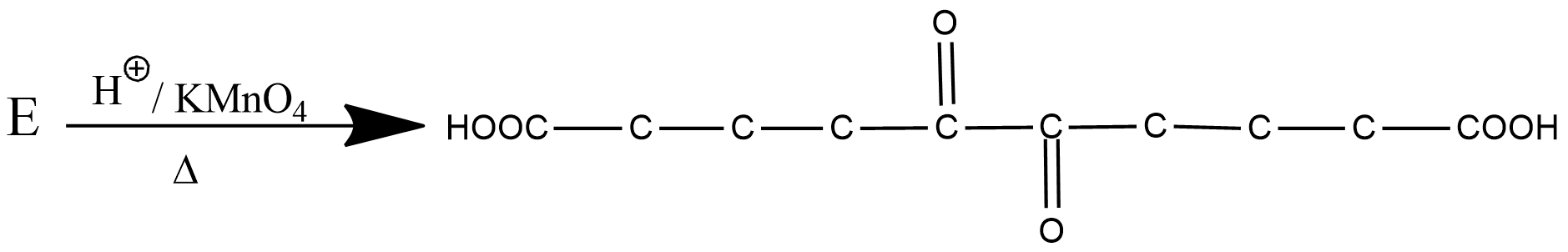
What is the structure of $$E$$?
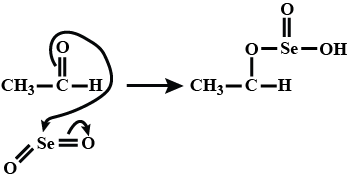
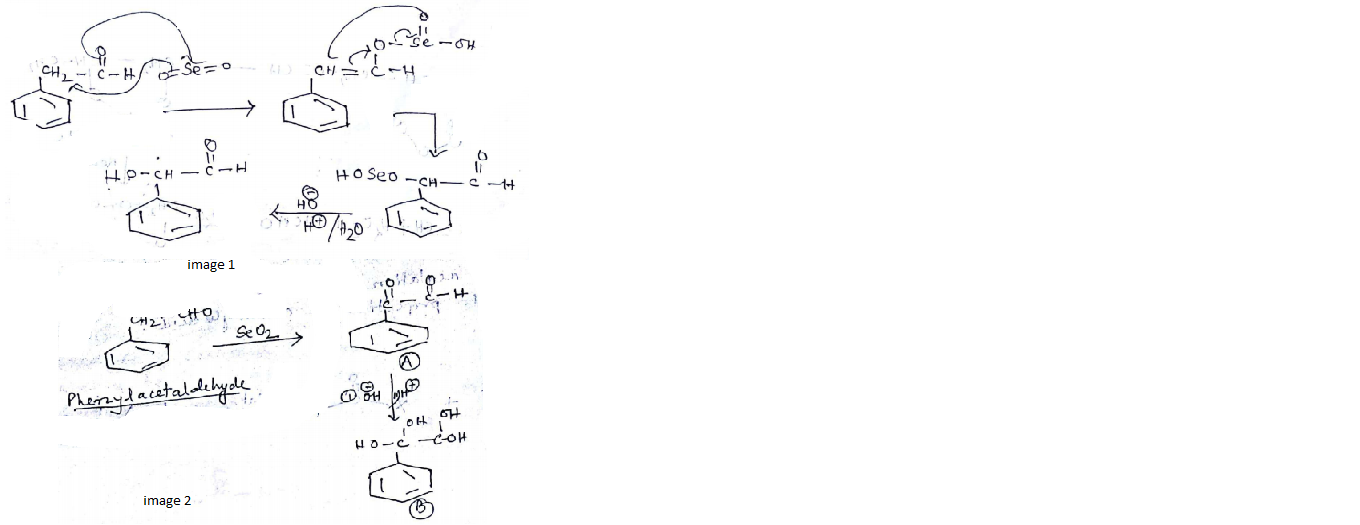
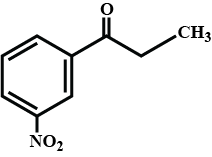
$$CH_3CHO\xrightarrow[\Delta]{SeO_2}$$ What is the product of this reaction:
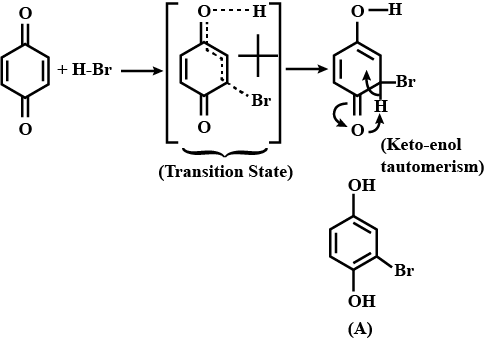
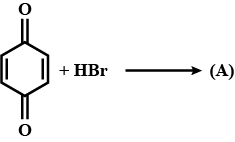
A is an alkene. Identify the reactant $$A$$ in the above reaction.
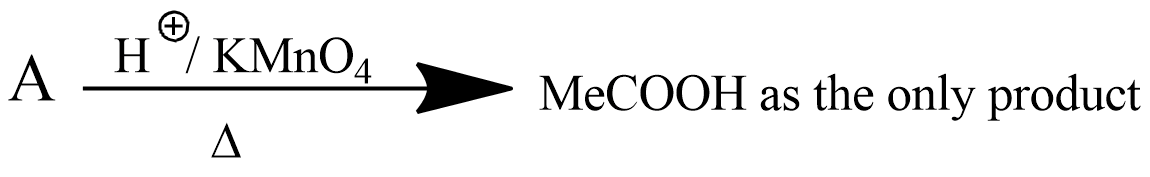
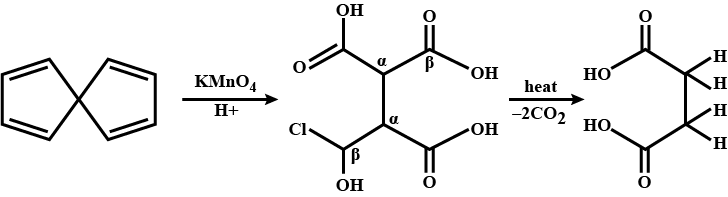
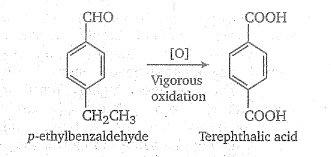
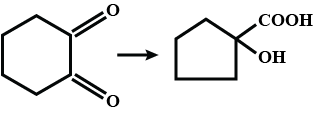
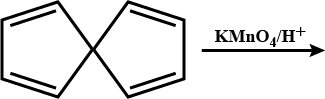
Convert the above compound and note that $$KMnO_4$$ can cause cleavage of the ring in the presence of the activating (-OH) group.
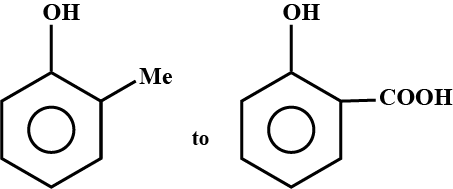
(I) (II)Convert I to II.
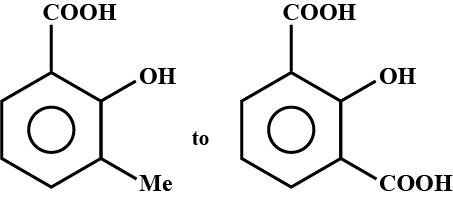
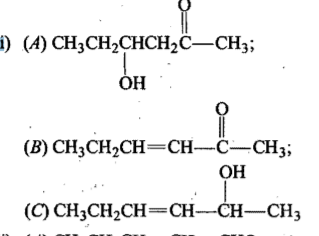
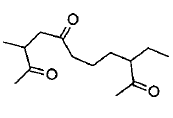
Propose structure/structures for the hydrocarbon that gives the following products on oxidative cleavage by $$KMnO_4/H^\oplus$$
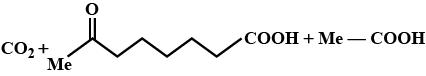
Identify $$E$$ in the above reaction.
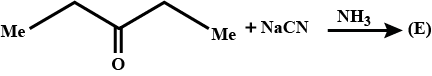
Complete the following.
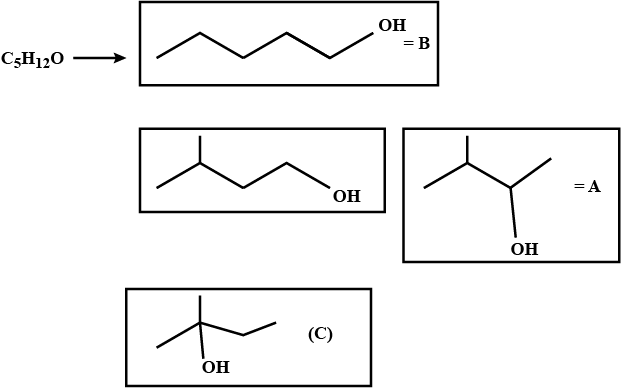
Identify A, B, and C.i) Isomeric alcohols (A), (B), and (C) $$\displaystyle \left ( C_{5}H_{12}O \right )$$.
ii) (A) and (B) react with chromic acid solution. (B) gives an acid (D).
iii) Reactivity with HBr is: $$\displaystyle C> A\gg B.$$ All give the same compound, $$\displaystyle C_{5}H_{11}Br\left ( E \right ).$$
iv) Only (A) is oxidised by NaOI.
ii) (A) and (B) react with chromic acid solution. (B) gives an acid (D).
iii) Reactivity with HBr is: $$\displaystyle C> A\gg B.$$ All give the same compound, $$\displaystyle C_{5}H_{11}Br\left ( E \right ).$$
iv) Only (A) is oxidised by NaOI.
Identify (A) and (B) in the given sequence of reaction $$PhCH_2CHO \overset{SeO_2}{\rightarrow}(A) \xrightarrow[(ii)H^+]{(i)con.OH^-}$$ (B)
Draw the structure of the following compound:
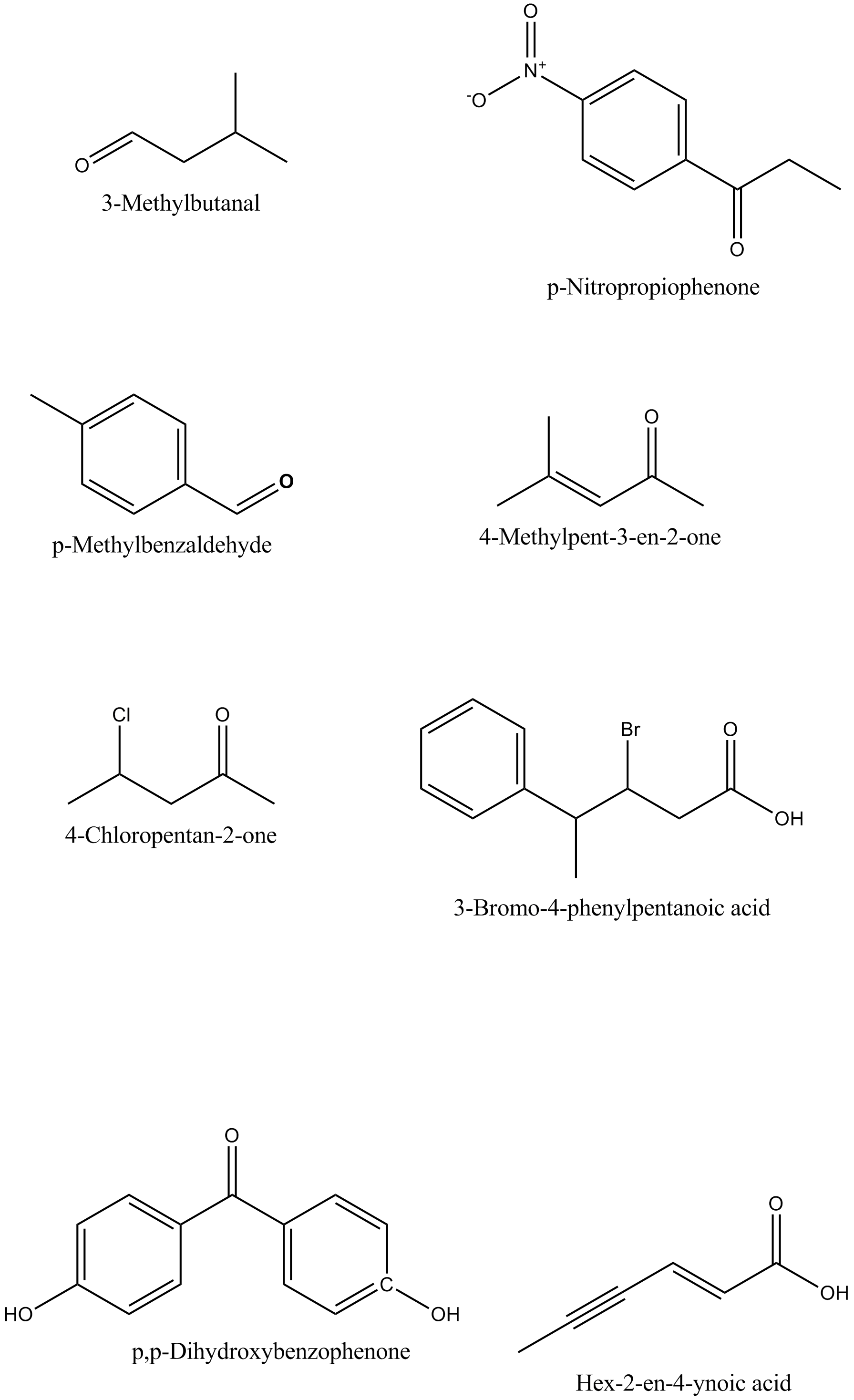
4-Chloropentan-2-one
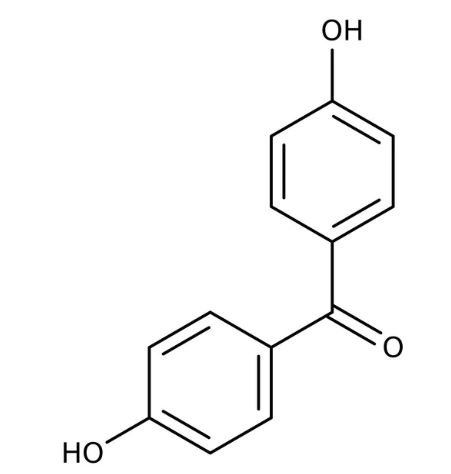
Draw the structures of following compounds:p-Nitropropiophenone
Draw the structure of the following compound :
p,p'-Dihydroxybenzophenone
Write structural formulae and names of four possiblealdol condensation products from propanal and butanal.III each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Draw the structure of the following compound.
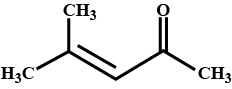
4-Methylpent-3-en-2-one
Complete the following reaction and write the structures of A to D.
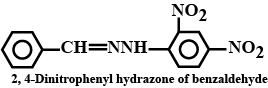
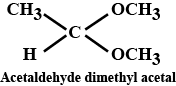
Draw structures of the following derivatives.
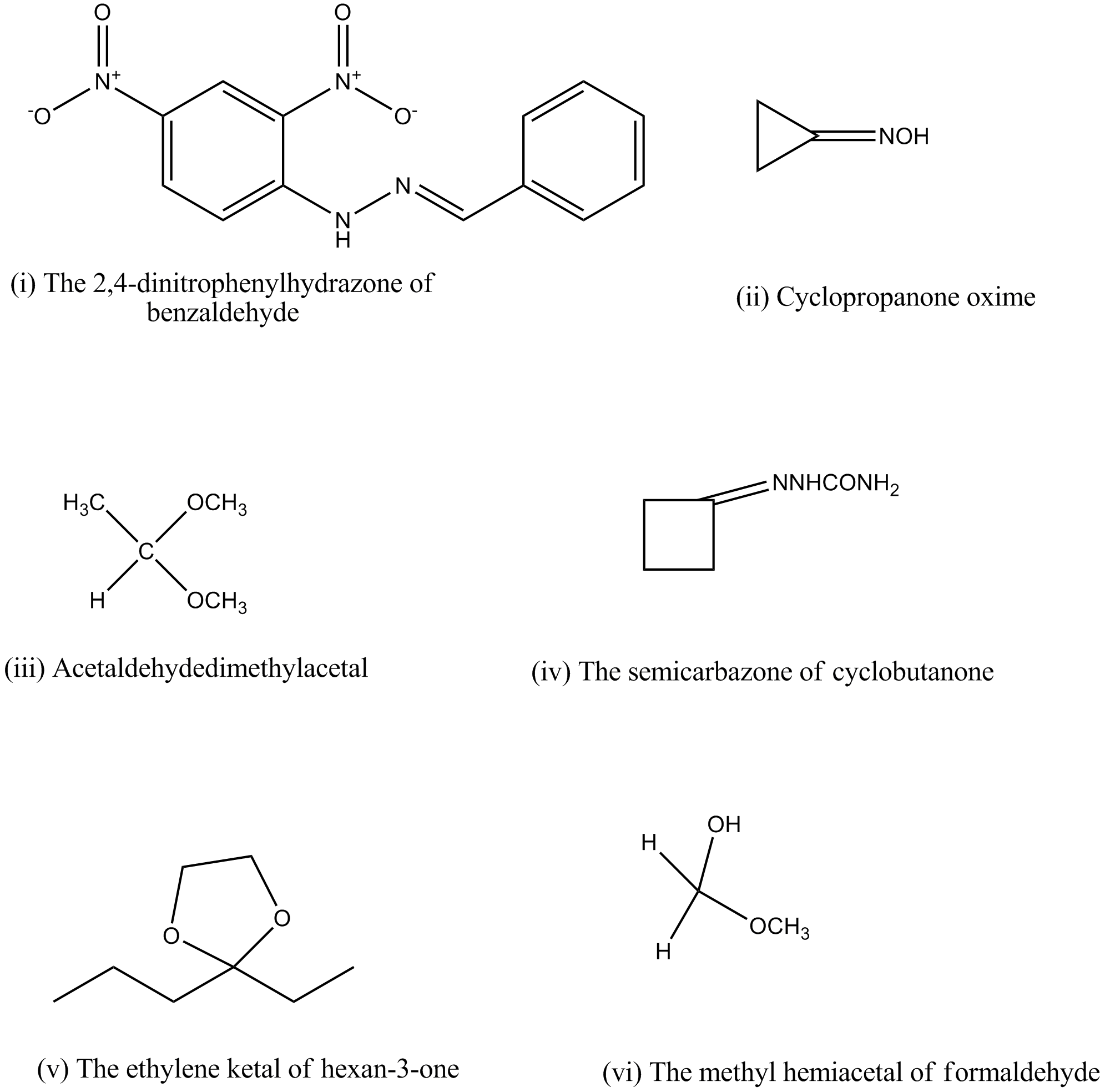
(i) The 2,4-dinitrophenylhydrazone of benzaldehyde
(ii) Cyclopropanone oxime
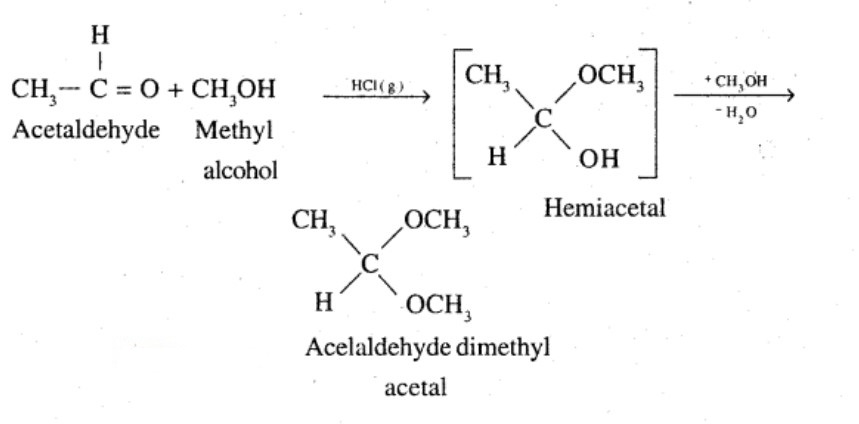
(iii) Acetaldehydedimethylacetal
(iv) The semicarbazone of cyclobutanone
(v) The ethylene ketal of hexan-3-one
(vi) The methyl hemiacetal of formaldehyde
Ethyl alcohol to trichloroacetic acid
How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom:
(i) Methyl benzoate (ii) m-Nitrobenzoic acid
(iii) p-Nitrobenzoic acid (iv) Phenylacetic acid
(v) p-Nitrobenzaldehyde.
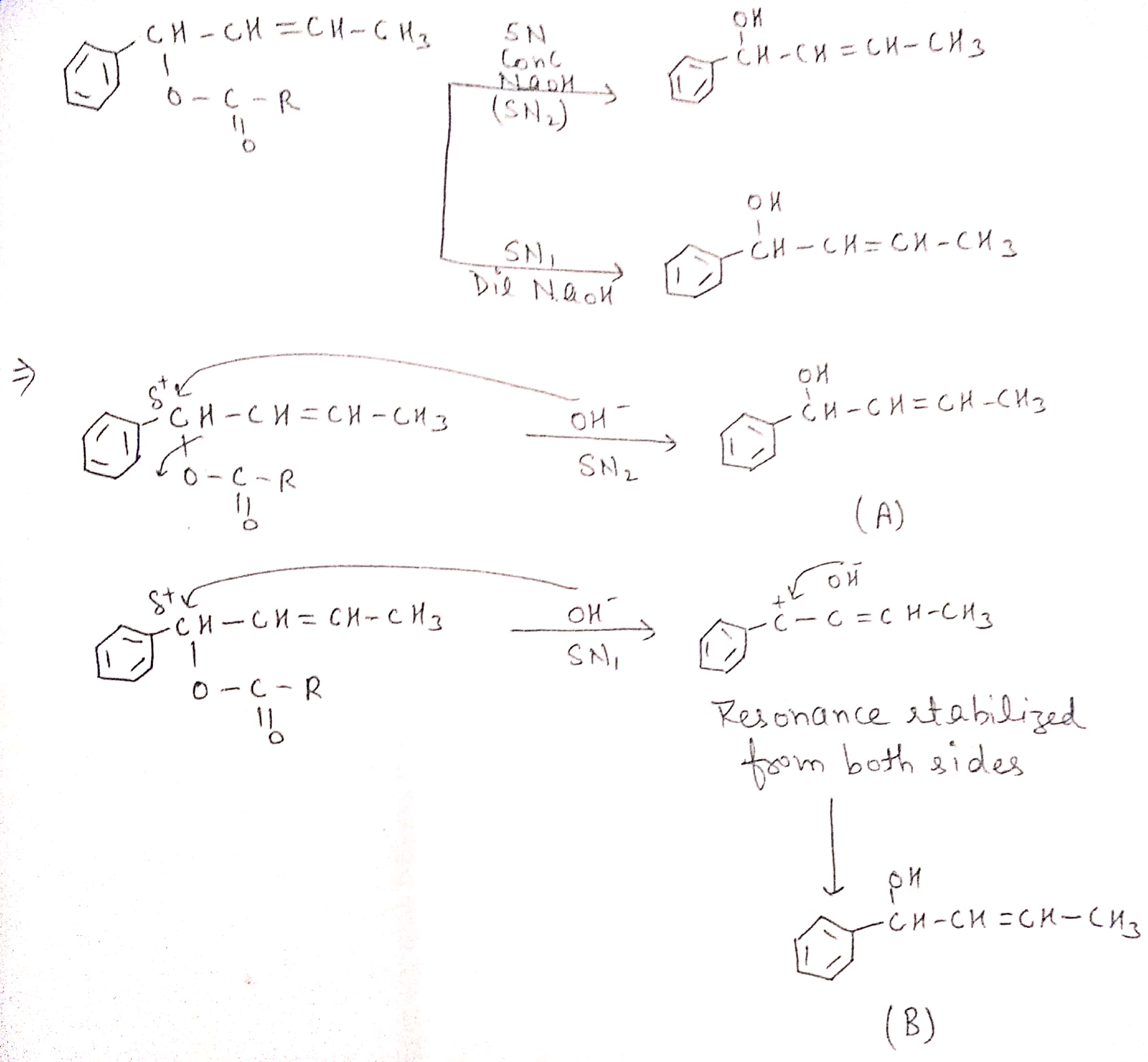
What are ambident nucleophiles? Explain with an example
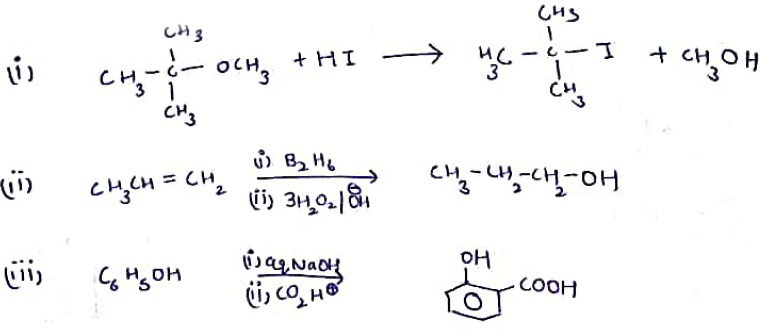
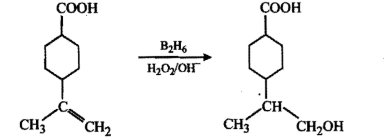
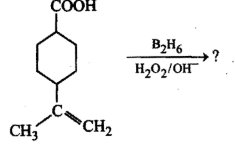
Write the main product(s) in each of the following reactions:(i) $$CH_3 -\!\!\!\underset{\,\,CH_3}{\underset{|}{ \overset{\quad CH_3}{\overset{|}{C}}} \!\!\!-}\, O - CH_3 - HI \to$$(ii) $$CH_3 - CH = CH_2 \xrightarrow[(ii)\, 3H_2O_2/OH^-]{(i)\, B_2H_6}$$(iii) $$C_6H_5 - OH \xrightarrow[(ii)\, CO_2, H^+]{(i)\, aq.NaOH}$$
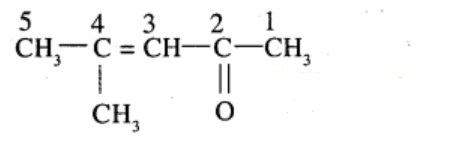
Write the IUPAC name of the compound :
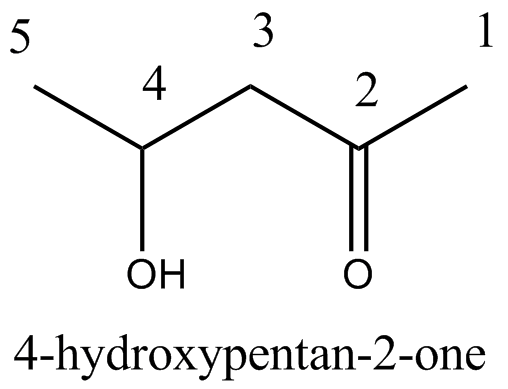
$$\begin{matrix} C{ H }_{ 3 }- & CH & -C{ H }_{ 2 }- & C & -C{ H }_{ 3 } \\ & | & & || & \\ & OH & & O & \end{matrix}$$
$$\begin{matrix} C{ H }_{ 3 }- & CH & -C{ H }_{ 2 }- & C & -C{ H }_{ 3 } \\ & | & & || & \\ & OH & & O & \end{matrix}$$
Name the reagents used in the following reactions:
(i) $$C{ H }_{ 3 }-CO-C{ H }_{ 3 }\xrightarrow { \quad \quad ?\quad \quad } \begin{matrix} & & C{ H }_{ 3 } & & \\ & & | & & \\ C{ H }_{ 3 } & - & C & - & C{ H }_{ 3 } \\ & & | & & \\ & & OH & & \end{matrix}$$
(ii) $$C{ H }_{ 3 }-COOH\xrightarrow { \quad \quad ?\quad \quad } ClC{ H }_{ 2 }-COOH$$
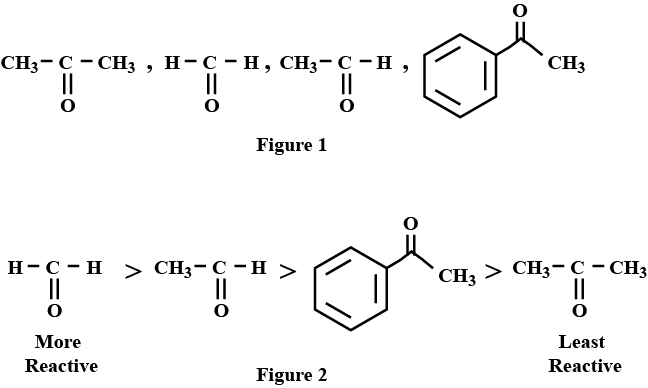
Arrange the following compounds in an increasing order of their reactivity in nucleophilic addition reactions: ethanol, propanal, propanone, butanone.
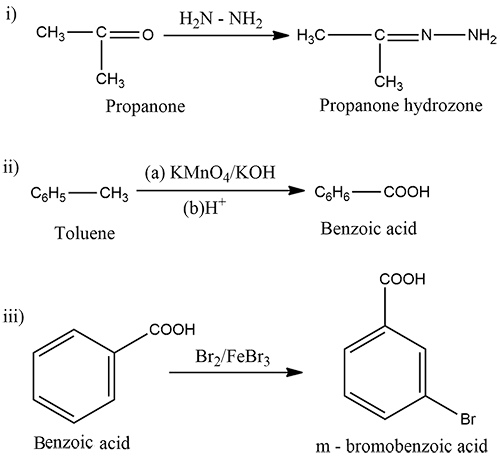
Predict the products of the following reactions.
(i) $$\displaystyle CH_{3}-C=O\xrightarrow{H_{2}N-NH_{2}}$$
|
$$CH_{3}$$
(ii)$$\displaystyle C_{6}H_{5}-CH_{3}\xrightarrow[(b)H^{+}]{(a)KMnO_{4}/KOH}$$
(iii) $$Ph-COOH \xrightarrow {Br_2/FeBr_3}$$
Why do aldehydes and ketones have high dipole moments?
How can the following conversation be brought about:
Propanoic acid to ethylamine.
$$\pi$$ bonds are formed by the _______ overlap of _______ orbitals.
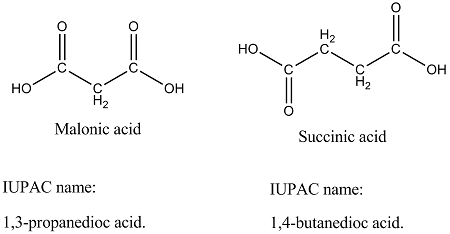
Give the structural formulae and IUPAC names of the following compounds
(a) Malonic acid
(b) Succinic acid.
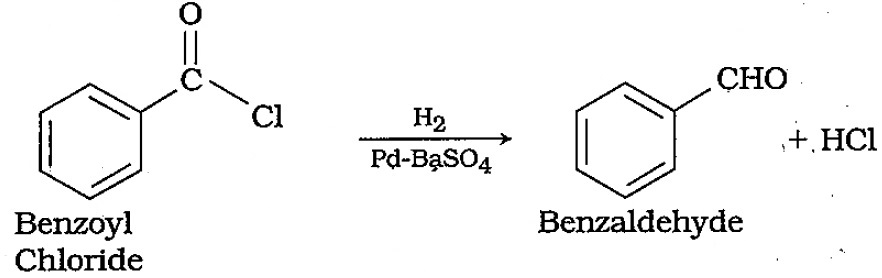
How are the following compounds prepared?Benzaldehyde from benzoyl chloride.
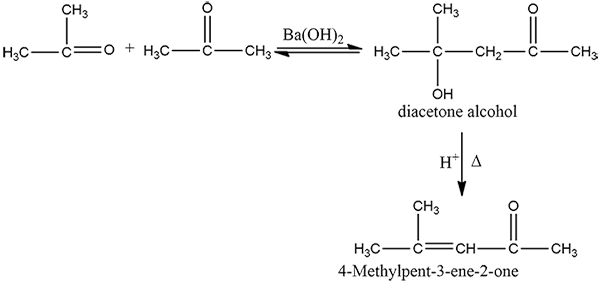
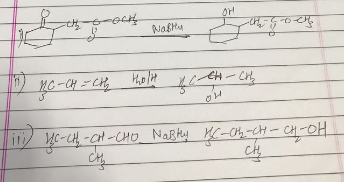
How is 4-methylpent-3-en-2-one obtained from propan-e-one?
State the IUPAC names of the following compounds :
(i) $$C{H}_{3} - C{H}_{2} - C{H}_{2} - OH$$
(ii) $$HCOOH$$
(iii) $$C{H}_{3} - C{H}_{2} - CH = C{H}_{2}$$.
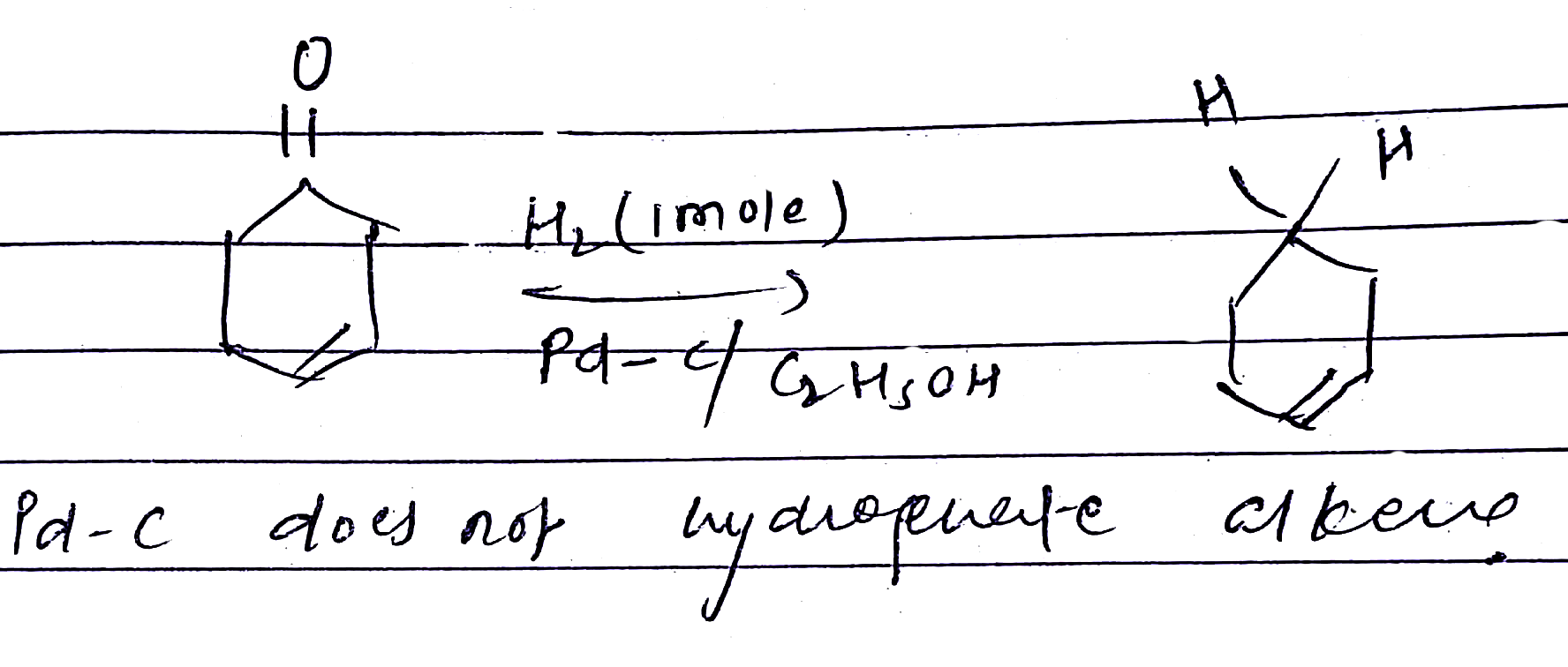
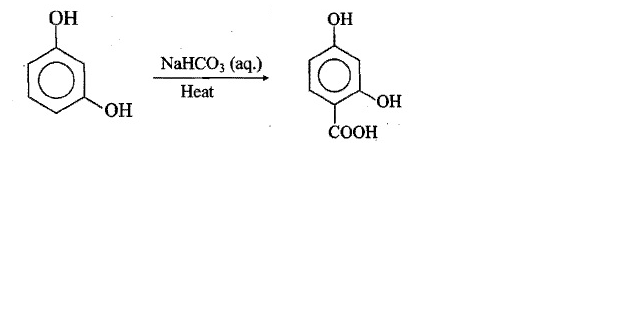
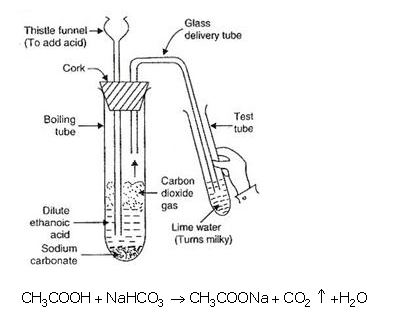
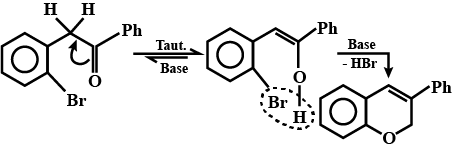
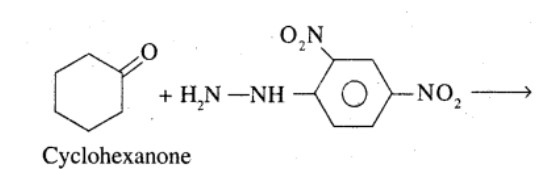
Look at the picture and identify what happens. Support your answer with equations.
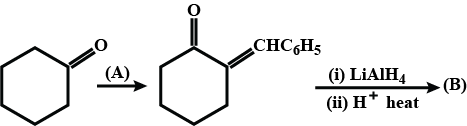
How is B formed from A?

Mention the hybridised state of carbonyl carbon atom.
How will you bring out the following conversion :
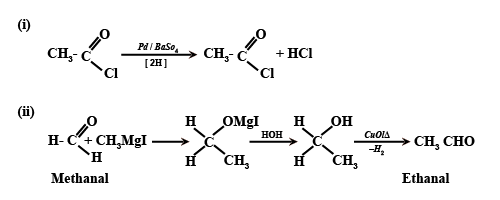
(i) Acetyl chloride to Acetaldehyde
(ii) Methanal to Ethanal.
Identify the substance underlined, in each of the following cases:
An organic compound containing - $$COOH$$ functional group.
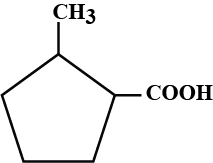
What is the IUPAC name of the compound?
What is the action of the following reagent on ethanoic acid?
$$LiAlH_4/H_3O^+$$.
Find the number of compounds from the list below that will be oxidised by Tollens reagent (Ammonical silver nitrate solution)
$$\displaystyle { H }_{ 3 }C-CHO\quad { H }_{ 3 }C-\overset { \overset { O }{ \parallel } }{ C } -{ CH }_{ 3 }\quad { H }_{ 3 }\underset { \underset { OH }{ | } }{ C } -\overset { \overset { O }{ \parallel } }{ C } -{ CH }_{ 3 }\\ { { H }_{ 3 }C }-{ CH }_{ 2 }C\equiv { CH }\quad { { H }_{ 3 }C-C\equiv C }-{ CH }_{ 3 }\quad Fructose$$
How do you convert the following?
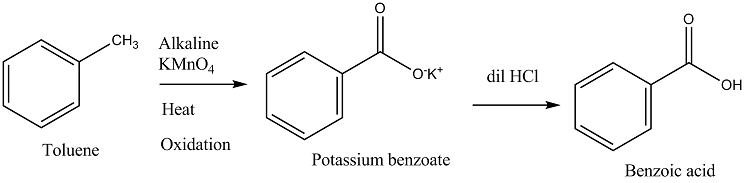
Toluene to Benzoic acid.
Toluene to Benzoic acid.
How are the following conversions carried out?
$$2 - methylbutan-1-01$$ into $$2-methylbutanoic\ acid$$.
Find the final product of the reaction.
Identify the organic compound : $$HOOC-(CH_2)_2 -COOH$$
Ketones can also be used as one component in the :
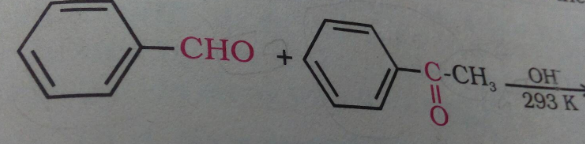
Give the IUPAC name of the given compound.
Which aldehyde smell like bitter almond?
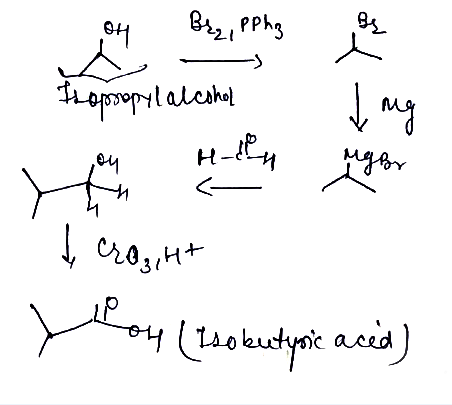
Isopropyl alcohol convert into isobuteryic acid.
Find:
In the reaction sequence, $$[X]$$ is: $$[X]\overset{KMnO_4/\overset{\ominus}{O}H}{\rightarrow} HOOC-(CH_2)_3-\overset{CH_3}{\overset{|}{C}}-H-COOH$$.
How will you convert Benzene to Benzoic acid?
Identify A and B

Write any two uses of $$40$$% solution of formaldehyde.
$$CH_3CH_2OH \xrightarrow {O} CH_3CHO \xrightarrow {O} CH_3COOH $$Give the oxidizing agent in the given reactions.
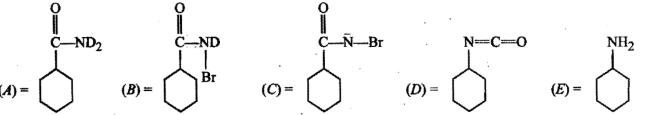
Write the structure of a nitrolic acid.
Identify the organic compound $$A$$ which is widely used as a preservative in pickles and has a molecular formula $$C_{2}H_{4}O_{2}$$. This compound reacts with ethanol to form a sweet-smelling compound $$B$$.
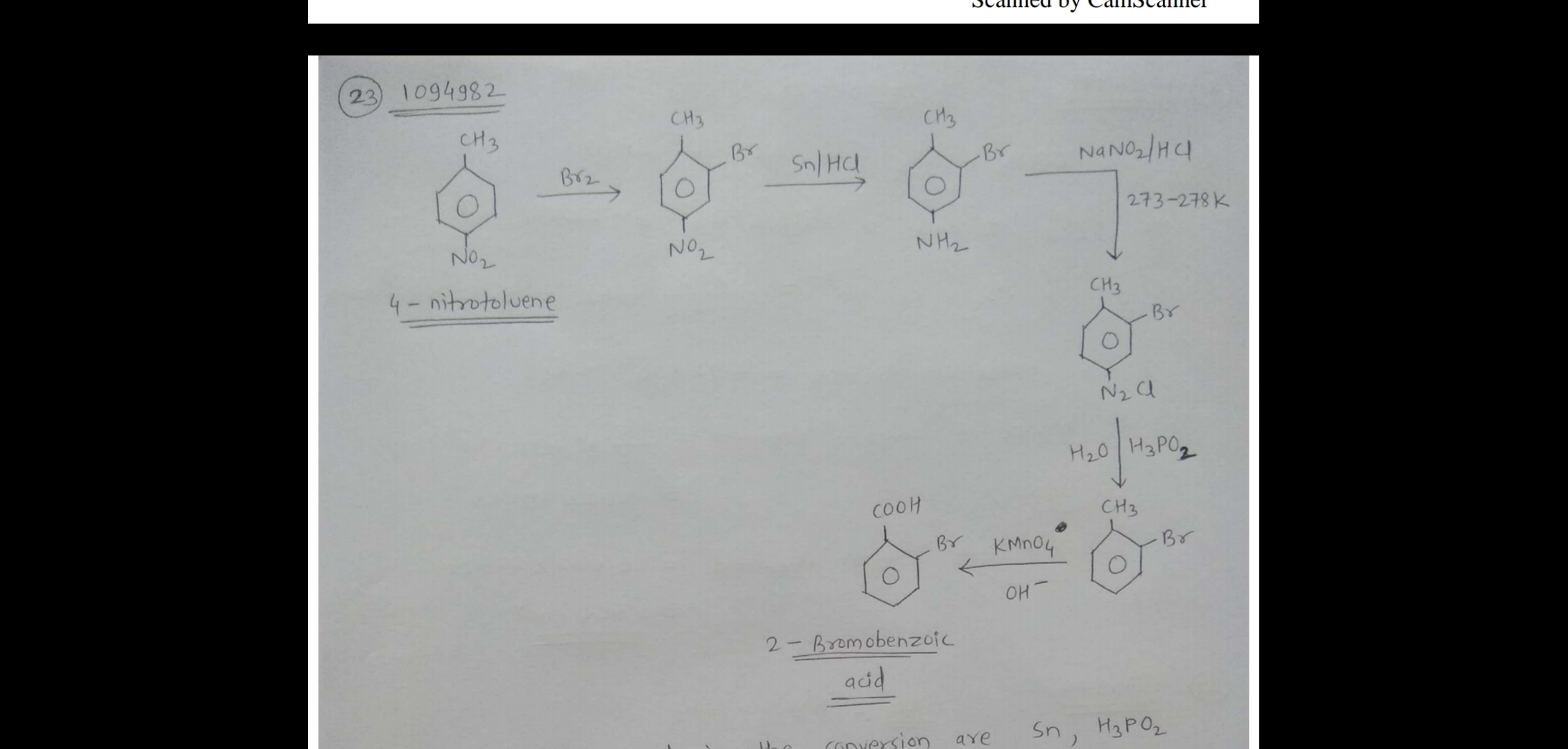
How will you convert $$4$$-nitrotoulene to $$2$$-bromobenzoic acid?
Based on $$pK_a$$ values the order of decreasing acidity of carboxylic acid molecules containing EWG must be?
$$CF_3COOH, \; CCl_3COOH, \; HCCl_2COOH, \; NO_2CH_2COOH, \; FCH_2COOH, \\ ClCH_2COOH, \; BrCH_2COOH, \; HCOOH, \; ClCH_2CH_2COOH, \; C_6H_5COOH, \; \\ C_6H_5CH_2COOH, \; CH_3COOH, \; CH_3CH_2COOH$$.
Write the name and formula of the first member of the carbon compounds having functional group $$-COOH$$.
Write the structures of aldehydes that are obtained on ozonolysis of (i) but-1-ene and (ii) but-2-ene.
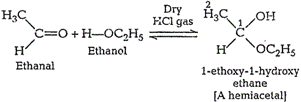
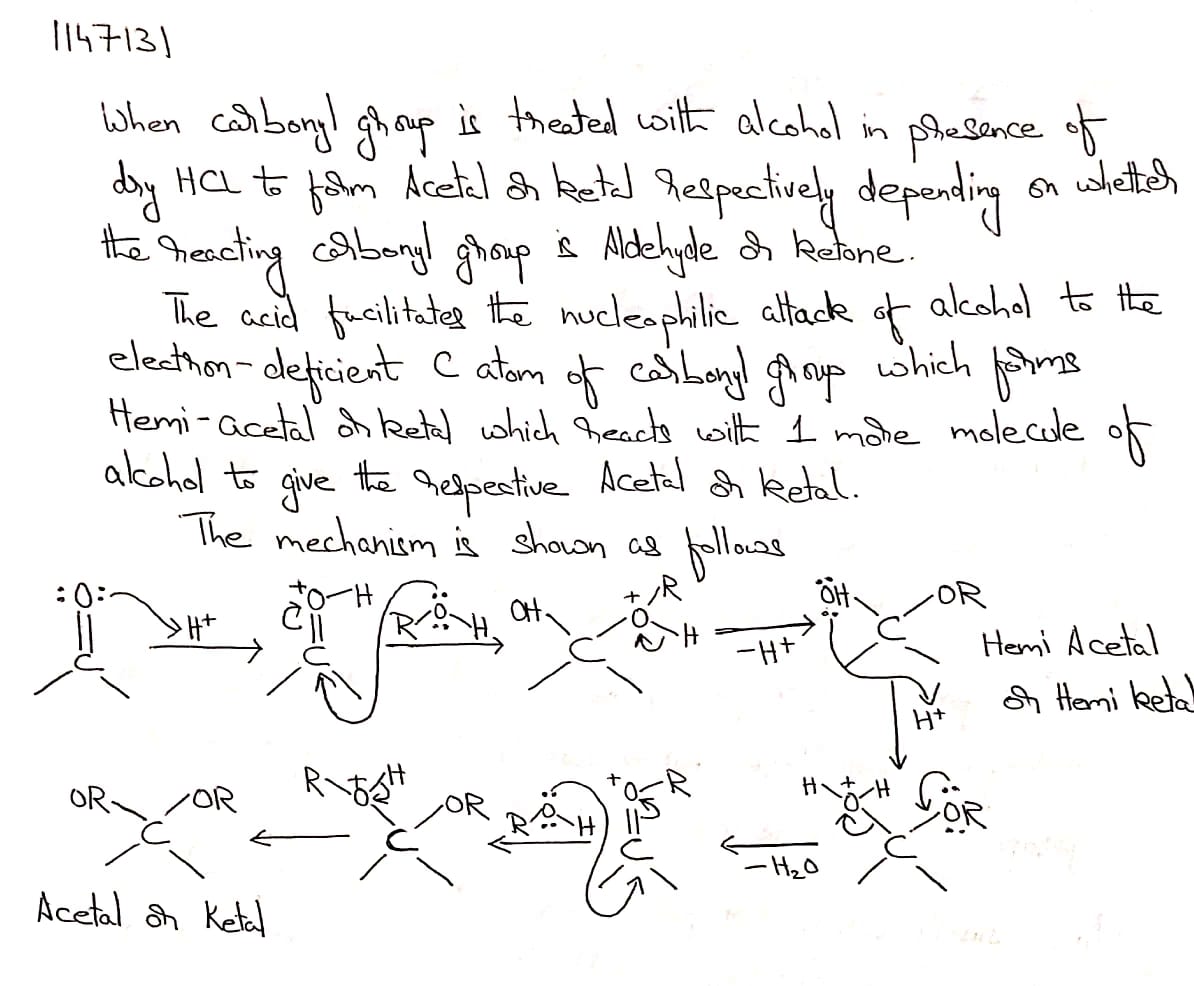
Name the product obtained when alcohol is added to a carbonyl group in presence of dry $${HCl}$$
Hydrocarbon $$'A'({C}_{4}{H}_{8})$$ on reaction with $$HCl$$ gives a compound $$'B'({C}_{4}{H}_{9}Cl)$$, which on reaction with $$1mol$$ of $${NH}_{3}$$ gives compound $$'C'({C}_{4}{H}_{11}N)$$. On reacting with $$Na{NO}_{2}$$ and $$HCl$$ followed by treatment with water. the compound $$C$$ yields an optically active alcohol, $$'D'$$. Ozonolysis of $$'A'$$ gives $$2$$ mol of acetaldehyde. Identify compound $$'D'$$.
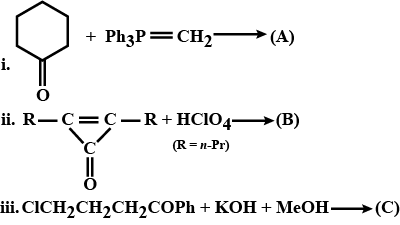
Which product is formed ?
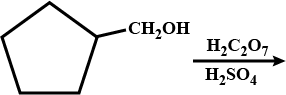
Convert : Ethentrityl $$\rightarrow $$ Ethanol
How is propanone converted into-
(i) Propan - 2 - ol
(ii) 2 - Methylpropan - 2 - ol?
(i) Propan - 2 - ol
(ii) 2 - Methylpropan - 2 - ol?
Complete the reaction :
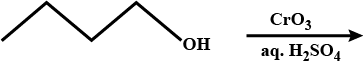
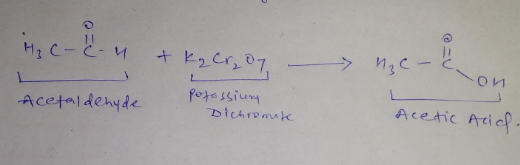
What is the action of acidified potassium dichromate on acetaldehyde?
$$NH_3$$ and its derivative do not show nucleophillic addition reactions with aldehydes and ketone in high acidic medium. Justify.
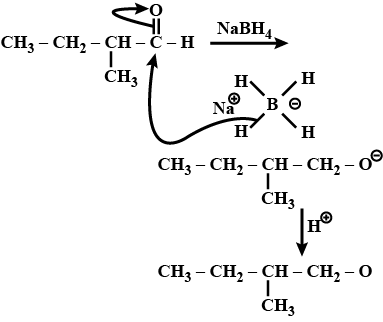
Write the structures of the products of the given reaction:$$CH_3-CH_2-\underset{\underset{\,\,\,\,CH_3}{|}}CH_CHO\xrightarrow{NaBH_4}$$
Image
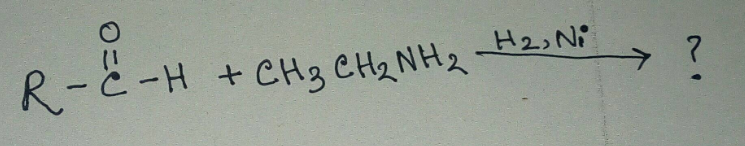
Aldehydes are more reactive toward nucleophillic addition reactions than ketones. Justify.
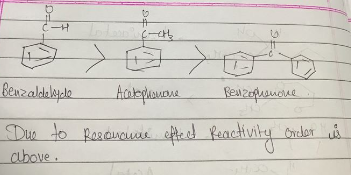
Arrange the following compounds according to increasing order of their reactivity.
acetophenone, benzaldehyde, benzophenone
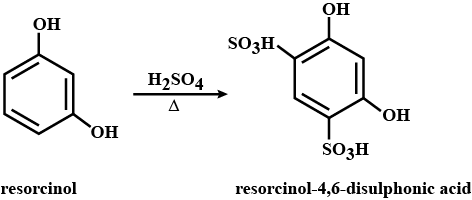
State what is formed when the compound is treated with $$H_2SO_4$$, heat?
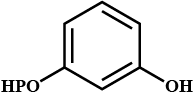
Conversion of acetic acid to acetone
Write structures of the products of the following reactions:
(i)$$CH_3-CH=CH_2 \xrightarrow []{H_2O/H}$$
(iii)$$CH_3-CH_2-\underset { |\\ CH_3 }{ CH } -CHO \xrightarrow []{NaBH_4}$$
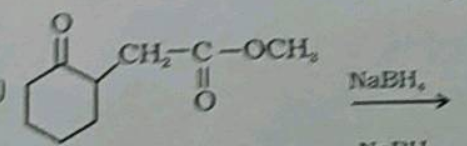
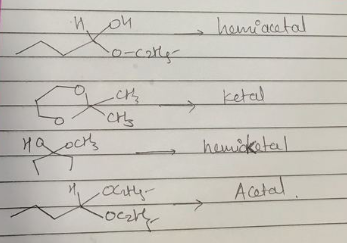
Classify the following compounds as acetals, ketals, hemiacetals and hemiketals.
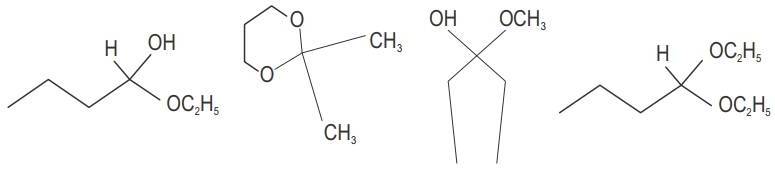
State whether the following statement is True or False:
Formic acid is not the typical acid of the monocarboxylic acid series.
State whether the following statement is True or False:
The IUPAC name of acetic acid is methanoic acid.
Write the final product in the given reaction:
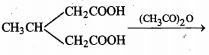
State whether the following statement is True or False:
Monocarboxylic acids are called fatty acids
Fill in the blanks:
Propionic acid and ethyl formate are _____ isomers.
Fill in the blanks:
Acid hydrolysis of alkyl nitriles gives ______
Complete the following equations by writing the missing (A), (B), (C), (D) etc
$$\underset { Ethylene }{ { H }_{ 2 }C } \xrightarrow [ ]{ HBr } (A)\xrightarrow [ ]{ KCN } (B)\xrightarrow [ ]{ { H }_{ 2 }O/{ H }^{ + } } (C)$$
How will you synthesise?
Crotonic acid from acetaldehyde
Answer the following:
Why the $$C=O$$ in $$RCOOH$$ is less reactive towards nucleophiles than in aldehydes and ketones?
How will you synthesise?
$$\alpha$$- Hydroxy propionic acid from acetaldehyde
How will you synthesise?
Acetamide from acetone
Give the common and IUPAC names of the dicarboxylic acids, $$HOOC{({CH}_{2})}_{n}COOH, n=0,1,2,3,4,5$$
Complete the following equations by writing the missing (A), (B), (C), (D) etc
$$\underset { Ethyl\quad alcohol }{ { C }_{ 2 }{ H }_{ 5 } } \xrightarrow [ { H }_{ 2 }{ SO }_{ 4 } ]{ { Na }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 } } (A)\xrightarrow [ { H }_{ 2 }{ SO }_{ 4 } ]{ { Na }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 } } (B)\xrightarrow [ ]{ NaH{ CO }_{ 3 } } (C)\xrightarrow [ ]{ soda\quad lime } (D)$$
Give the structural formula, common names and IUPAC names for the first six straight-chain aliphatic carboxylic acids.
Complete the following equations by writing the missing (A), (B), (C), (D) etc
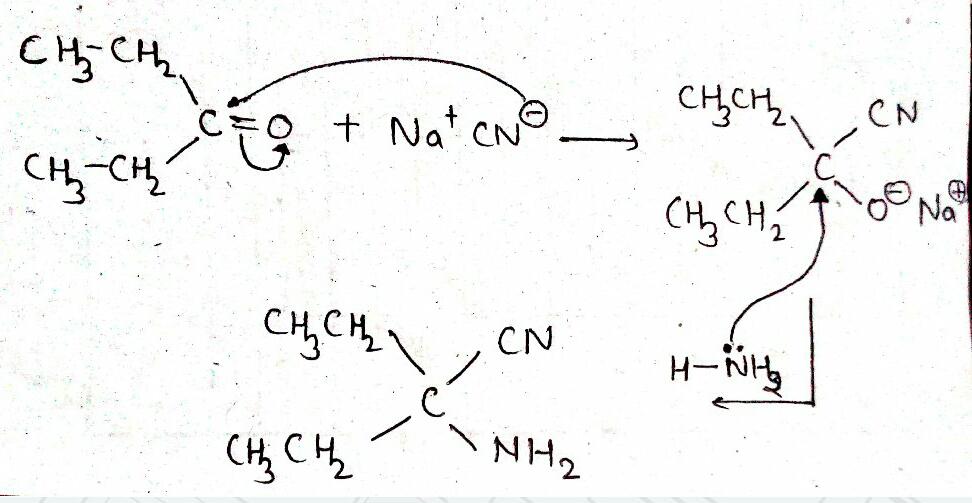
$${ CH }_{ 3 }CHO\xrightarrow [ ]{ HCN } (A)\xrightarrow [ ]{ { H }_{ 2 }O/{ H }^{ + } } (B)\xrightarrow [ { H }_{ 2 }{ SO }_{ 4 } ]{ K_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 } } (C)$$
Complete the given reactions:
Complete the given reactions:
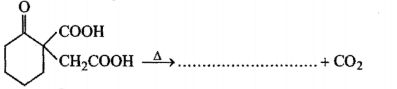
Fill in the blanks :
The carbonyl group in aldehydes and ketones undergoes ........... addition reactions.
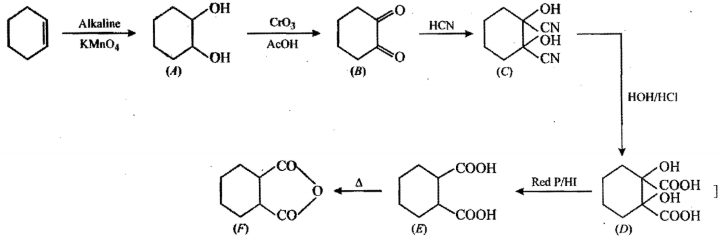
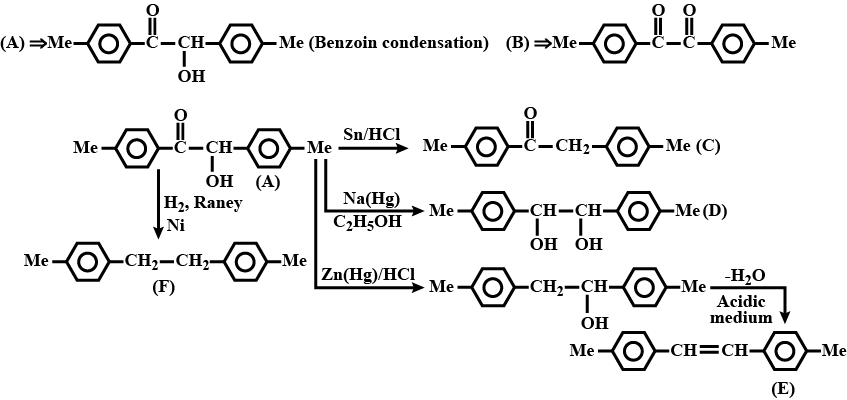
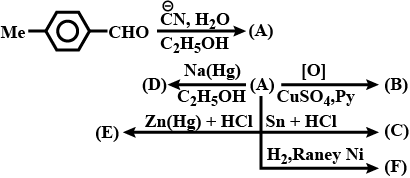
Identify (A) to (F) in the given series of reaction:

What are (A) and (B) in the given reaction?
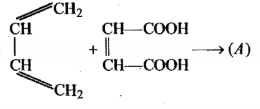
Complete the given reaction:
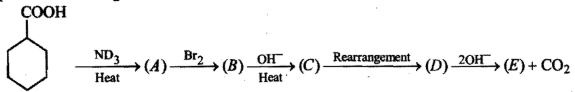
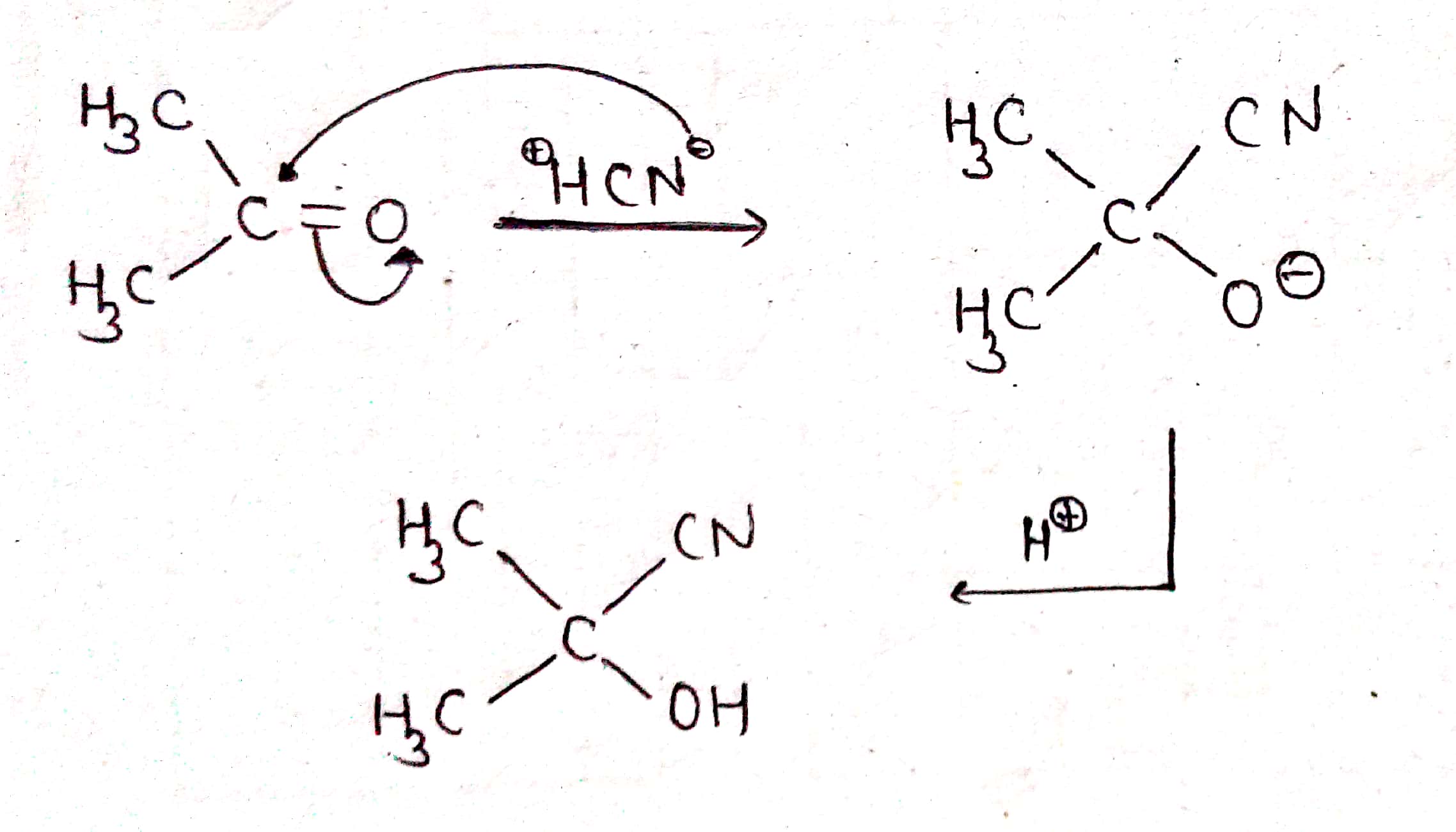
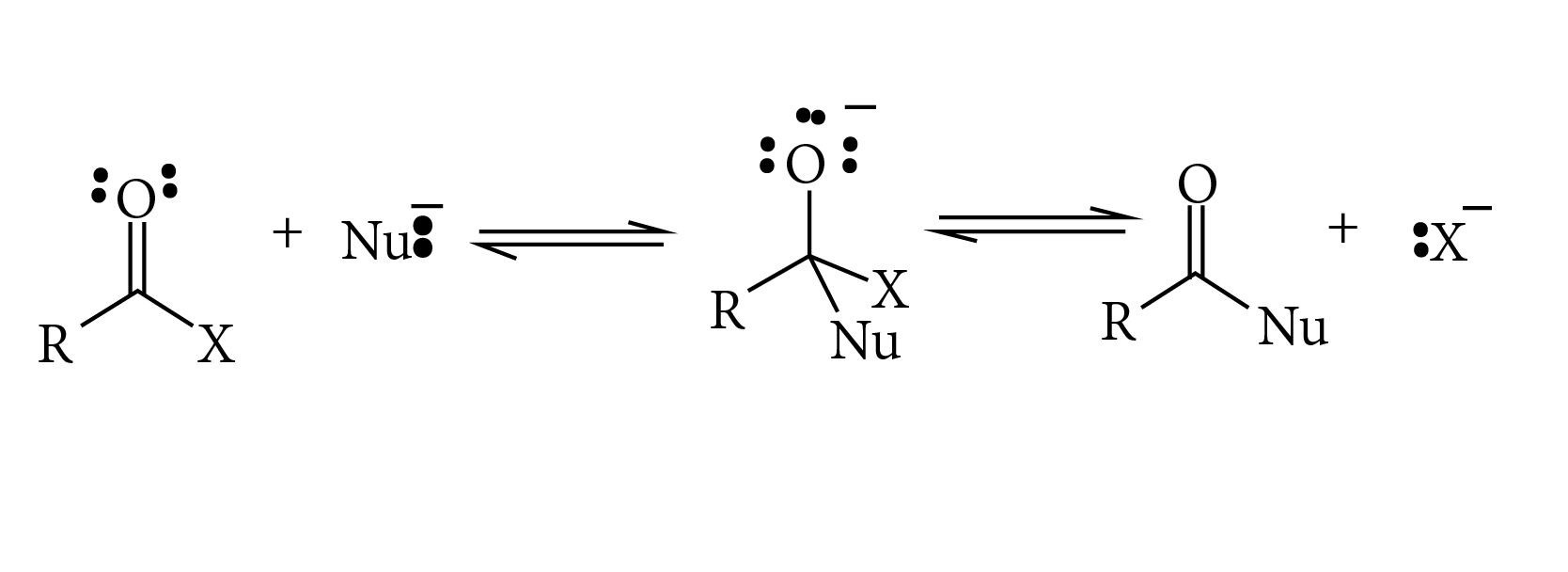
Give the mechansim of :
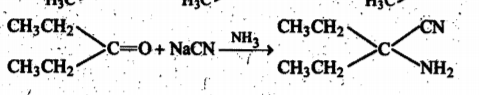
Give the mechanism of :
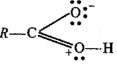
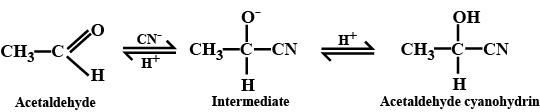
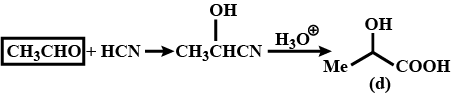
(i) addition of HCN to acetaldehyde
Identify the (A), (B), in the following reactions sequence:
$$ (CH_3)_2C+O +C_6H_5NH_2 \xrightarrow {H^+ } (A) \xrightarrow [Heat] { H_3O^+} (B) $$
Identify the (A), (B), (C) and in the following reactions sequence:
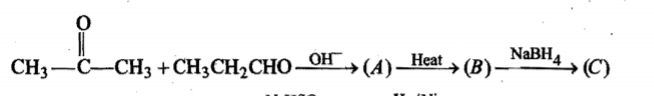
Give the mechansim of :
(ii) Addition of HCN to acetone
Identify the unknown compounds:
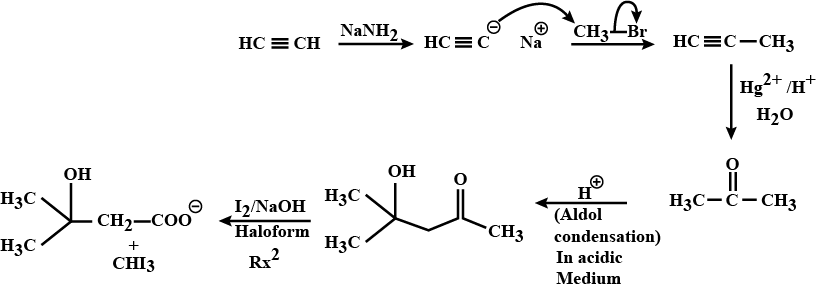
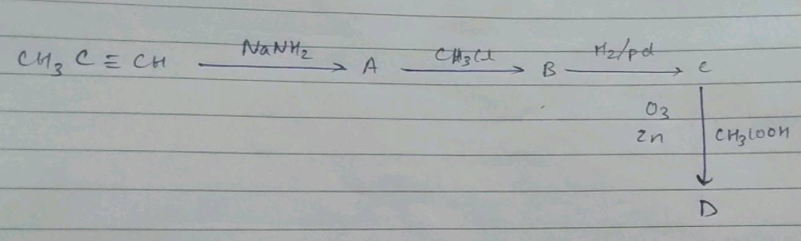
$$C_{6}H_{5}C\equiv CH \xrightarrow {NaNH_{2}, MeI} (A) \xrightarrow {Na/NH_{3}(l)} (B)$$.
$$C_{6}H_{5}C\equiv CH \xrightarrow {NaNH_{2}, MeI} (A) \xrightarrow {Na/NH_{3}(l)} (B)$$.
Identify the final product in the above reaction.
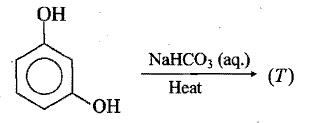
Name the final product of the following reaction:
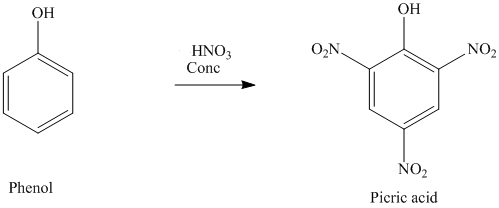
Phenol is treated with nitric acid in the presence of $$H_{2}SO_{4}$$.
Complete the following equations:
$$C_{6}H_{5}CH_{3}\xrightarrow {Air/ V_{2}O_{5}}$$.
How will you obtain?
Adipic acid from benzene.
Identify the unknown compounds:
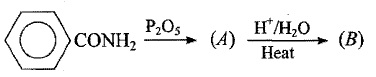
How will you prepare the following compounds from benzene?
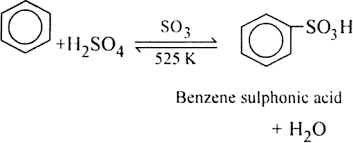
Benzene sulphonic acid.
Give the name and structural formula of one homologue of $$HCOOH$$.
(a) When ethanoic acid reacts with sodium hydrogencarbonate, then a salt $$X$$ is formed and a gas $$Y$$ is evolved. Name the salt $$X$$ and gas $$Y$$. Describe an activity with the help of a labeled diagram of the apparatus used to prove that the evolved gas is the one you have named. Also, write the chemical equation of the reaction involved.
(b) Give any two uses of ethanoic acid.
Write the formulae of : (a) methanoic acid; and (b) ethanoic acid.
(a) What would be observed on adding a $$5\%$$ alkaline potassium permanganate solution drop by drop to some warm ethanol in a test-tube? Write the name of the compound formed during the chemical reaction. Also write chemical equation of the reaction which takes place.
(b) How would you distinguish experimentally between an alcohol and a carboxylic acid on the basis of a chemical property?
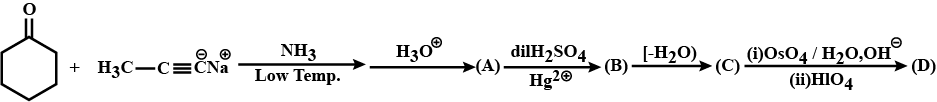
Provide the structures of compounds A, B, C, and D in the above questions.

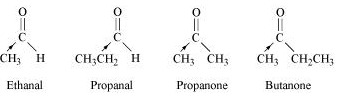
Write the IUPAC names of following ketones and aldehydes. Wherever possible, give common names also.
- $$Ph - CH = CH - CHO$$
Complete the above :
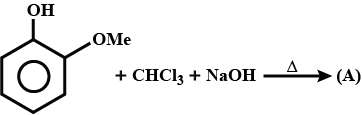
Write the IUPAC names of following ketones and aldehydes. Wherever possible, give common names also.
- $$CH_3(CH_2)_5CHO$$
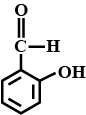
Write the structure of $$2-$$ hydroxybenzaldehyde.
Name the reagents used in the following reaction:
Oxidation of primary alcohol to a carboxylic acid.
Oxidation of primary alcohol to a carboxylic acid.
Give the formula of the following functional groups
(a) Aldehyde
(b) Ketone
Do the following conversions in not more than two steps:
Propanoic acid to $$2-$$hydroxypropanoic acid
Arrange the following compounds inn increasing order of their property as indicated:
$$CH_3COCH_3, C_6H_5-CO-C_6H_5, CH_3CHO$$ (reactivity towards nucleophilic addition reaction)
Write the IUPAC names of above ketones and aldehydes. Wherever possible, give common names also.

Provide the structures of compounds A, B, C, and D in the above questions.

Convert: Methanol to ethanoic acid
Which of the following are carboxylic acids:
$$ C_{2}H_{4}O_{2} , \quad C_{2}H_{4}O, \quad C_{2}H_{6}O, \quad C_{3}H_{6}O_{2} $$
Write the structure of the following compound : $$3-$$ oxopentanal.
Give reasons:
Propanone is less reactive than ethanal towards nucleophilic addition reactions.
Propose the mechanism for the following reaction:
$$CH_3\ CHO+\ HCN \xrightarrow [ ]{ { H }_{ 2 } } \begin{matrix} { CH }_{ 3 }- & CH- & CN \\ & | & \\ & OH & \end{matrix}$$
Give the IUPAC name of the following organic compounds:
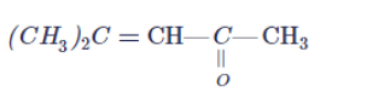
Arrange the following compounds in increasing order of their reactivity in nucleopjilic addition reactions.
Benzaldehyde, $$p-$$Tolualdehyde, $$p-$$Nitrobenzaldehyde, Acetophenone.
Arrange the following compounds in increasing order of their property as indicated:
Acetaldehyde, Acetone, Methyl tert-butyl ketone ( reactivity towards $$HCN$$)
Would you expect benzalsehyde to be more reactive or less reactive in nucleophilic addition reaction than propanal? Explain your answer.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Ethanal, Propanal, Propanone, Butanone
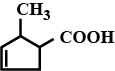
Give the IUPAC name of the following organic compounds:
Write the structural formula and IUPAC name of the compound: di-sec.
butylketone.
Write the IUPAC name of the following:
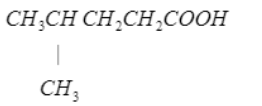
The monoamino monocarboxylic acids have two $$ pK_a $$ values. Explain.
How would you bring about the conversion of Propanol to propanoic acid? Name the process and write reaction involved.
Draw the structure of the following derivatives:
The 2,4-Dinitrophenylhydrazone of benzaldehyde
Draw the structure of the following derivatives:
Cyclopanone oxime
Suggest a reagent for the conversion of ethanol to ethanoic acid.
What are carboxylic acids? Give their formula
Draw the structure of the following derivatives:
Acetaldehyde dimethyl acetal
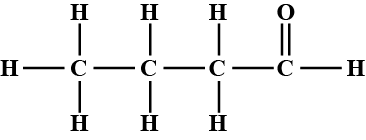
write the name and structure of an aldehyde with four carbon atoms in its molecule.
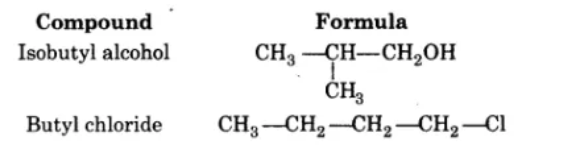
Given the $$IUPAC$$ names of the following compounds numbered (i) to (v). The $$IUPAC$$ name of thr compounds on the left are to guide you for given the correct $$IUPAC$$ names of the compounds on the right.

Identify the odd one out and justify.
Acetic acid, carbonic acid, hydrochloric acid, nitric acid
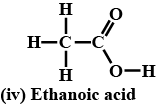
Give the structural formulae and IUPAC name of acetic acid. What is glacial acetic acid?
Write the IUPAC name of the following structural formulae.
$$ CH_{3} CH_{3} - COOH $$
Write structural formulae for the following IUPAC name.
butanone
Write the common name, IUPAC name and formula of one monocarboxylic acid and one dicarboxylic acid
Write the structural formulae for the following IUPAC
butanoic acid
Write the structural formulae for the following IUPAC name .pent-2-one
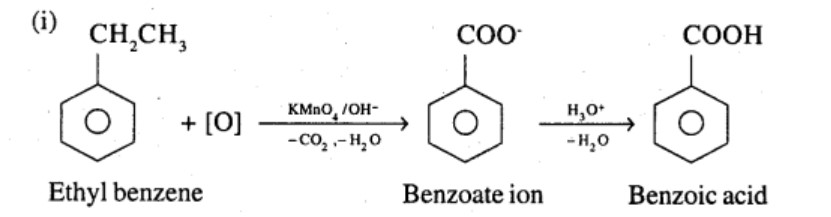
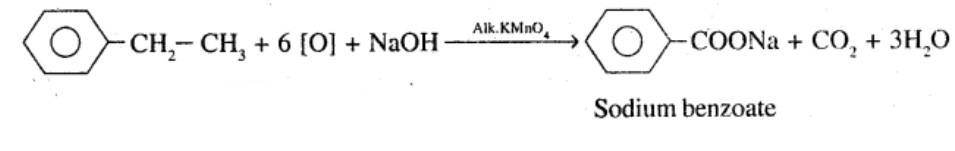
Show how each of the following compounds can be converted to benzoic acid,
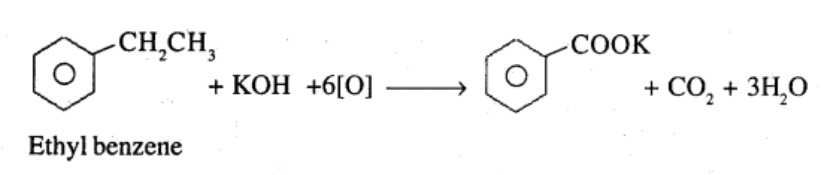
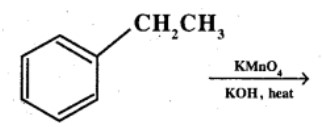
Ethylbenzene
Give the IUPAC names of the following compounds:
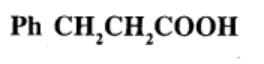
What product is obtained when ethyl benzene is oxidized with alkaline $$KMnO_4$$?
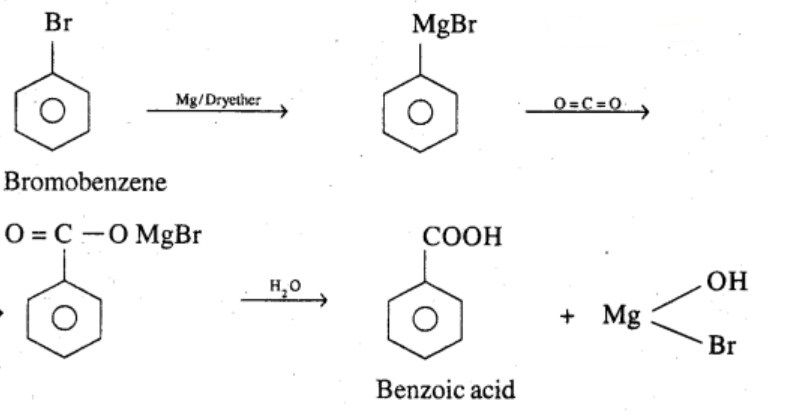
Show how each of the following compounds can be converted to benzoic acid,
Bromobenzene
Give the IUPAC names of the following compounds:
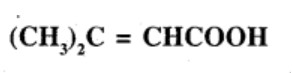
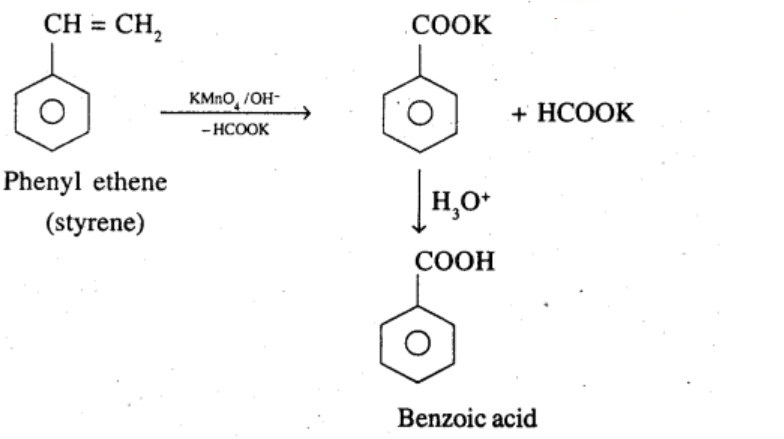
Show how each of the following compounds can be converted to benzoic acid,
Phenylethene (Styrene)
Give the names of the functional group
> C = O
Draw structures of the following derivatives.
Acetaldehydedimethylacetal
Complete each synthesis by giving missing starting material, reagent or products
Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
$$CH_3CH_2CHBrCH_2CH(CH_3)CHO$$
Write down the IUPAC name of the given compound.
$$CH_3-CH_2-CH_2-COOH$$
Write the structural formula of the following compounds.
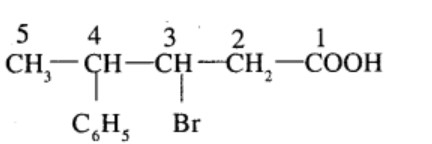
$$3$$-Bromo-$$4$$-phenyl pentanoic acid
Write the uses of methanoic acid.
Write the structural formula and IUPAC name of the following compounds:
(i) Formic acid
(ii) Ethyl acetate
(iii) Ethyl methyl ether
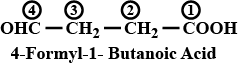
Write the IUPAC name of $$OHC-CH_2-CH_2 - COOH$$
Write the IUPAC name of glycerol and crotonic acid.
Write the IUPAC names of
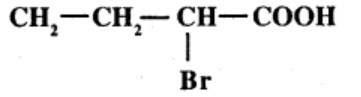
Write the structural formula of the following compounds.
Hex -$$2$$-en-$$4$$- ynoic acid
Draw the structure of $$4$$ methyl pent -$$3$$ ene-$$2$$one.
Write down the IUPAC name of the compound given below:
$$CH_3 - CH_2 - CH_2 - CH_2 - CH_2 - COOH$$
List out the uses of ethanoic acid.
Write the IUPAC name of the following organic compound:
$$CH_3 - COOH$$
$$3$$ mL, of ethanol is taken in a test tube and warmed gently in a water bath. A $$5\%$$ solution of alkaline potassium permanganate is added first drop by drop to this solution, then in excess.
Write chemical equation of this reaction.
Give hybridization state of each carbon in the following compounds:
$$(CH_3)_2CO $$
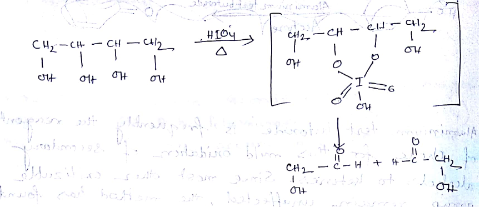
| Column I ( organic compounds oxidised by $$\displaystyle HIO_{4}$$ ) | Column II ( products of $$\displaystyle HIO_{4}$$ oxidation ) |
Consider the reaction in column-I and match them with product properties in column-II
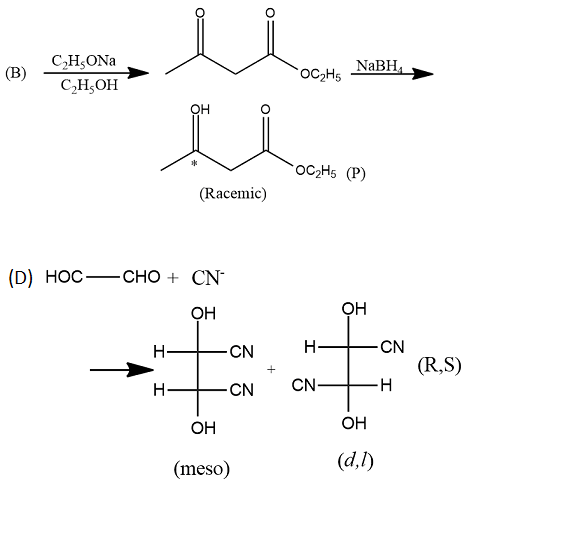
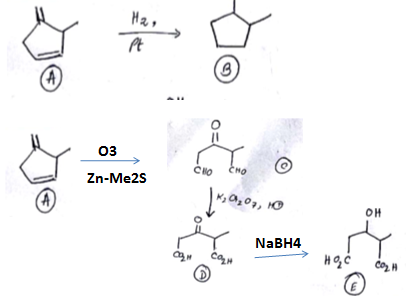
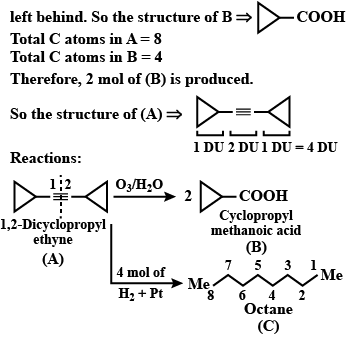
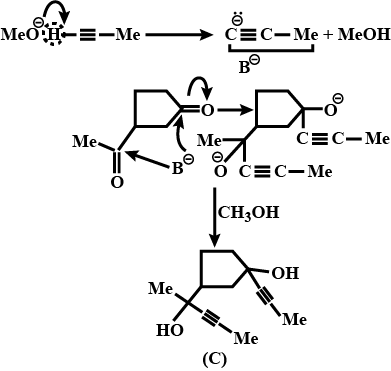
An organic compound has molecular formula A $$\left ( C_{7}H_{10} \right )$$ and known to decolourized $$Br_{2}$$-water solution.A on reduction with $$H_{2}/Pt$$ produces B $$\left ( C_{7}H_{14} \right )$$. B on monochlorination with $$Cl_{2}/hv$$ produces four isomeric monochloro derivatives $$C_{7}H_{13}Cl$$. A on ozonolysis followed by work-up with Zn-dimethyl sulphide affords two and only two products, one being methanal and other C $$\left ( C_{6}H_{8}O_{3} \right )$$. C is oxidised with acidic solution of dochromate to yields D $$\left ( C_{6}H_{8}O_{5} \right )$$ which is soluble in $$NaHCO_{3}$$. D is reduced with $$NaBH_{4}$$ to yield E $$\left ( C_{6}H_{10}O_{3} \right )$$ which may be resolved into enantiomers. Identify A to E.
Identify (A) To (C)
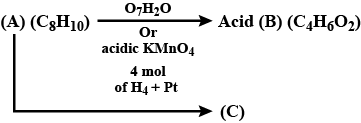
Identify the products.
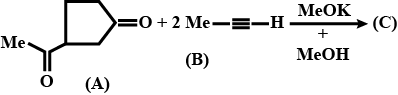
Match the Compounds given in List-I with their Uses in List-II.
The compound which would undergo nucleophilic substitution fastest would be
$$CH_3CH_2CONH_2$$
$$CH_3CH_2COOCH_3$$
$$CH_3CH_2COCl$$
How many equivalents of HCHO are formed by oxidative cleavage of one equivalent of D-glucose by excess of HIO$$_4$$ in water?
Draw the structures of the following compounds.
(i) 3-Methylbutanal (ii) p-Nitropropiophenone
(iii) p-Methylbenzaldehyde (iv) 4-Methylpent-3-en-2-one
(v) 4-Chloropentan-2-one (vi) 3-Bromo-4-phenylpentanoic acid
(vii) p,p-Dihydroxybenzophenone (viii) Hex-2-en-4-ynoic acid
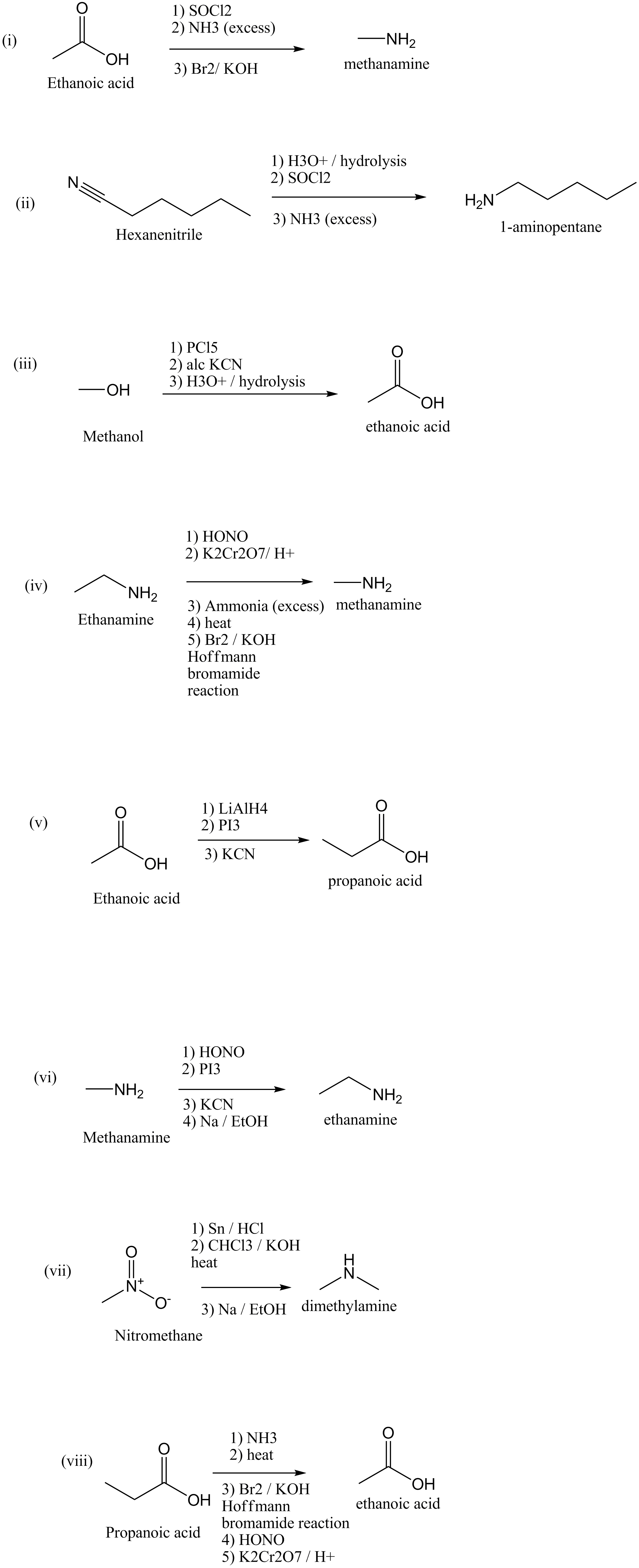
How will you convert:
(i) Ethanoic acid into methanamine
(ii) Hexanenitrile into 1-aminopentane
(iii) Methanol to ethanoic acid
(iv) Ethanamine into methanamine
(v) Ethanoic acid into propanoic acid
(vi) Methanamine into ethanamine
(vii) Nitromethane into dimethylamine
(viii) Propanoic acid into ethanoic acid?
An organic compound with molecular formula $${C}_{9}{H}_{10}O$$ forms 2, 4-DNP derivative, reduces Tollen's reagent and undergoes Cannizaro reaction. On vigorous oxidation it gives a dicarboxylic acid which is used in the preparation of terylene. Identify the organic compound.
Aldehydes, ketones and acids contain $$CO$$ group.
(a) Name the product obtained by the reaction between Acetic acid and Ethanol.
(b) Give any two tests to distinguish between aldehydes and ketones.
Give one chemical test each to distinguish between the following pairs of compounds:
(1) Ethanol and acetic acid
(2) Acetaldehyde and benzaldehyde
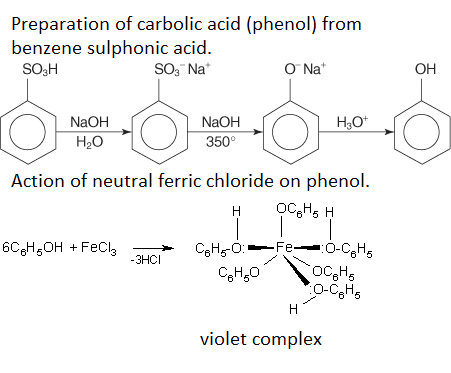
Prepare carbolic acid from benzene sulphonic acid. Write a chemical equation for the action of neutral ferric chloride on phenol.
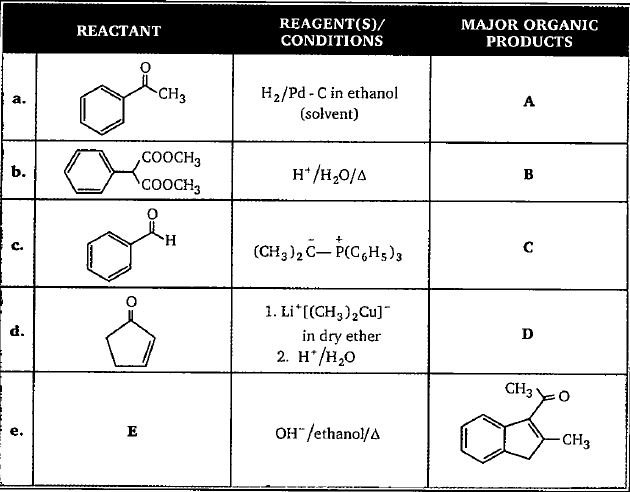
Find A, B, C, D, and E in the given table.
Name the product obtained when alcohol is added to the carbonyl group in the presence of dry $$HCl$$?
$$\xrightarrow[]{LiAlH_4} A..............................$$
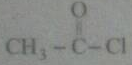
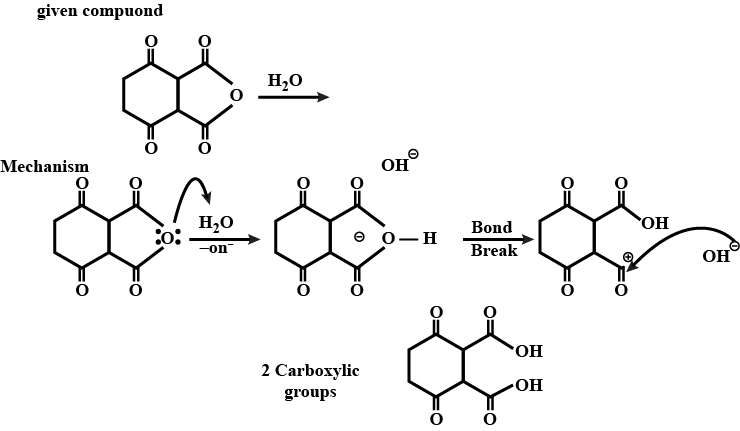
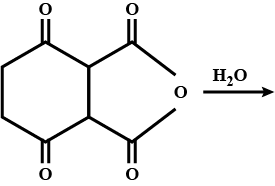
How many -COOH the product has?
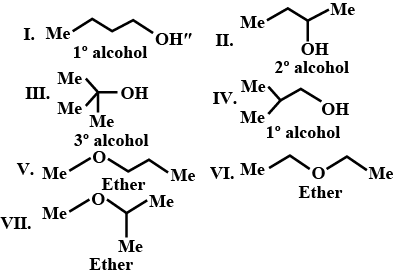
There are seven isomeric compounds with the formula $$C_{4}H_{10}O$$. Write their structure and identify their functional groups.
Write the structures of the following compounds.
$$4$$-Fluoroacetophenone
What is the functional groups in the following molecules?
$$CH_{3}COOH$$
Arrange the following in the increasing order of their reactivity towards nucleophilic addition reaction :
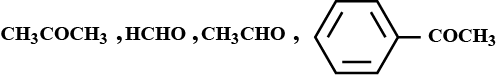
Answer the following questions:
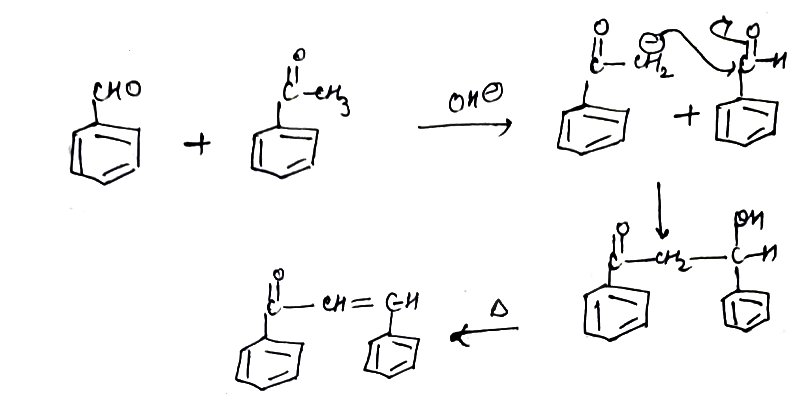
Explain the mechanism of aldol addition reaction.
Mention two uses of carboxylic acids.
Explain the mechanism of aldol addition reaction.
Mention two uses of carboxylic acids.
Match the following:
| (A) Acetic acid | (1) Soda lime | (M) $$Cl{CH}_{2}COOH$$ |
| (B) Formic acid | (2) Poisonous | (N) Dicarboxylic acid |
| (C) Decaboxylation | (3) Phosphorus | (O) Soap |
| (D) Hell-Volhard-Zelinsky reaction | (4) Kolbe's synthesis | (P) Fermentation of sugar |
| (E) Oxalic acid | (5) Saponification | (Q) Carbon monoxide |
| (F) Electrolysis of $${CH}_{3}COOK$$ | (6) Vinegar | (R) Alkane |
| (G) $${CH}_{3}COO{C}_{2}{H}_{5}+NaOH$$ | (7) Dehydration | (S) Reducing acid |
| (H) Formic acid +conc. $${H}_{2}{SO}_{4}$$ | (8) Red ants | (T) Ethane |
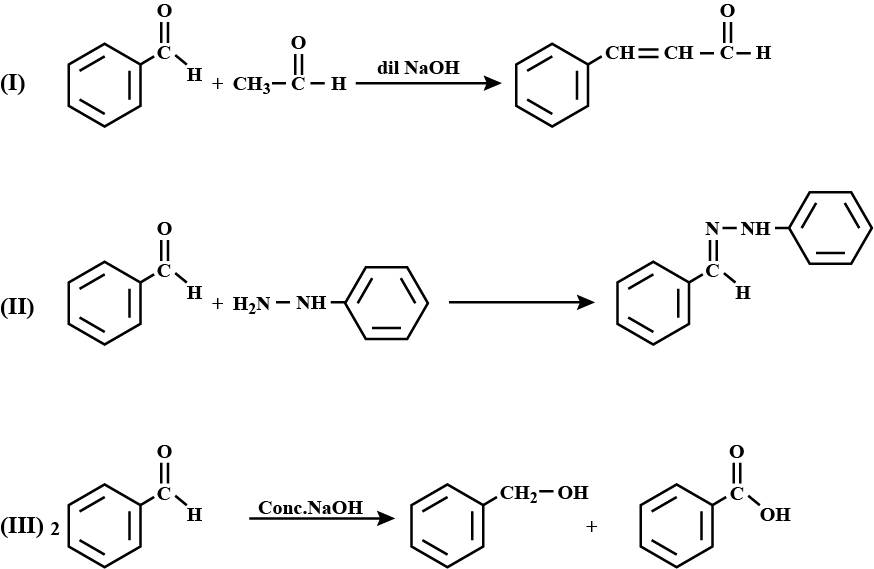
Write the products formed when benzaldehyde reacts with the following reagents :
(i) $$CH_3 CHO$$ in presence of dilute $$NaOH$$
(ii) $$NH_2NH-C_6H_5$$
(iii) Conc. $$NaOH$$
Two isomeric compounds (A) and (B) have molecular formula $${C}_{6}{H}_{10}$$. Oxidation of (A) gives mixture of butanoic and acetic acids, whereas compound (B) gives hexa-1,6-dioic acid. What are (A) and (B)?
Complete the given reactions:
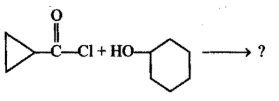
Show the steps to carry out the following transformation:
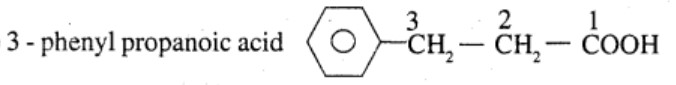
Ethylbenzene $$\rightarrow$$ 2-phenyl propionic acid
Ethylbenzene $$\rightarrow$$ 2-phenyl propionic acid
Fill in the blanks :
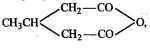
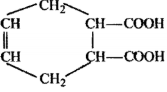
Trimer of acetaldehyde has a structure ........... .
Complete the given reactions:
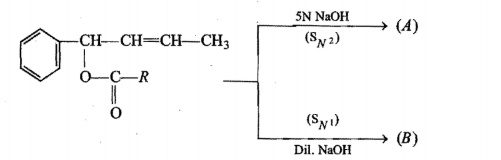
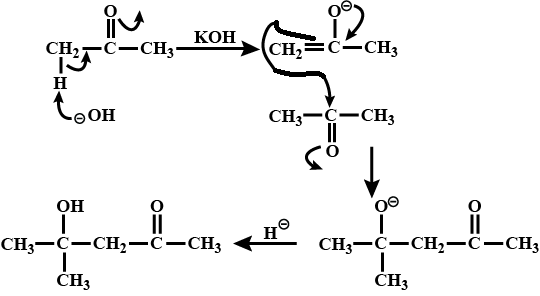
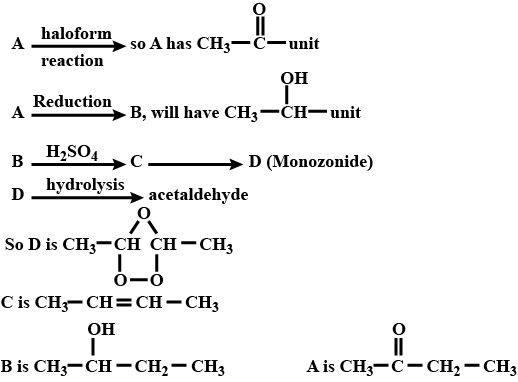
A ketone (A) which undergoes haloform reaction gives compound (B) on reduction. (B) on heating with $$H_2SO_4$$ gives compound (C), which forms monozonide (D). (D) on hydrolysis in the presence of Zn dust gives only acetaldehyde. Identify(A), (B) and (C).
Amongst o-hydroxybezaldehyde and p-hydroxy-benzaldehyde which is more soluble in water?
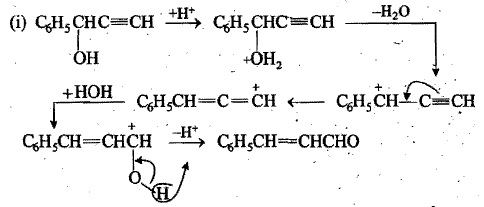
Write the intermediate steps for the following reaction:
$$C_{6}H_{5}CH(OH)C\equiv CH \xrightarrow {H_{3}O^{+}}C_{6}H_{5}CH = CHCHO$$.
Match the following
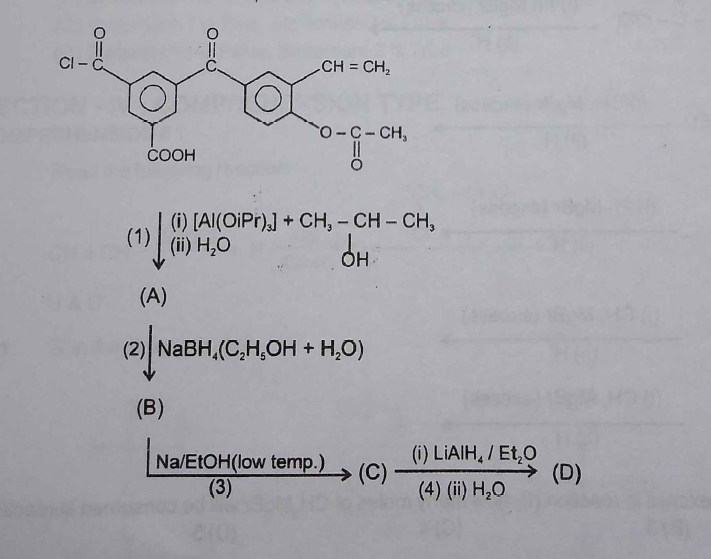
complete the reaction

Complete the above:

Convert
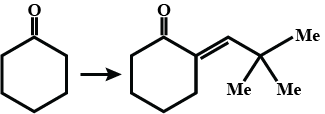
Name and complete the above reaction.

Convert $$CH_3CHO$$ into $$CH_3CH(OH)COOH$$
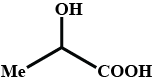
Complete the above :
Complete the above :
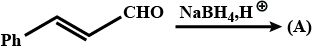
Give the structure of products (A) & (B).

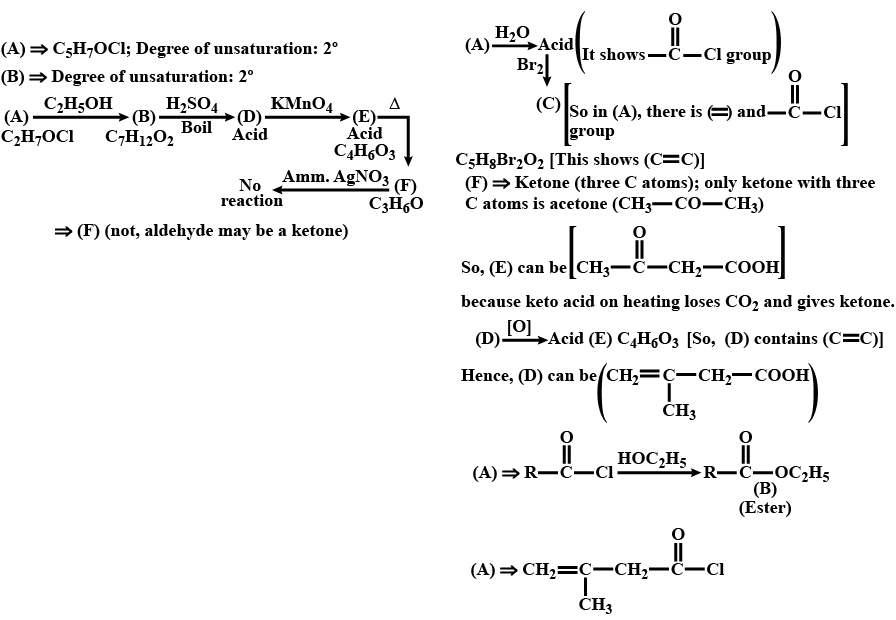
An organic compound $$(A) (C_5H_7OCl)$$ reacts rapidly with ethanol to give $$(B) (C_7H_{12}O_2).(A)$$ also reacts with water to produce an acid which reacts with bromine to give $$(C) (C_5H_8Br_2O_2). (B)$$ on boiling with $$H_2SO_4$$ forms an acid (D). When (D) is oxidised with $$KMnO_4,$$ an acid $$(E) (C_4H_6O_3)$$ is produced. On mild heating , (E) gives $$(F)(C_3H_6O)$$ which cannotb be oxidised by ammoniacal $$AgNO_3.$$ Identify the compounds (A) to (F).
Complete the following reaction::
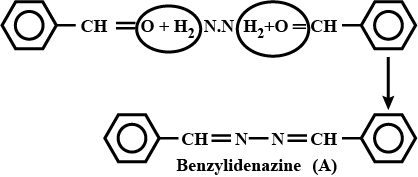
$$2PhCHO + NH_2NH_2 \longrightarrow$$
Complete the above :
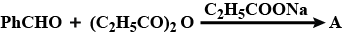
Complete the above:
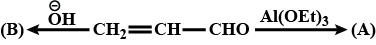
Convert the following:
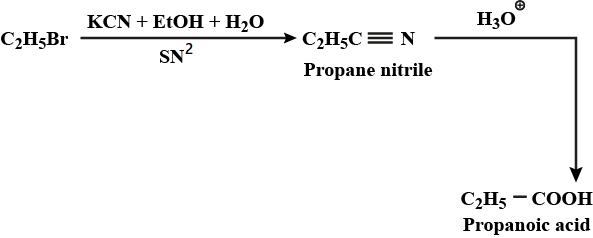
Ethyl bromide to propanoic acid
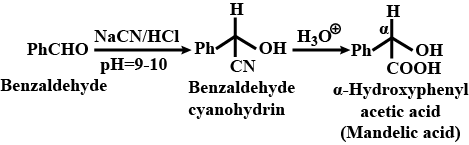
How will you bring about the following conversions in not more than two steps ?
Benzaldehyde to $$\alpha- Hydroxyphenylacetic$$ acid
Complete each synthesis by giving the missing starting material, reagent, or products.

Provide the structures of compounds A, B, C, and D in the above questions.
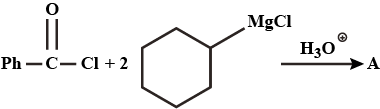
What would be the major product in the above reaction ?
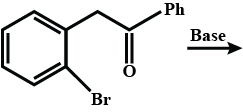
Complete the above reactions with appropriate structures of products / reagents.
Write the products (s) in the following reactions:
(i) & (ii) - Refer Image(iii) $$CH_2 = CH - CH_2 - CN \xrightarrow[(b) H_2O]{(a) DIBAL-H}$$
Write chemical reaction to affect the following transformations:
Benzyl alcohol to phenylethanoic acid
Write chemical reaction to affect the following transformations:
Butan $$-1-ol$$ to butanoic acid
Answer the following questions
Give reasons for the following:
Ethanal is more reactive than acetone towards nucleophilic addition reaction.
Complete the above, giving the structures of principal organic products.
$$pK_3$$ of chloroacetic acid is lower than $$pK_3$$ of acetic acid. Explain.
A compound $$'X'(C_2H_4O)$$ on oxidation gives $$'Y'(C_2H_4O_2). 'X'$$ undergoes haloform reaction. On treatment with $$HCN\ 'X'$$ forms a product $$'Z$$ which on hydrolysis gives $$2-$$ hydroxy propanoic acid.
Write down structures of $$'X'$$ and $$'Y$$'.
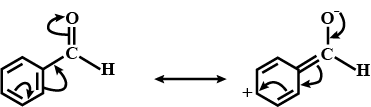
Alkenes $$(> C = C <)$$ and carbonyl compounds $$(> C = 0)$$, both contain a $$\pi $$ bond but alkenes show electrophilic reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents.
Tollens' reagent?
An organic compound $$A$$ on treatment with ethyl alcohol gives carboxylic acid $$B$$ and compound $$C$$. Hydrolysis of $$C$$ under acidic conditions given $$B$$ and $$D$$. Oxidation of $$D$$ with $$KMnO_{4}$$ also gives $$B$$, $$B$$ on heating with $$Ca(OH)_{2}$$ gives $$E$$ with molecular formula $$C_{3}H_{6}O$$. $$E$$ does not give Tollen test or reduce Fehling solution but forms $$2, 4-$$ dinitrophenyl hydrazone. Identify $$A, B, C, D, E$$.
Answer the following questions
How would you account for the following:
Aldehydes are more reactive than ketones towards nucleophilies.
Answer the following questions
How would you account for the following:
The aldehydes and ketones undergo a number of addition reactions.
Answer the following questions
A ketone $$A$$ which undergoes haloform reaction gives compound $$B$$ on reduction. $$B$$ on heating with sulphuric acid gives compound $$C$$, which forms mono-ozonide $$D$$. The compound $$D$$ on hydrolysis in presence of zinc dust gives only acetaldehyde . Write the structures and $$IUPAC$$ names of $$A, B$$ and $$C$$ Write down the reactions involved.
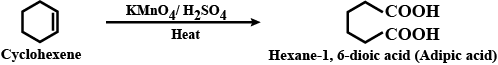
Write chemical reaction to affect the following transformations:
Cyclohexene to hexane $$-1, 6-$$ dioic acid
An aldehyde as well as a ketone can be represented by the same molecular formula, say $$ C_{3}H_{6}O $$. Write their structures and name them. State the relation between the two in the language of science
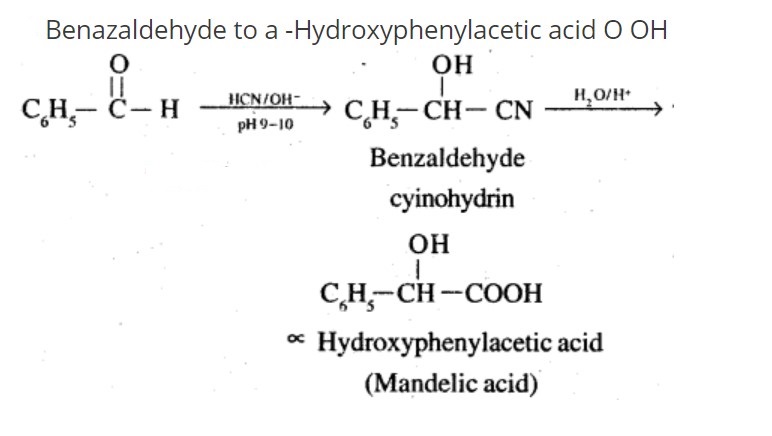
How will you bring about the following conversions in not more than two steps?
Benzaldehyde to a -Hydroxyphenylacetic acid
Predict the product of the following reactions:
Complete each synthesis by giving missing starting material, reagent, or products.
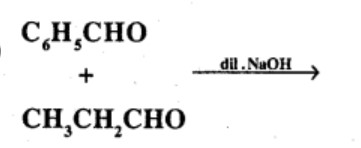
Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
Draw structural formula for each of the following compounds:
ethanal
What is used to describe these compounds taken together?
How will you covert:
Methonal to ethanoic acid
Write the following decreasing order of nucleophilic addition.
$$ CH_3CHO , CH_3COCH_3 , HCHO , C_2H_5 COCH_3 $$
How can ethanol be converted into ethanoic acid?
Draw the structural formula for each of the following compounds: "acetone"
Explain the following reactions and give equations also :
Addition product obtained by addition of alcohol to aldehydes and ketones
Molecular formula of some compounds are given in the box.$$C_{2}H_{4}$$ , $$C_{6}H_{14}$$, $$CH_{3}-CH_{2}-Cl$$ , $$CH_{3}-COOH$$, $$ C_{6}H_{6}$$
Which compound can be used in food?
Arrange the following in decreasing ease of acid-catalysed esterification:
Class 12 Engineering Chemistry Extra Questions
- Alcohols,Phenols And Ethers Extra Questions
- Aldehydes,Ketones And Carboxylic Acids Extra Questions
- Amines Extra Questions
- Biomolecules Extra Questions
- Chemical Kinetics Extra Questions
- Chemistry In Everyday Life Extra Questions
- Coordination Compounds Extra Questions
- Electrochemistry Extra Questions
- General Principles And Processes Of Isolation Of Elements Extra Questions
- Haloalkanes And Haloarenes Extra Questions
- Polymers Extra Questions
- Solutions Extra Questions
- Surface Chemistry Extra Questions
- The D-And F-Block Elements Extra Questions
- The P-Block Elements Extra Questions
- The Solid State Extra Questions