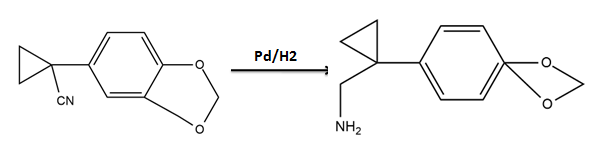
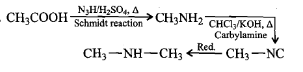
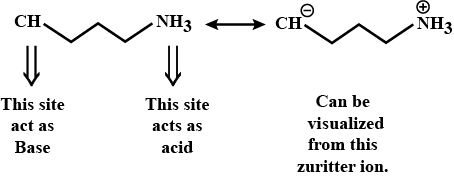
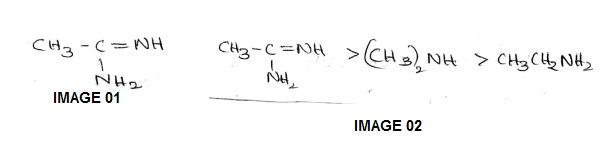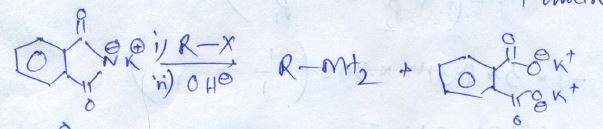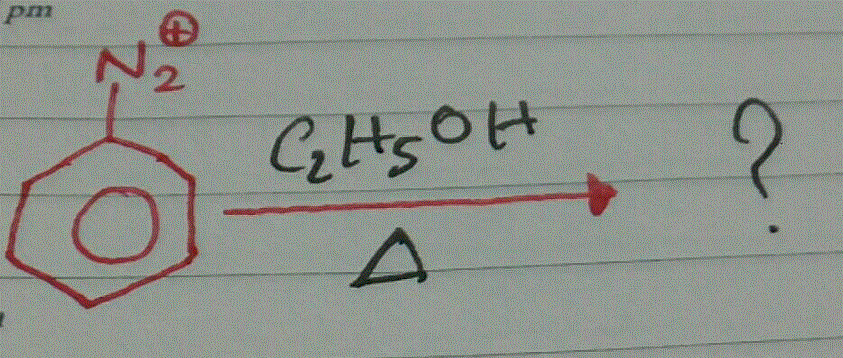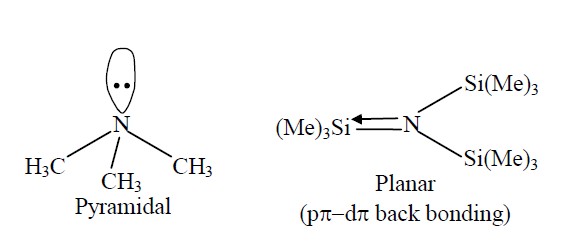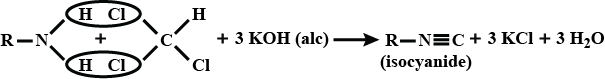Amines - Class 12 Engineering Chemistry - Extra Questions
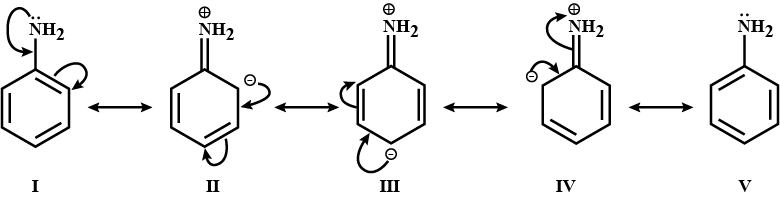
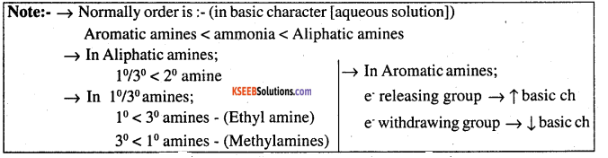
pKb of aniline is more than that of methylamine.
If this is true enter 1, if false enter 0.
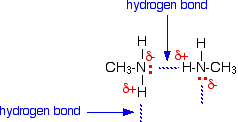
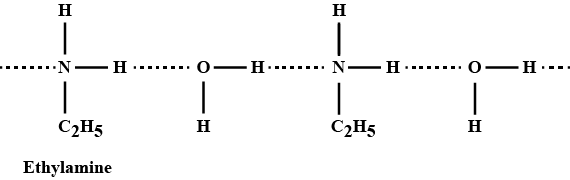
Ethylamine dissolves in water due to intermolecular H-bonding.
If this is true enter 1, if false enter 0.
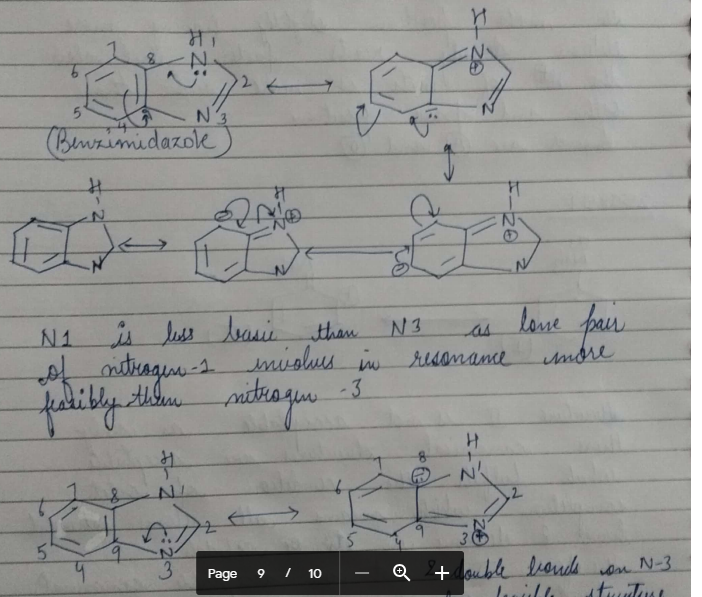
Discuss the basic strength of two nitrogens in benzimidazole.
What is the reason that aromatic diazonium salt is more stable than aliphatic diazonium salt?
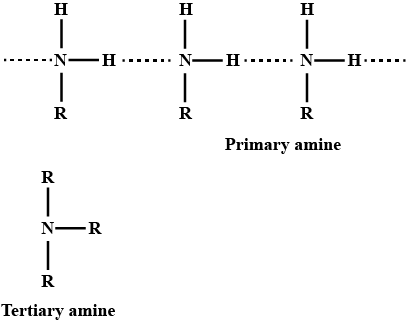
Primary amines have higher boiling points than tertiary amines
If this is true enter 1, if false enter 0.
Ethylamine is not soluble in water whereas aniline is.
If this is true enter 1, if false enter 0.
Diazonium salts of aliphatic amines are more stable than those of aromatic amines.
If this is true enter 1, if false enter 0.
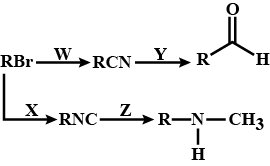
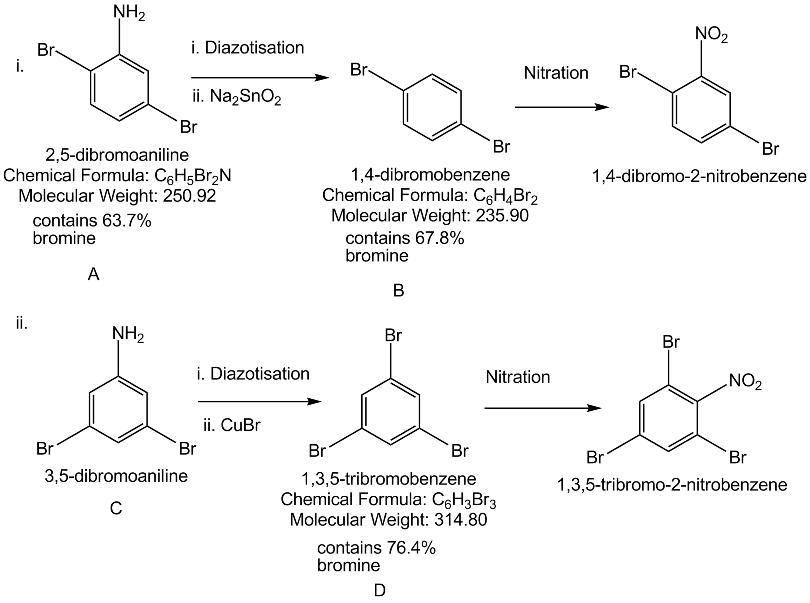
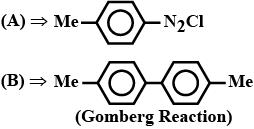
Predict W,X, Y and Z
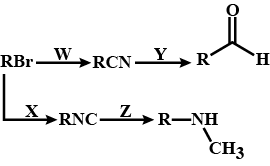
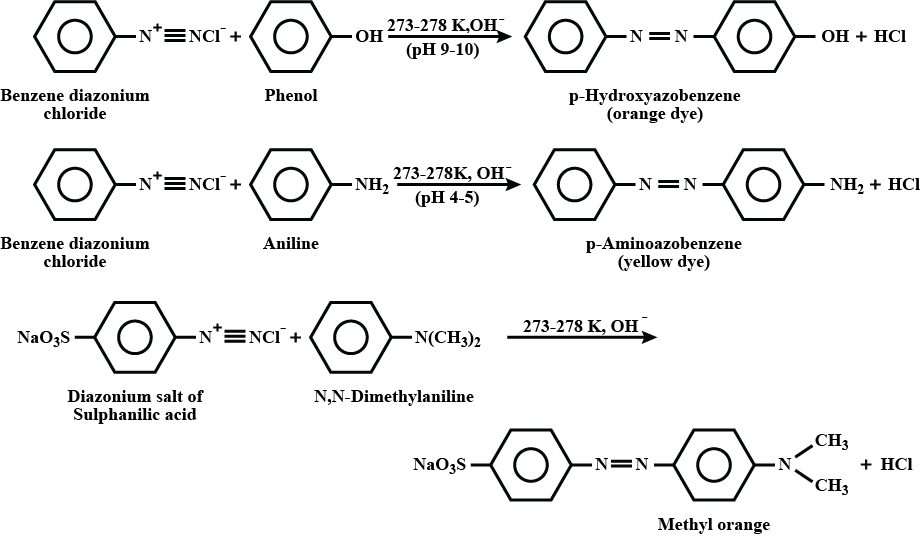
What are coupling reaction?
Write IUPAC name of the given compound:C6H5−NH−C6H5
Write down IUPAC name of CH3−CH3|NH.
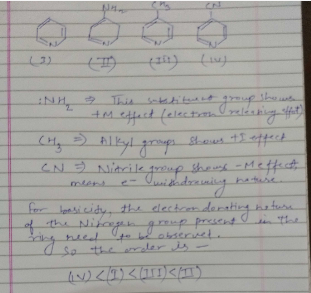
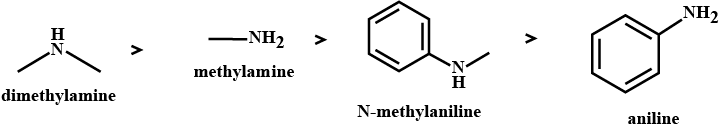
The correct order of basicities of the following compounds is
2)CH3CH3NH2
3)(CH3)2NH
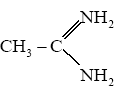
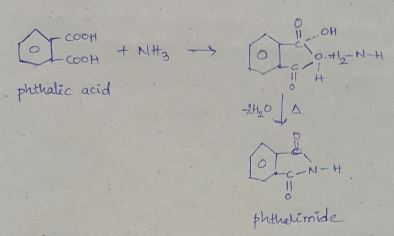
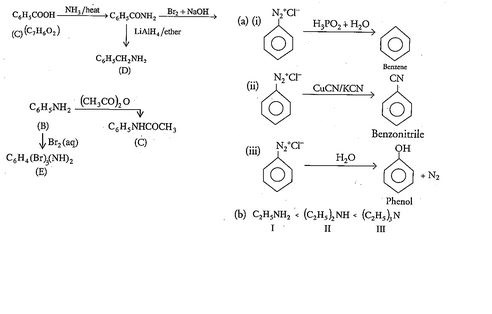
Phthalic acid +NH3→DΔ→E
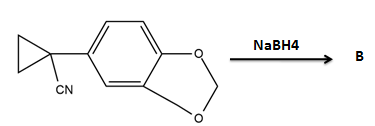
What is B?
2
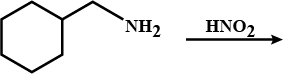
What are the possible products?
Write the reactions of (i) aromatic and (ii) aliphatic primary amines with nitrous acid.
Give a plausible explanation for each of the following:
i. Why are amines less acidic than alcohols of comparable molecular masses?
ii. Why do primary amines have higher boiling points than tertiary amines?
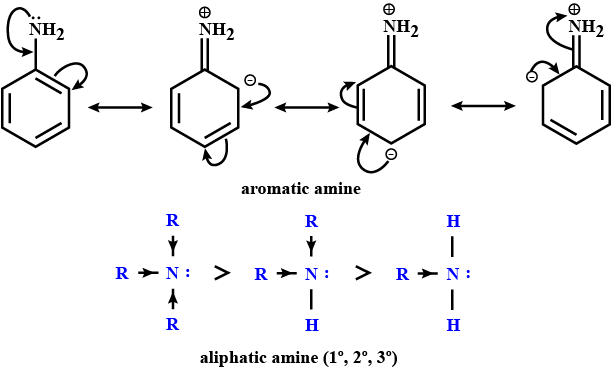
iii. Why are aliphatic amines stronger bases than aromatic amines?
Convert CH3COOH into (CH3)2NH.
Among (CH3)3N and CH3NH2, amine with ________ methyl groups attached has the higher boiling point.
How many of the following are soluble in water?(i) Ethylamine (ii) Aniline
In the given compound which site acts as an acid and which as a base?
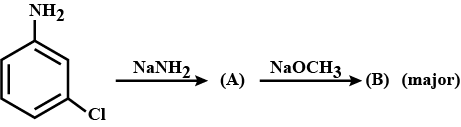
Write the compound (A) and (B) formed in this.
Arrange the following
(i) In decreasing order of the pKb values: C2H5NH2,C6H5NHCH3,(C2H5)2NH and C6H5NH2
(ii) In increasing order of basic strength: C6H5NH2,C6H5N(CH3)2,(C2H5)2NH and CH3NH2
(iii) In increasing order of basic strength:
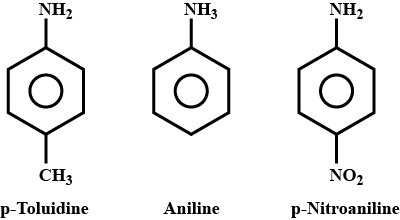
(a) Aniline, p-nitroaniline and p-toluidine
Amines are more acidic than alcohols of comparable molecular masses.
If this is true enter 1, if false enter 0.
(a) Illustrate the following reaction giving suitable example(s) in each case:
(i) hoffmann bromamide degradation reaction
(ii) diazotisation
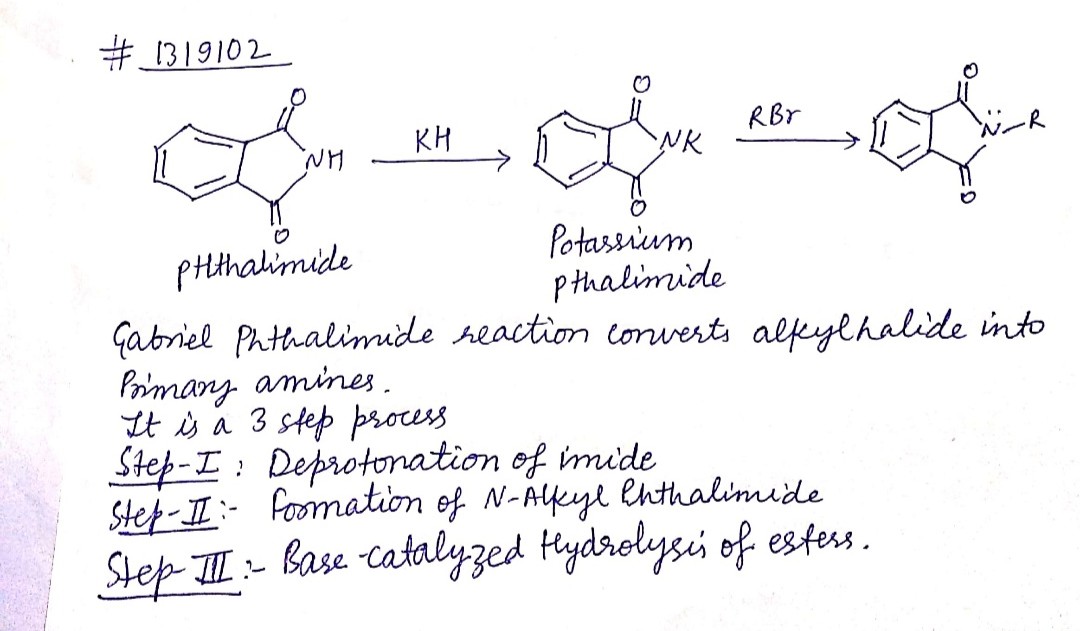
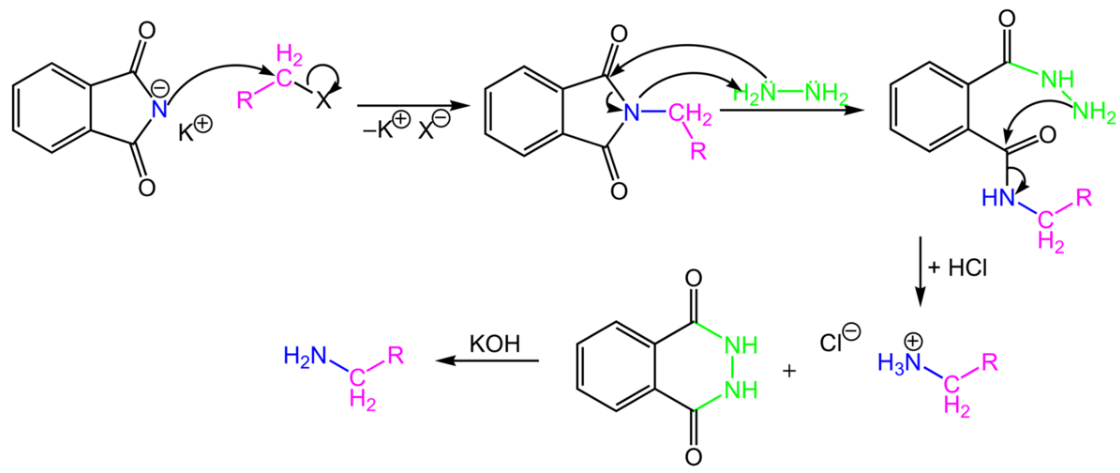
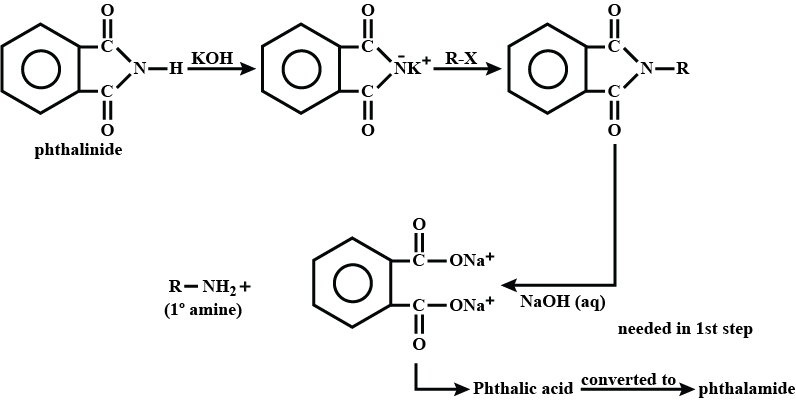
(iii) gabriel phthalimide synthesis
(b) Distinguish between the following pairs of compounds:
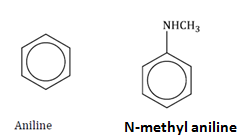
(i) aniline and N-methylaniline
(ii) (CH3)2NH and (CH3)3N
OR
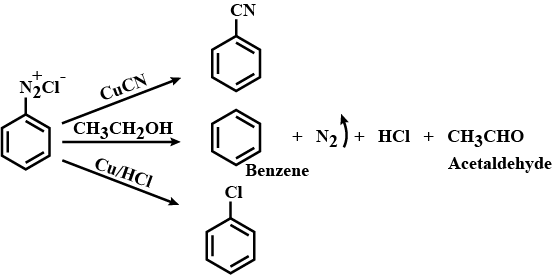
(a) Write the structures of main products when benzene diazonium chloride (C6H5N+2Cl−) reacts with the following reagents:
(i) CuCN/KCN
(ii) H2O
(iii) CH3CH2OH
(b) Arrange the following:
(i) C2H5NH2,C2H5OH,(CH3)3N -in the increasing order of their boiling point
(ii) aniline, p-nitroaniline, p-methyl aniline - in the increasing order of their basic strength
The conversion of primary aromatic amines into diazonium salts is known as ______.
Arrange the following in the increasing order of their basic strength in aqueous solution: CH3NH2,(CH3)3N,(CH3)2NH
Account for the following:
(i) Primary amines (R−NH2) have higher boiling point than tertiary amines (R3N).
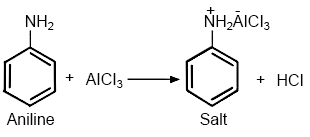
(ii) Aniline does not undergo Friedel-Crafts reaction.
(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
Arrange the following in the decreasing order of their basic strength in aqueous solution:
CH3NH2,(CH3)2NH,(CH3)3N and NH3
An aromatic primary amine A with molecular formula C6H7N undergoes diazotisation to give B. B when the aqueous solution of B is boiled it gives C. Identify A,B and C.
Arrange the following compounds in increasing order of their basic strength. Explain reason
C2H5NH2,NH3,C6H5NH2
Explain the following reactions:
(i) Diazotization
(ii) Carbylamine reaction
How can urea be detected by Biuret test?
What are functional groups? Name the class of compounds containing −NH2 as the functional group.
Identify the reagents X, Y and Z.
C2H2ClX→C2H5CNY→C2H5CH2NH2Z→C2H5CH2NHCOCH3
Arrange the following in decreasing order of their basic strength:
C6H5NH2,C2H5NH2,(C2H5)2NH,NH3
C6H5NH2,C2H5NH2,(C2H5)2NH,NH3
Give reasons of the following:
CH3NH2 is more basic than C6H5NH2
Write a note on Hofmann bromamide degradation.
What is Oil of mirbane.
The basic character of Amine is due to the presence of ________________ on Nitrogen atom.
Methyl amine is __________ basic than ammonia.
Write the name of reaction:
C6H5NH2+CHCl3+3KOHAlcoholic→SolutionC6H5NC+3KCl+3H2O
Write the name of reaction:
C6H5N2ClCuCl/HCl→C6H5Cl+N2
Write the name of following compounds:
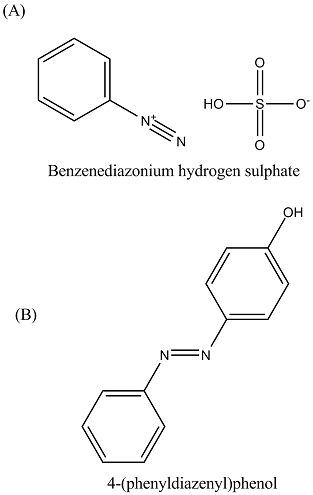
(A) C6H5N⊕2HSO⊖4(B) Ref. image

Write the reactions involved in the Diazotisation.
Write the following reactions:
(i) Williamson's synthesis
(ii) Mendius reaction
(iii) Friedel Craft's Alkylation
(iv) Haloform reaction
(v) Gattermann reaction
(vi) Carbylamine reaction
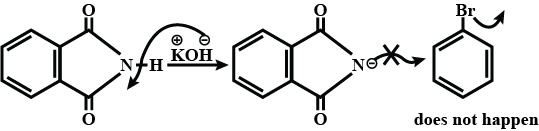
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
How are ethylamine and ethyl methyl amine distinguished by using nitrous acid?
Arrange the following in increasing order of their basic strength:
CH3NH2(I),(CH3)2NH(II),(CH3)3N(III),C6H5CH2NH2(IV)
Which is more basic?
What will be product of the reaction?
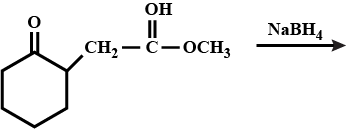
CH3−NH2 CH3−NH−CH3 CH3−N|CH3−CH3 I II III
Compare boiling points of the given compounds:
Arrange the basic strength of the following compounds.
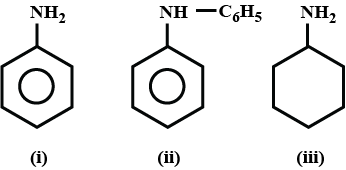
question
Which reaction shows that amine is base?
Why diazonium salts do not show positive Lassaigne's test for nitrogen?
Rearrange the following in an increasing order of their basic strengths :
C6H5NH2,C6H5N(CH3)2, (C6H5)2NH and CH3NH2.
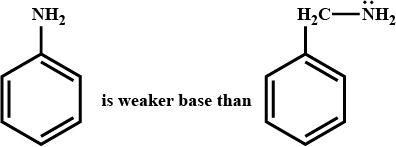
Which is more basic and why?
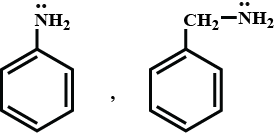
Explain.
Classify the given amines a primary, secondary or tertiary:
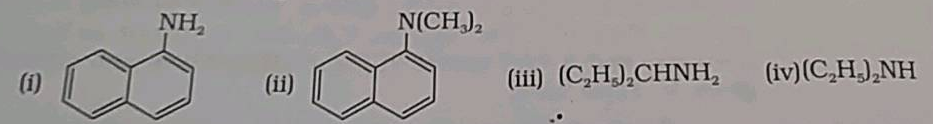
Arrange the given amines in the order of their decreasing basicity:
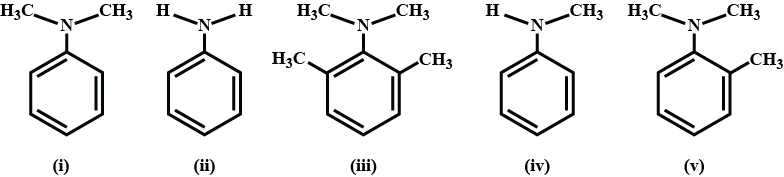
Which among the following are the correct statements?Statement 1: Methyl-amine in water reacts with ferric chloride to precipitate hydrated ferric oxide.Statement 2: Methylamine is more basic than ammonia.
Justify the formation of given products and compare basic character of major organic product and aniline.
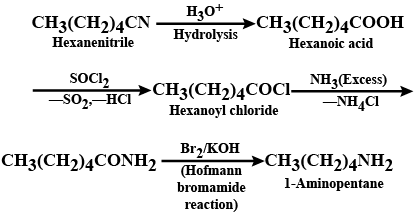
1) Butanenitrile to propanamine
2) propanenitrile to propanamine
Arrange the following in the increasing order of their basic strength.
CH3NH2,(CH3)2NH,C6H5NH2,C6H5CH2NH2
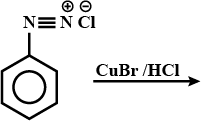
Write the structures of the main products when benzene diazonium chloride reacts with the following reagents:
(a) CuCN (b) CH3CH2OH (c) Cu/HCl
In each of the following pair of compounds, which is more basic in aqueous solution? Give an explaination for your choice:
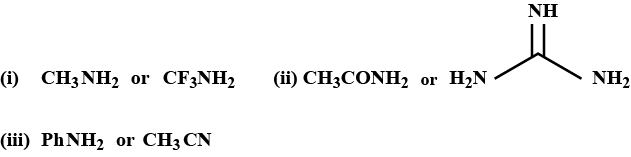
Arrange the following compound according to their basic strength (with suitable explanation):
C6H5NH2,C6H5NHMe,C6H5NMe2,o−MeC6H4NH2
Compare the basic strengths of the given compounds.

Arrange the following in increasing order of their basic strength:
C2H5NH2,C6H5NH2,NH3,C6H5CH2NH2 and (C2H5)2NH
Arrange the following in the increasing order of basic strength
C6H5NH2,C6H5NHCH3,C6H5CH2NH2
Final product for this reaction is:
Arrange the following in increasing order of their basic strength:
C2H5NH2,(C2H5)2NH,(C2H5)3N,C6H5NH2
Which is more basic? Explain in simple words.
Arrange the following amines in the decreasing order of their basic nature :
(a) Aniline, propane-1-amine and N-methyl ethanamine
(b) Benzene-1-diamine, ammonia and 4-aminobenzoic acid.
(c) N- Methylaniline, phenylmethylamine and N-Phenylaniline
(a) Aniline, propane-1-amine and N-methyl ethanamine
(b) Benzene-1-diamine, ammonia and 4-aminobenzoic acid.
(c) N- Methylaniline, phenylmethylamine and N-Phenylaniline
How does the formation of 2∘ and 3∘ amines can be avoided during the preparation of 1∘ amines by alkylation?
Basic nature order of the following is:
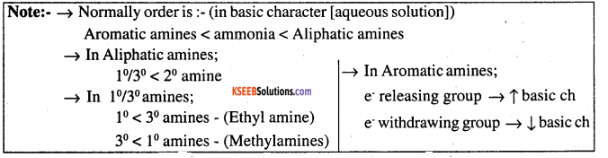
Why are aliphatic amines stronger bases than aromatic amines?
Complete the following acid-base reactions and name the products:
(i)CH3CH2CH2NH2+HCl→
(ii)(C2H5)N+HCl→
Assign reason:
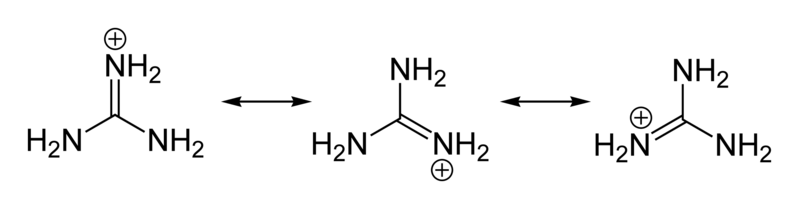
a. There are two NH2 groups in semi carbazide only one is involved in the formation of semicarbazide.
The major product (X) of the reaction is :
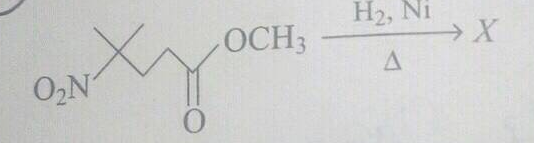
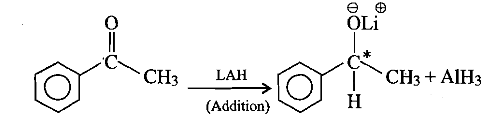
What is A?

What is the product of the reaction if the reactant is treated with ammonia ?
Arrange the following in the increasing order of their boiling point:
C2H5OH, (CH3)2NH, C2H5NH2
Arrange the following compounds in decreasing order of basic strength ( In gaseous phase):
NH3, (C2H5)2NH, (CH3)3N.
Give reason:
The pKb of aniline is greater than methylamine.
Give reason:
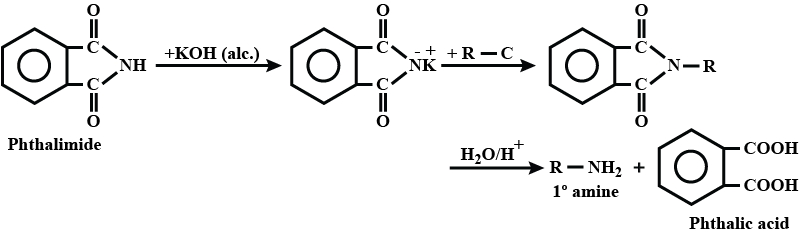
Explain Gabriel Phtalimide reaction.
What is the action of sodium nitrite and hydrochloric acid on ethanamine, N-ethylethanamine and N, N-diethylethanamine?
Complete and balance the following reaction :
C2H5NH2+HCl⟶
The correct order of increasing basic nature for the following compound is
Find the total no. of sigma and pi bonds in the given compound.
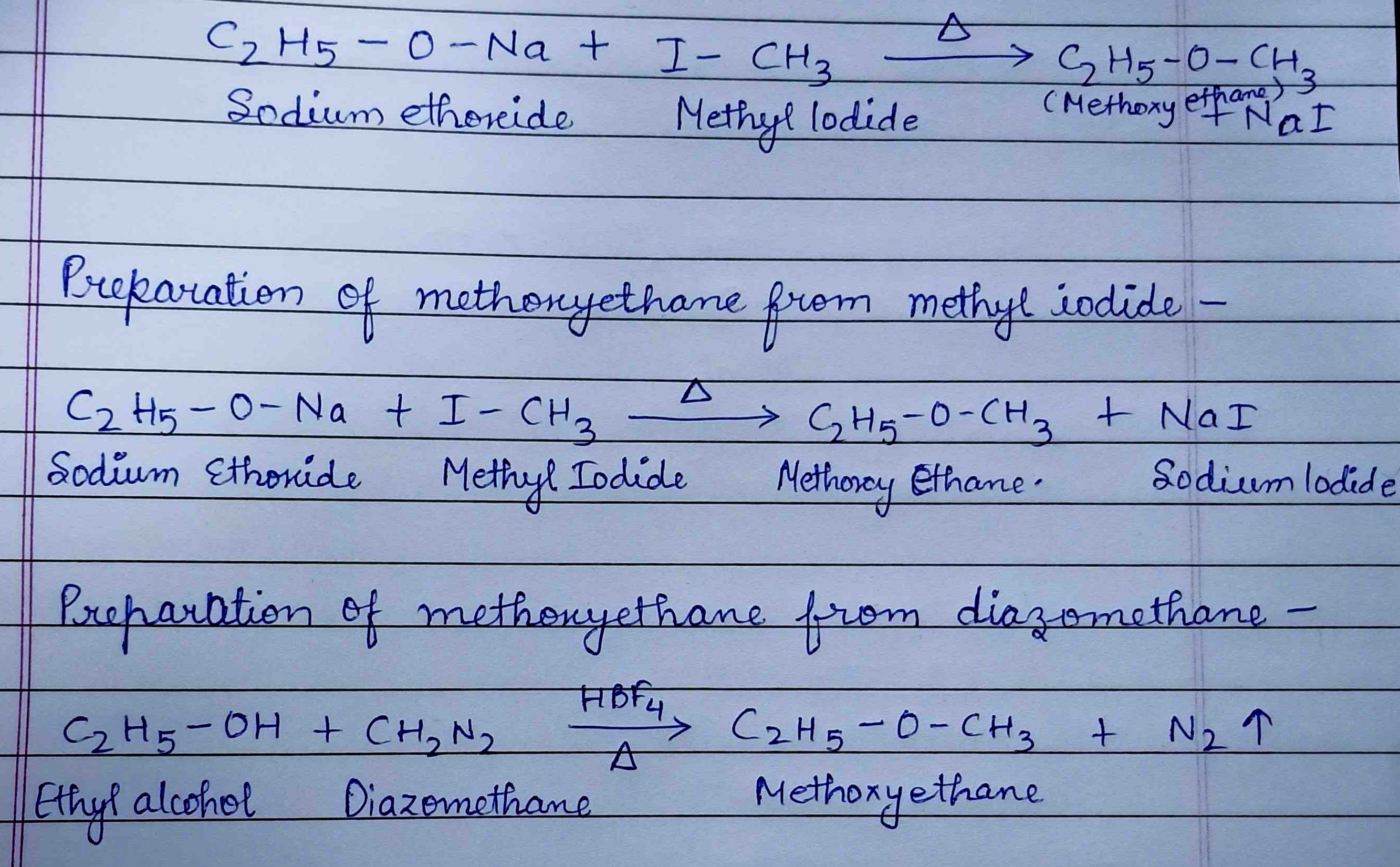
How is methoxyethane prepared from
(i) Methyl lodide (ii) Diazomethane.
What are amines?
Give reasons:
Aliphatic amines are stronger bases than ammonia.
Lower amines are soluble in water due to__________
(CH3)3CNH2 is a______________ amine
Ethylamine is __________ basic than ammonia.
Basic nature of amine is due to the presence of ________ on nitrogen atom.
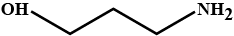
Give (a) conjugate acid (b) conjugate base of HO(CH2)3.NH2.
Fill in the blanks:
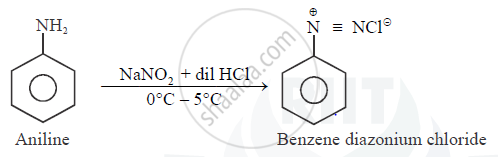
Aniline when treated with sodium nitrite and HCl at 0∘C forms benzene diazonium chloride. This reaction is termed is _______.
Write short note on Basic nature of amines.
Name the final product of the following reaction:
Aniline reacts with sodium nitrite and hydrochloric acid at 0∘.
Explain Me4+NOH− is more basic than Me3N.
Explain tertiary amine has lowest boiling point of a group of isomeric amines.
Compare the basicities of (a) CH3NH2, (b) (CH3)2NH, (c)NH3, (d) C6H5NH2.
Explain why are amines basic in nature?
Explain the boiling point of methylamine is −6.3°C, whereas ethane's boiling point is much lower at −88.6°C
Explain Dimethylamine is a stronger base than trimethyl amine.
Explain there is decreasing order in basicity of: CH3NH2>CH3N=CHCH3>CH3CN.
Identify the unknown compounds:
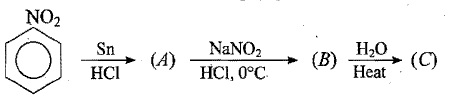
Compare the basicities of:
C2H5NH2, (C3H7)3, CH3CONH2, CH3N−HNa+.
Compare the basicities of:Methylamine, Dimethylamine, Aniline, N-Methylaniline.
Compare the basicities of
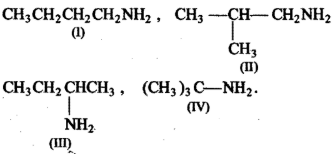
For an amine RNH2, write the expression for Kb to indicate its strength.
Give plausible explanation for: Why do primary amines have higher boiling points than tertiary amines?
Account for: pKb of aniline is more than that of methylamine.
Arrange the following in increasing order of base strength:
methylamine, dimethylamine, aniline, N−methylaniline.
Give the decreasing order of basic strength of their following:
a.NH3
b.NH2OH
c.NH2−NH2
Account for: Ethylamine is soluble in water, whereas aniline is not.
Which of the following is the weakest base?
a. NH3
b. C6H5NH2
c. C6H5CH2NH2
d. CH3NH2
Give the decreasing order of acidic strength of the following :
a. CH4
b.H2O
c.HF
d.NH3
In the following case rearrange the compounds as directed:
- In an increasing order of pKb values:
C2H5NH2,C6H5NHCH3,(C2H5)2NH and C6H5−NH2
Arrange the following:
In decreasing order of the pKb values:
C2H5NH2,C6H5NHCH3,(C2H5)2NH, and CH5NH2
Classify as primary, secondary or tertiary amines.
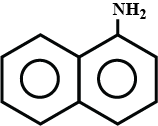
Arrange the following:
In increasing order of solubility in water:
C6H5NH2,(C2H5)2NH,C2H5NH2.
Classify as primary, secondary or tertiary amines.
(C2H5)2CHNH2
In the given pairs which one is more basic and why?
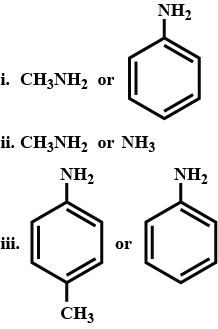
Write structural formulea for the following IUPAC name.
ethanamine
Write short notes on the following:
Diazotisation
Classify as primary, secondary or tertiary amines.
(C2H5)2NH
For an amine RNH2, write the expression for Kb to indicate its base strength.
(DBS2000)
Write the structures of (CH3)3N and (Me3Si)3N. Are they isostructural? Justify your answer.
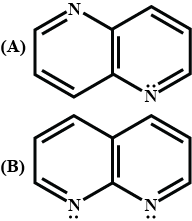
Out of the given compounds A and B, the number of N atoms in the compound which is more basic are:
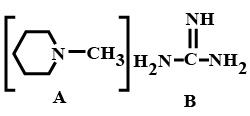
The total number of basic groups in the following form of lysine is:
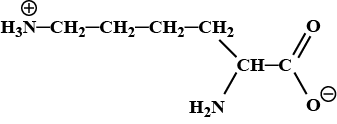
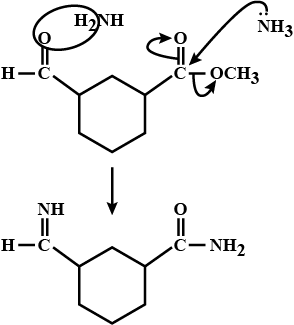
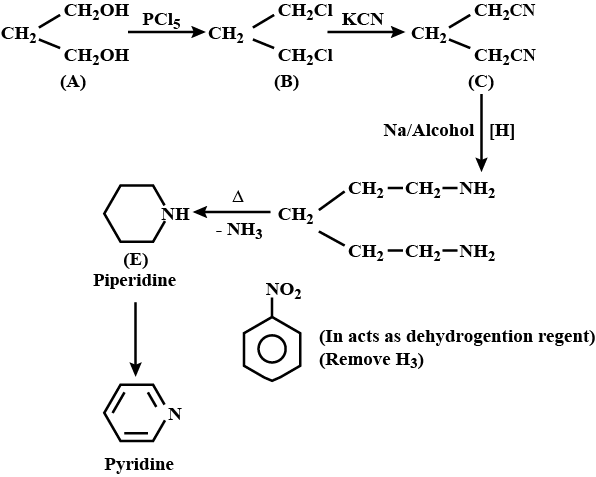
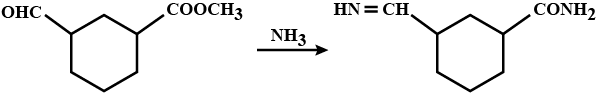
An organic compound (A) composed of C, H, and O gives a characteristic colour with ceric ammonium nitrate. Treatment of (A) with PCl_5 gives (B) which reacts with KCN to form (C). The reduction of (C) with warm Na/C_2H_5OH produces (D) which on heating gives (E) with the evolution of ammonia. Pyridine is obtained in treatment of (E) with nitrobenzene.
Match.
Compound (C) is___________.
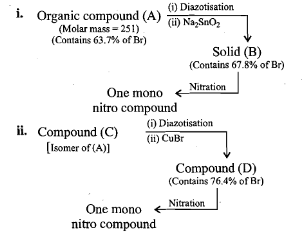
Compound (D) is :
Match the reactions in Column I with appropriate options in Column II.
Among methylamine and aniline, the number of C atoms in compound with lower pK_b is:
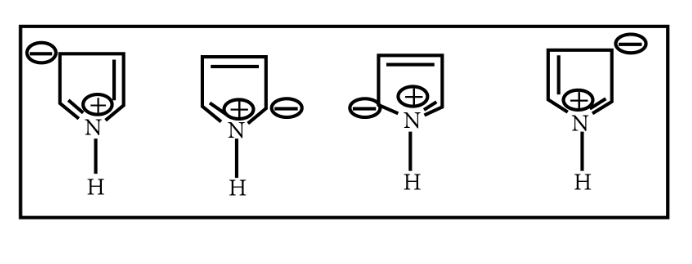
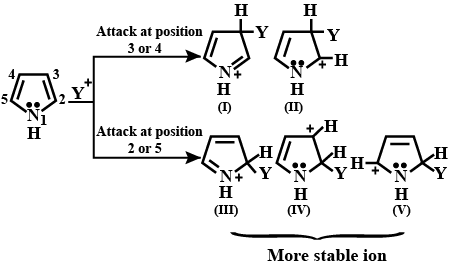
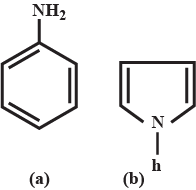
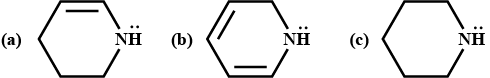
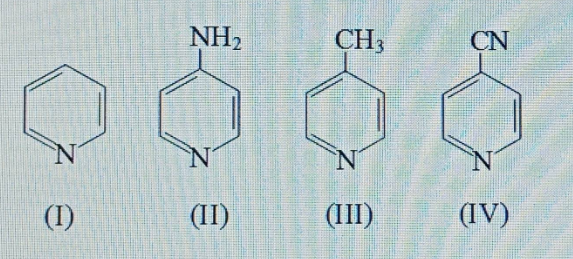
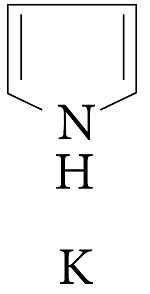
Pyrroles are five-membered nitrogen-containing heterocyclic compounds present in heme which is a constituent of hemoglobin. Draw all the significantly contributing resonance structures of pyrrole (K).
How many resonance structures can be found?
Alkyl cyanides ({CH}_{3}CN) when treated with hydrogen in presence of Pt or with LiAl{H}_{4} produces same carbon number compound. What is the formula of that compound?
Write short notes on the following:
Carbylamine reaction
Why can aromatic primary amines not be prepared by Gabriel phthalimide synthesis?
Give plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
(ii) Why do primary amines have higher boiling point than tertiary amines?
(iii) Why are aliphatic amines stronger bases than aromatic amines?
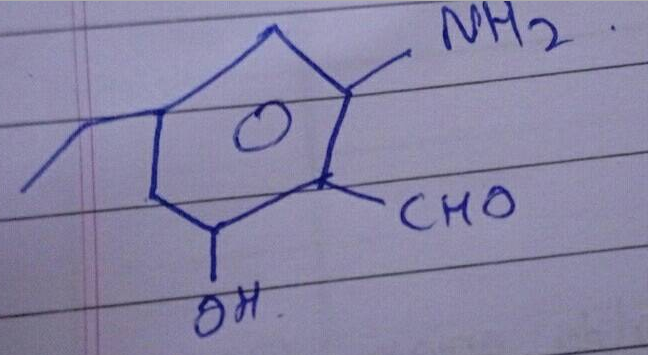
An aromatic compound 'A' of molecules formula C_{7}H_{6}O_{2} undergoes a series of reaction as shown below. Write the structures of A,B,C,D and E in the following reactions:
OR
(a) Write the structures of main products when benzene diazonium chloride reacts with the following regents:
(i) H_{3}PO_{2}+H_{2}O
(ii)CuCN/KCN
(iii)H_{2}O
(b) Arrange the following in the increasing order of their basic character in an aqueous solutions:
C_{2}H_{5}NH_{2},(C_{2}H_{5})_{2}NH,(C_{2}H_{5})_{3}N
(c) Give a simple chemical test to distinguish between the following pair of compounds:
C_{2}H_{5}-NH_{2}\;and\;C_{6}H_{5}-NH-CH_{3}
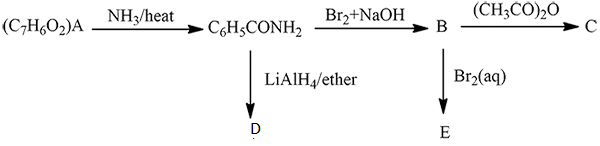
Arrange the following in the decreasing order of their basic strength in aqueous solutions. CH_3NH_2,\ (CH_3)_2NH,\ (CH_3)_3 N, \,NH_3
Amines are classified as primary, secondary and tertiary.
(a) Write the IUPAC name of the following compound:
N{H}_{2} - {\left(C{H}_{2}\right)}_{6} - N{H}_{2}
(b) Which is stronger base - C{H}_{3}N{H}_{2} or {C}_{6}{H}_{5}N{H}_{2}? Why?
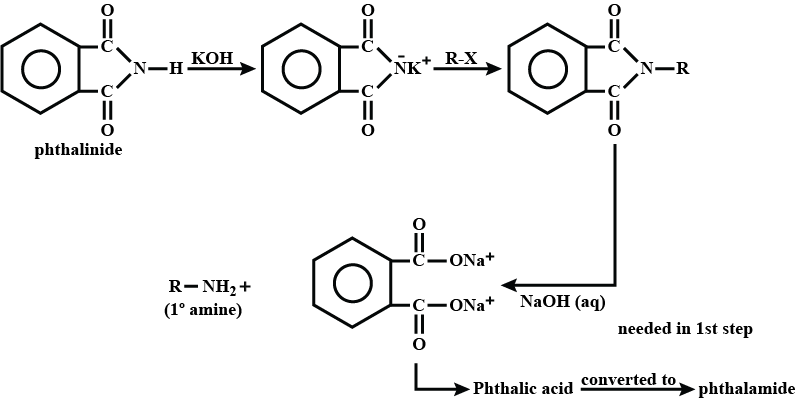
Write a note a Gabriel phthalimide synthesis.
What are biodegradable polymers and non-biodegradable polymers? Write one example of each.
Explain cationic detergents.
What are biodegradable polymers and non-biodegradable polymers? Write one example of each.
Explain cationic detergents.
Arrange the following in the increasing order of basicity:
{CH}_{3}{NH}_{2}, {({CH}_{3})}_{2}NH, {({CH}_{3})}_{3}N
{CH}_{3}{NH}_{2}, {({CH}_{3})}_{2}NH, {({CH}_{3})}_{3}N
Account for the following.
pK_a value of 4-nitrobenzoic acid is lower than that of benzoic acid.
Which of the following is most basic?(Write 1 if I is the answer)
Arrange the following in the increasing order of their pK_b values.
C_6H_5NH_2, C_2H_5NH_2, C_6H_5NHCH_3.
Give a simple chemical test to distinguish between Aniline and N, N-dimethylaniline.
Give reasons (CH_3)_2NH is more basic than (CH_3)_3N in an aqueous solution.
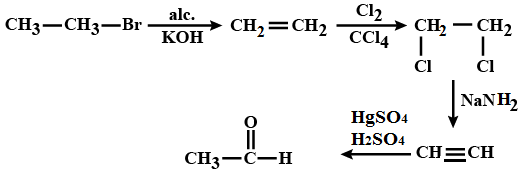
Identify the compound 'D' in the following series of reactions.
CH_3-CH_2-Br \xrightarrow{alc. KOH} A \xrightarrow {Cl_2/ CCl_2}B \xrightarrow{NaNH_2} C \xrightarrow[1\% HgSO_4]{40\%H_2SO_4}D
In the following pair which one is more basic and why? { CH }_{ 3 }{ NH }_{ 2 } or { C }_{ 6 }{ H }_{ 5 }{ NH }_{ 2 }
Arrange the following compound in decreasing order of basic strength NH_{3}, (C_{2}H_{s})_{2}NH, (CH_{3})_{3}N.
In pyrrole
The electron density is maximum on
Account for the following:
(a) Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
(b) On reaction with benzene sulphonyl chloride, primary amine yields product soluble in alkali whereas secondary amine yields product insoluble in alkali.
Give reason:
Aromatic primary amines cannot be prepared by Gabriel's phthalimide synthesis.
Explain the following with at least one example :
Diazotisation.
Write short note on Gabriel phthalimide synthesis.
Compare the basicicties of (a) H_2C=CHCH_2NH_2. (b) CH_3CH_2CH_2NH_2, (c) HC=CCH_2NH_2.
Compare the basicities of (a) Ho(CH_2)_2HH_2, (b) HO(CH_2)_3 NH_2, (c) CH_3CH_2NH_2.
Primary amines are formed by the use of phthalimide. which is the name of the reaction?
Explain why amines are more basic than amides?
Amongst the following, the total number of compounds soluble in aqueous HCl is:
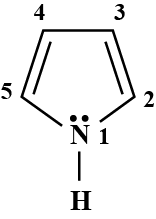
Give the decreasing order of basic character at a, b, c, d in the following compounds:
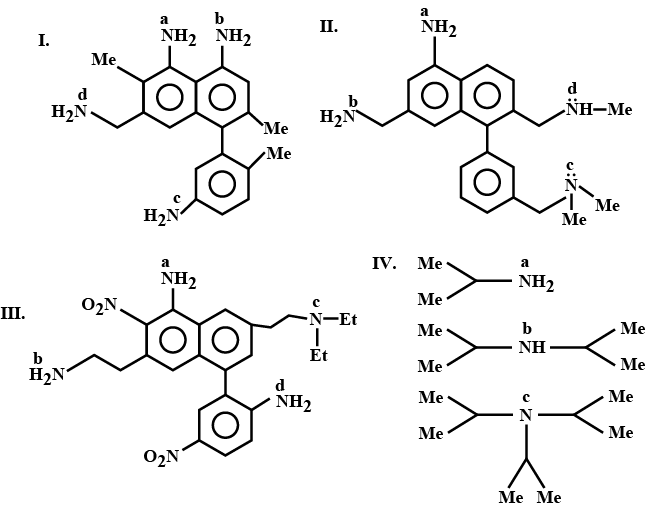
There are four amides with the formula C_{3}H_{7}NO.
a. Write their structures.
b. Identify the amide which has lower melting point and boiling point than the other three.
Which of the following statements are correct?
a. In gas phase, the basic character of amine is {3}^{0} > {2}^{0} > {1}^{0}, Due to the +I effect of (R-), the availability of LP \bar{e}'s on N increases.
b. In aqueous medium, the basic character of amines is Me_{2}NH>Me_{3}N>MeNH_{2}>NH_{3}.
c. In an aqueous medium, the addition of protons increases crowding and thus strains setup, which is the highest in 3^{0} amine decreases its basic character.
d. In an aqueous medium, the ammonium ions in solution are stabilised not only by alkyl groups but also by H-bond donation to the solvent.
Gabriel synthesis is used for the preparation of:
a. 1^{0} amine
b. 2^{0} amine
c. 3^{0} amine
d. All can be prepared
Arrange the following compounds in an increasing order of basic strengths in their aqueous solutions:
{ NH }_{ 3 },{ CH }_{ 3 }{ NH }_{ 2 },{ \left( { CH }_{ 3 } \right) }_{ 3 }N, \left(CH_{3}\right)_{2}NH
Give reason for the following in one or two sentences. 'Dimethylamine is a stronger base than trimethylamine'.
Account for: Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Complete the following.
Mention the chief use of quaternary ammonium salts derived from long chain amines.
Give a plausible explanation for: Why are aliphatic amines stronger bases than aromatic amines?
Give a plausible explanation for why are amines less acidic than alcohols of comparable molecular masses?
What is the best reagent to convert nitrile to primary amine?
Complete the following:
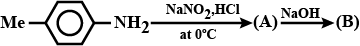
Convert: Hexanenitrile into 1-aminopentane
Account for the following:
Although trimethylamine and n-propylamine have the same molecular weight, but the former boils at a lower temperature (276K) than the latter (322K). Explain why.
\left[ A \right] ,\left[ B \right] ,\left[ C \right] ,\left[ D \right] ,\left[ E \right] ,\left[ F \right] are amines each of which forms a hydrochloride containing 32.42% chlorine. \left[ A \right] ,\left[ B \right] ,\left[ C \right] ,\left[ D \right] evolve {N}_{2} on reaction with H{NO}_{2}, but \left[ E \right] and \left[ F \right] do not. Give structures of \left[ A \right] to \left[ F \right] with reasons.
Why do primary amines have higher boiling points than the tertiary amines?
Illustrate the following reactions by giving chemical equations in each case:
- Gabriel phthalimide synthesis
Give reasons for the following:
Primary amines have higher boiling point than tertiary amines.
Give possible explanation for the following
- Amides are more acidic than amines
Give reasons for the following:
{({CH}_{3})}_{2}NH is more basic than {({CH}_{3})}_{3}N in an aqueous solution.
Illustrate the following reaction:
Coupling reaction:
Amino acids behave like salts rather than simple amines or carboxylic acids. Explain.
How will you covert:
Nitromethane into dimethlyamine
How will you covert:
Hexanenitrile into 1 - aminopentane
Arrange the following:
In decreasing order of basic strength in gas phase:
C_6H_5NH_2, (C_2H_5)_2NH, (C_2H_5)_3N and NH_3
Which of the following amines has larges value of pK_b ?
p-Toluidine, Aniline, p- Nitroaniline.
Write short notes on the following:
Gabriel phthalimide synthesis
Account for the following:
Garbriel phthalimide synthesis is preferred for synthesising primary amines.
Account for the following:
Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Which is more soluble in water, NH_3 or methylamine?
Arrange the following:
In increasing order of boiling point:
C_2H_5OH, (CH_3)_2NH, C_2H_5NH_2
How is phenyl amionmethane obtained from phenyl nitrile. (AISB 2004)
Alkylamines are stronger base than ammonia. Explain.
Explain why silver chloride is soluble in aqueous solution of methyl amine.
(CBSE 1997, 1998, 2002, 2004)
Why is an alkyl amine more basic than ammonia (CBSE Delhi 2009)
What does K_b value for an amine stand for? (AISB 2000)
Why are aqueous solution of amines basic in nature ? (DSB 2006)
Write one chemical reaction each to illustrate the following
Hoffmann's bromamide reaction
Garbriel phthalimide synthesis (CBSE 2008)
(a) Arrange the following in an increasing order of basic strength in water:
C_2H_5NH_2, (C_2H_5)_2NH, (C_2H_5)_3N and NH_3
(b) Arrange the following in increasing order of basic strength in the gas phase
C_2H_5NH_2, (C_2H_5)_2NH, (C_2H_5)_3N and CH_3NH_2. (CBSE Delhi 2008)
Lassaigne's test is not shown by diazonium salts. Why?
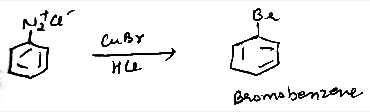
How are the following conversions carried out ?
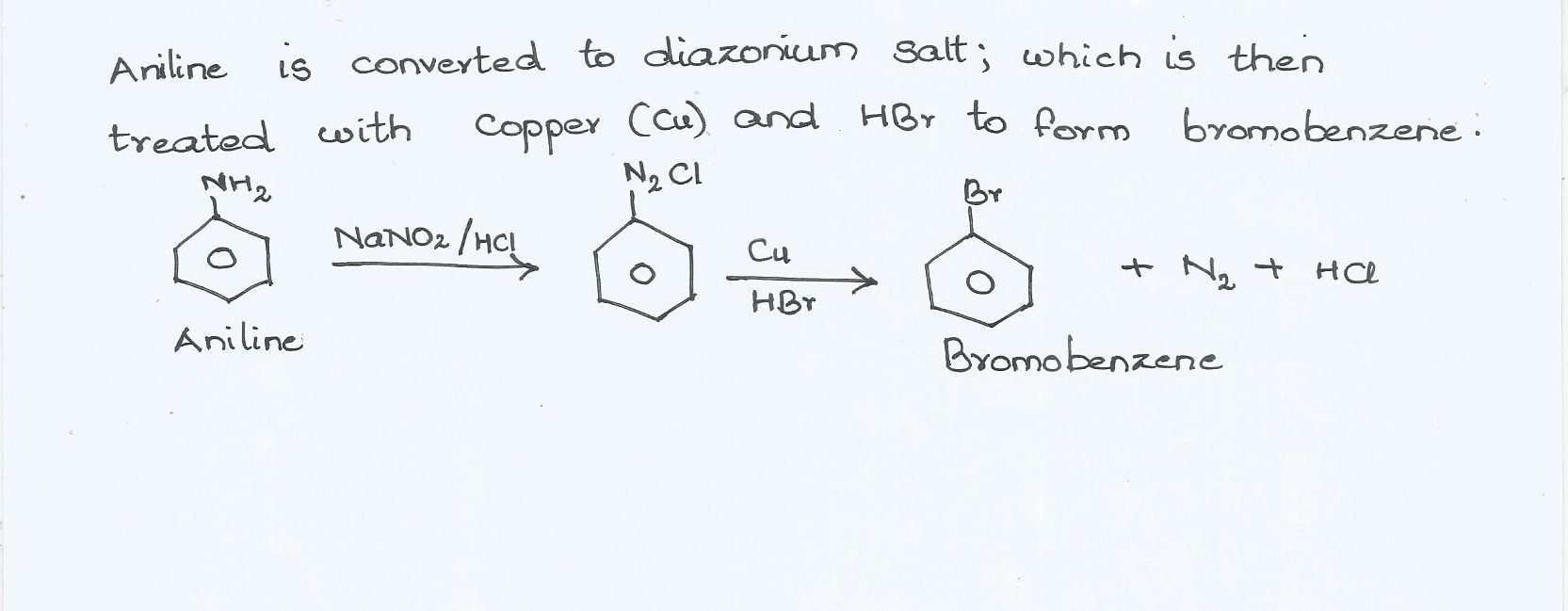
Aniline into bromobenzene
Class 12 Engineering Chemistry Extra Questions
- Alcohols,Phenols And Ethers Extra Questions
- Aldehydes,Ketones And Carboxylic Acids Extra Questions
- Amines Extra Questions
- Biomolecules Extra Questions
- Chemical Kinetics Extra Questions
- Chemistry In Everyday Life Extra Questions
- Coordination Compounds Extra Questions
- Electrochemistry Extra Questions
- General Principles And Processes Of Isolation Of Elements Extra Questions
- Haloalkanes And Haloarenes Extra Questions
- Polymers Extra Questions
- Solutions Extra Questions
- Surface Chemistry Extra Questions
- The D-And F-Block Elements Extra Questions
- The P-Block Elements Extra Questions
- The Solid State Extra Questions