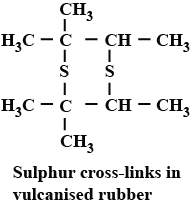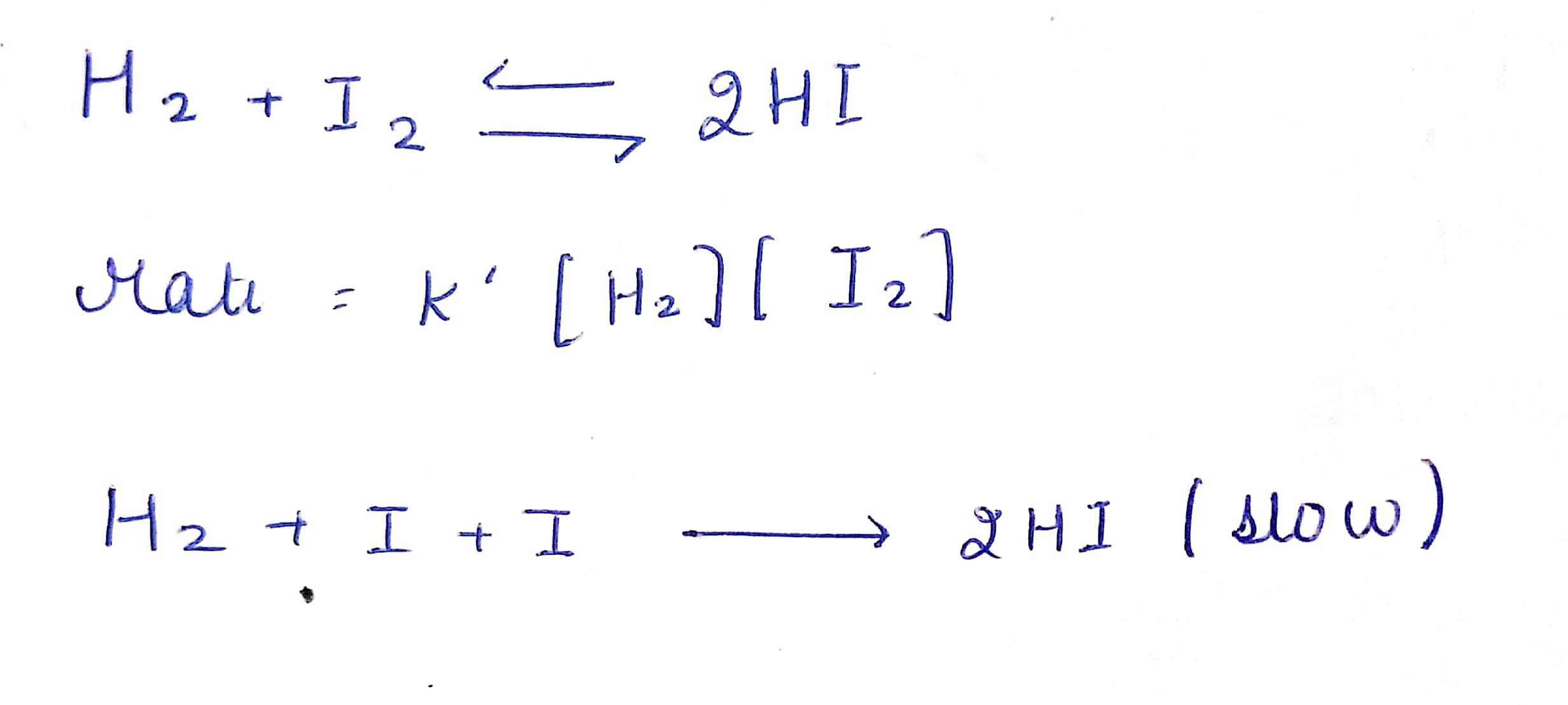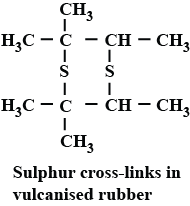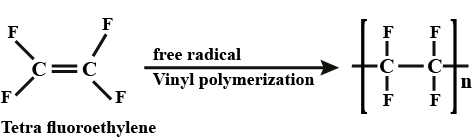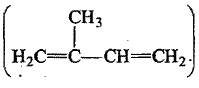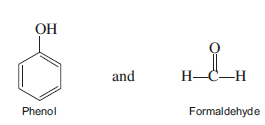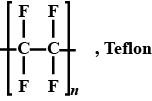Polymers - Class 12 Engineering Chemistry - Extra Questions
Write any two differences between step growth polymerisation and chain growth polymerisation.
What is the code number on plastic bottle HDPE?
What is the utility of vulcanisation of rubber? How is vulcanisation carried out?
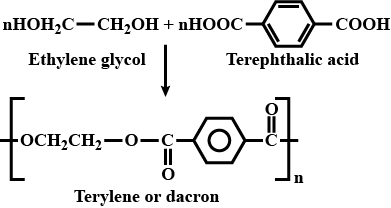
Which catalyst is used in the preparation of dacron?
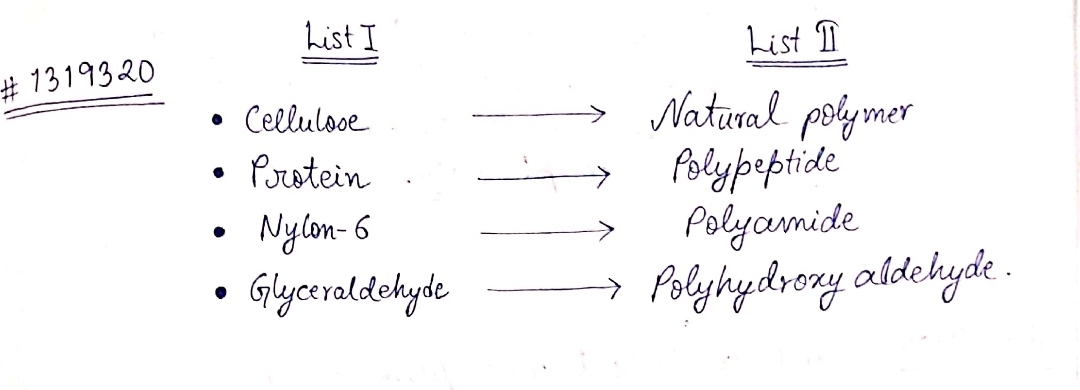
Match the following:
explane this reaction$$H_{2}+I_{2}$$ = 2HI
Match the List I with the List II.
Illustrate the following terms :
(a) Copolymerization (b) Vulcanization
(a) Copolymerization (b) Vulcanization
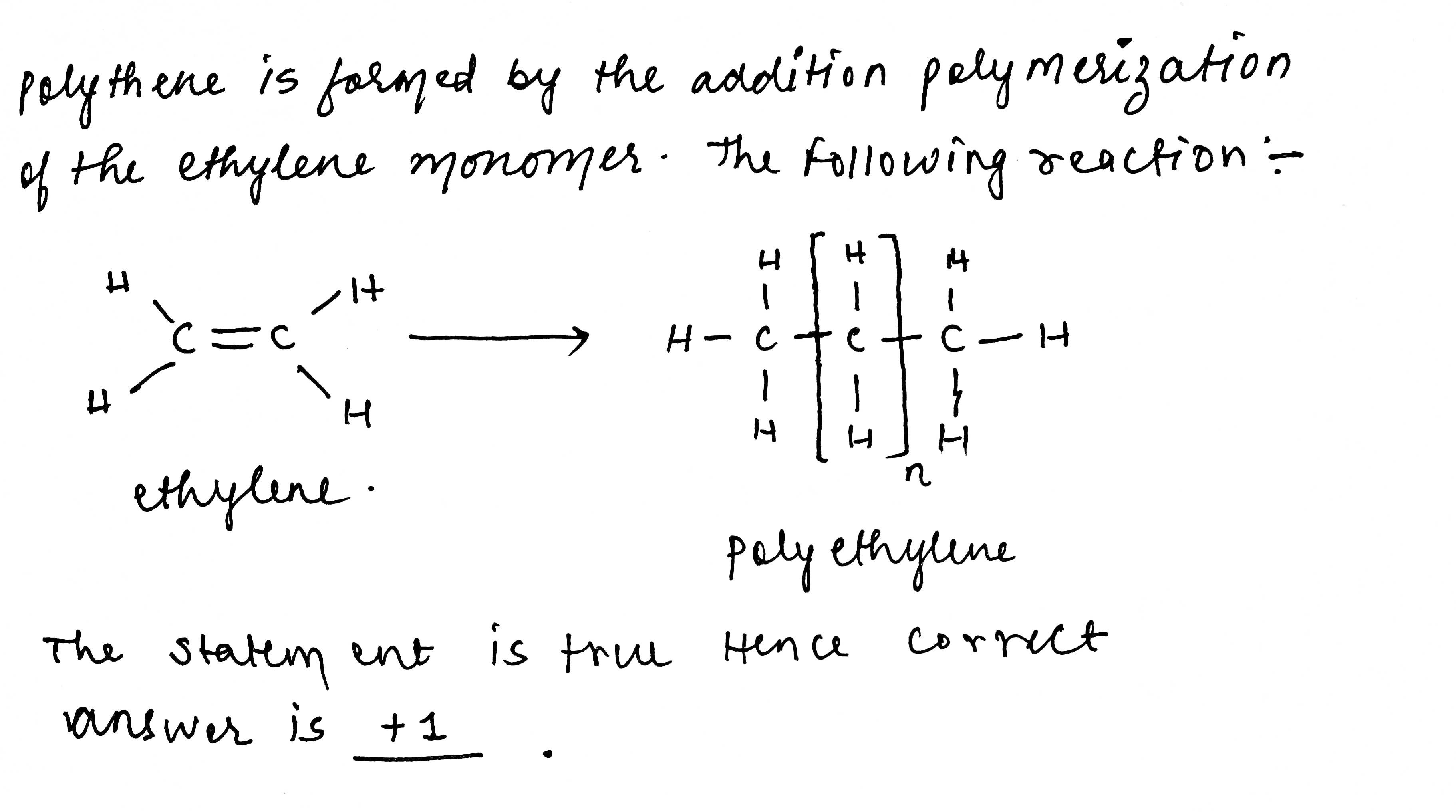
Statement: The monomer used for the preparation of polythene is ethylene.
If true enter 1 else 0.
If true enter 1 else 0.
Why is teflon used as a tape for sealing purpose?
Write two names of polymer which are biodegradable.
Why teflon is used to make nonstick cooking pans and other cookwares ?
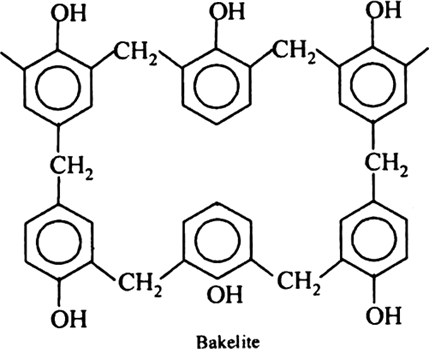
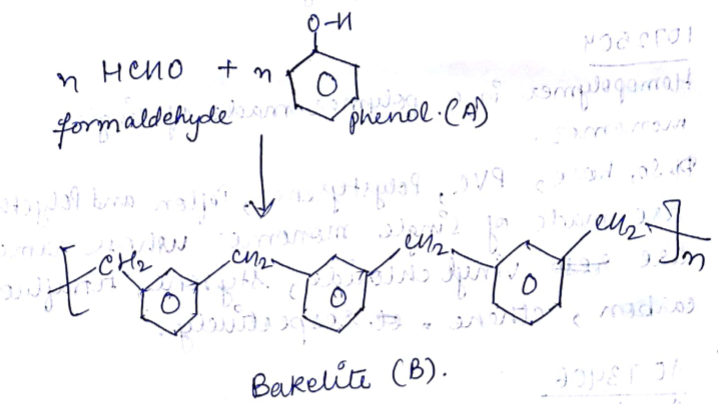
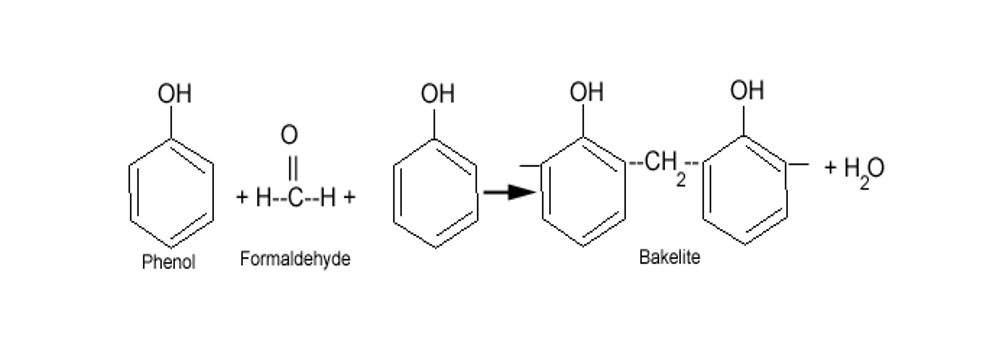
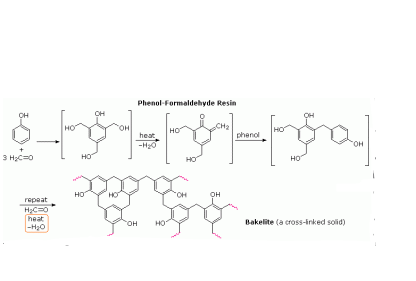
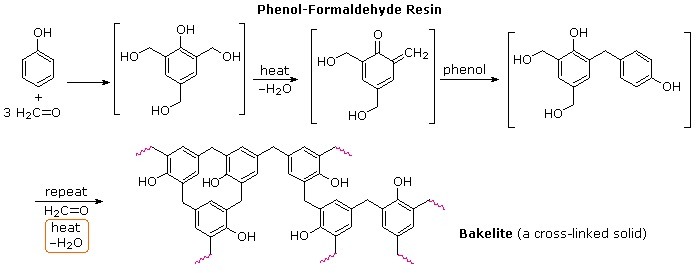
What do you know about Bakelite?
How do you explain the functionality of a monomer?
Define the term polymerisation.
What are natural and synthetic polymers? Give two examples of each type.
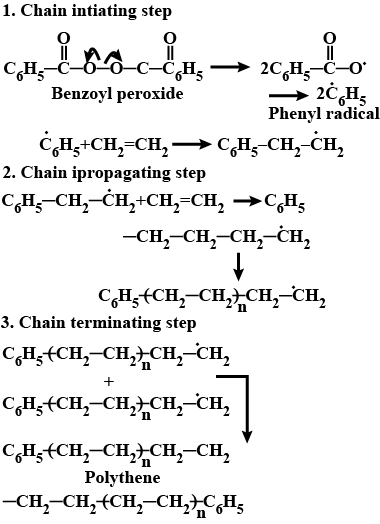
Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Discuss the main purpose of vulcanisation of rubber.
Explain the terms polymer and monomer.
Distinguish between the terms homopolymer and copolymer and give an example of each.
Define the term polymerisation
Write the free radical mechanism for the polymerisation of ethene.
Categorise the materials of the following products into 'can be recycled' and 'cannot be recycled':
Telephone instruments, plastic toys, cooker handles, carry bags, ball point pens, plastic bowls, plastic covering on electrical wires, plastic chairs, electrical switches.
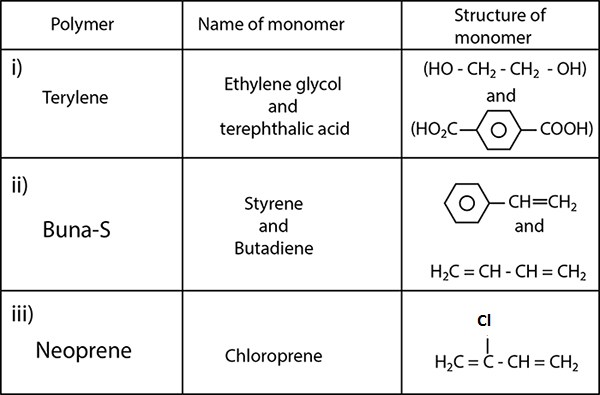
Write the names and structures of the monomers of the following polymers:
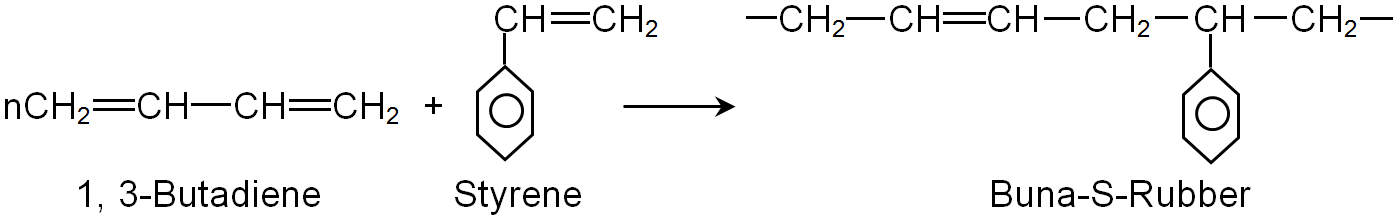
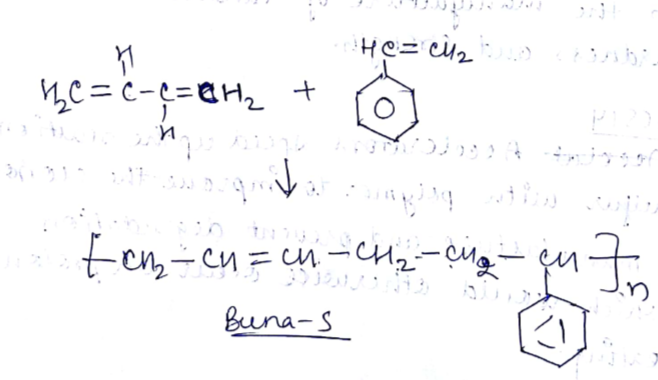
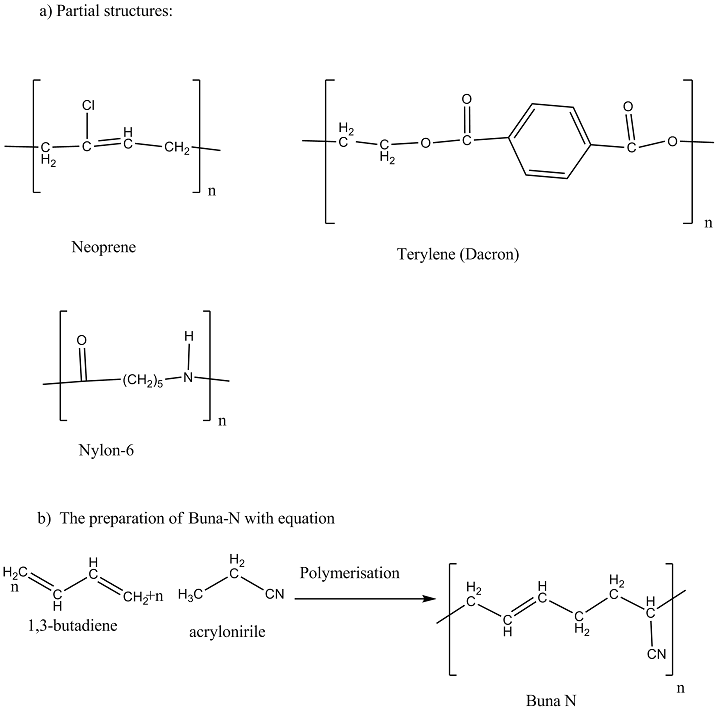
(i) Buna-S (ii) Buna-N (iii) Dacron (iv) Neoprene
(i) Buna-S (ii) Buna-N (iii) Dacron (iv) Neoprene
What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
Which of the following is a fibre?
Nylon, Neoprene, PVC
Write the name of monomers used for getting the following polymers:
(i) Bakelite (ii) Neoprene
Write the names and structures of the monomers of the following polymers.
(i) Bakelite (ii) Nylon-$$6,6$$ (iii) Polythene
(i) Bakelite (ii) Nylon-$$6,6$$ (iii) Polythene
Write the name and structures of the monomers of the following polymers.(i) Terylene
(ii) Buna-S
(iii) Neoprene
(ii) Buna-S
(iii) Neoprene
As plastics and synthetic fibres consist of repeating monomer units, they are known as __________.
Write the name of monomers used for getting the following polymers:
(i) Teflon (ii) Buna-N
Write the names and structures of the monomers of the following polymers.
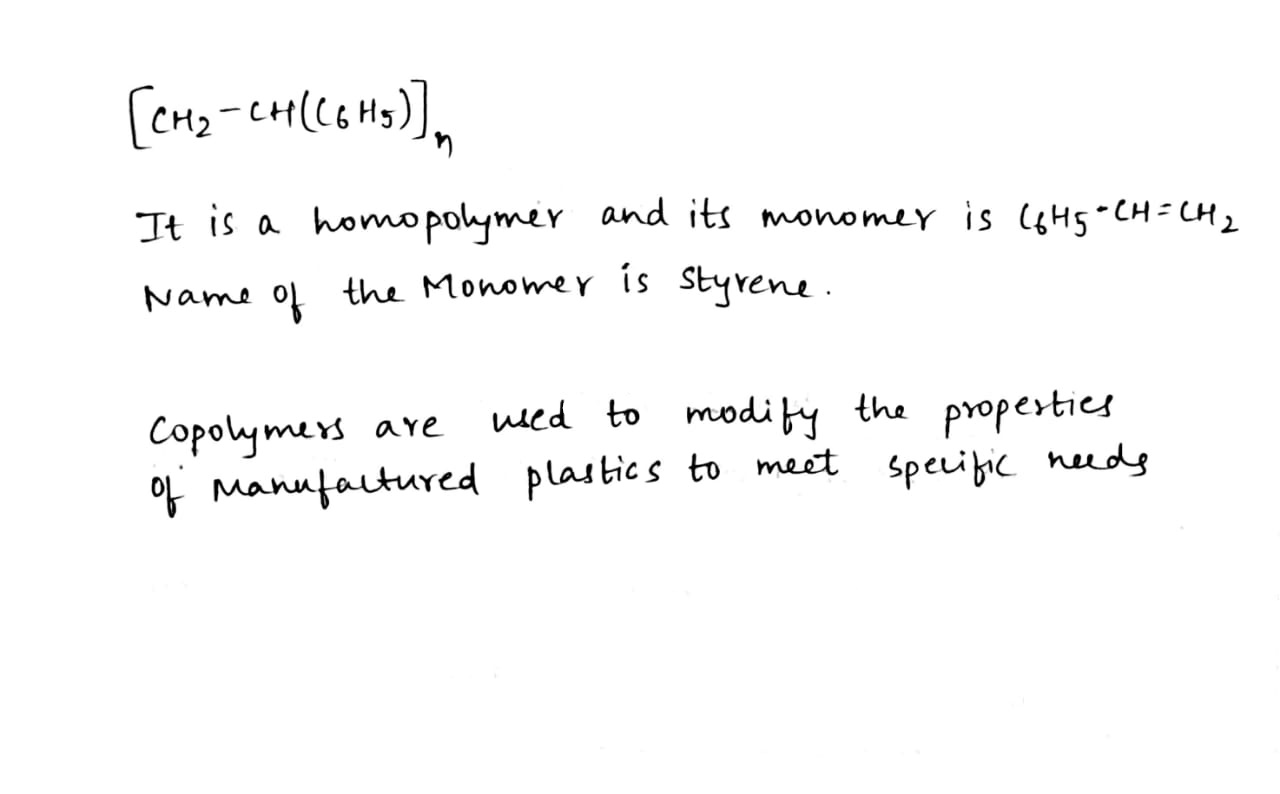
(i) Polystyrene
(ii) Dacron
(iii) Teflon
(i) Polystyrene
(ii) Dacron
(iii) Teflon
Give one example of a condensation polymer.
State True or False.
Nylon has high elasticity and tensile strength.
Nylon has high elasticity and tensile strength.
We use PET bottles. PET jars for storing sugar, dal, salt etc., what is this PET exactly?
What are broad-spectrum antibiotics ?
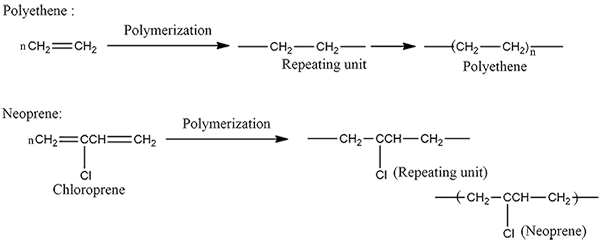
How are polythene and neoprene prepared ?
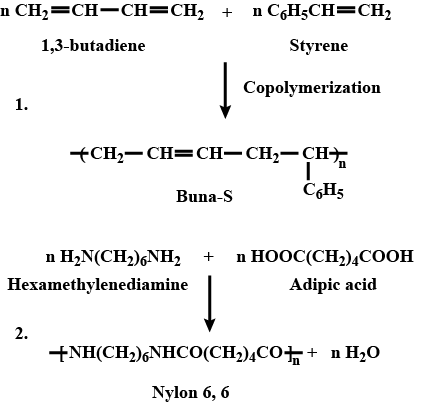
Write names and chemical formulae of monomers used in preparing Buna-S.
Write the names of monomers of the following polymers :
(a) Bakelite
(b) Terylene
What is vulcanization of rubber?
Write the names of the monomers of PHBV polymer.
Give the complete name for $$PHBV$$. How it is useful?
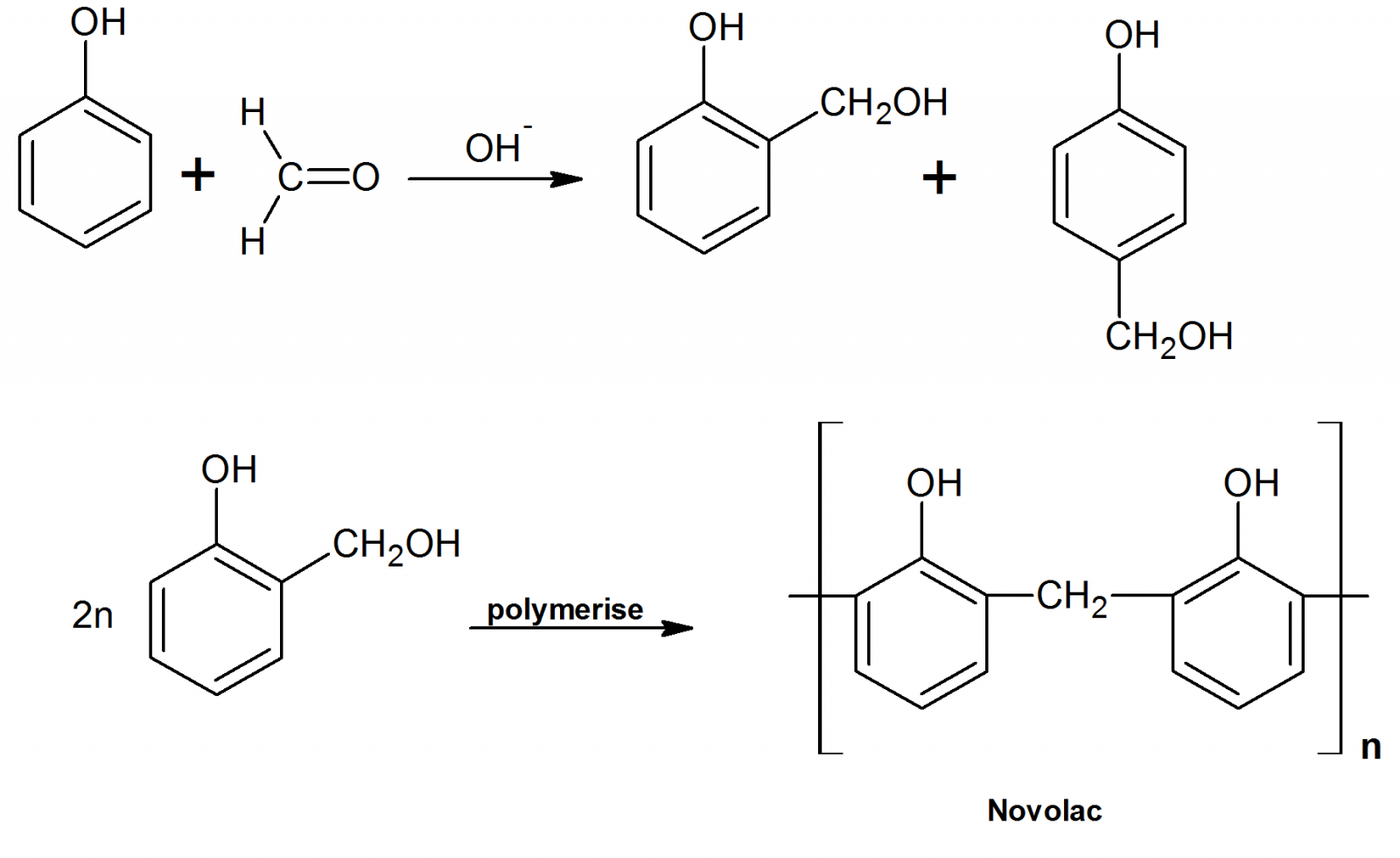
Write the chemical reaction to prepare novolac polymer.
What is PHBV? How is it useful to man?
.(a) Explain the addition polymer by an example.
(b) Write the equation of preparation of synthetic rubber.
(c) Give the names of monomers used for obtaining dacron.
(a) What is addition polymer? Give example.
(b) What is PHBV? How is it useful to man?
What is PHBV ? How is it useful to man ?
Explain that vulcanised rubber is an elastomer.
Write the names and structures of monomers used for getting the following polymers.
(i) Teflon
(ii) Bakelite
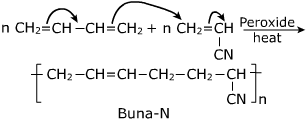
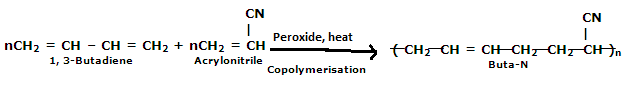
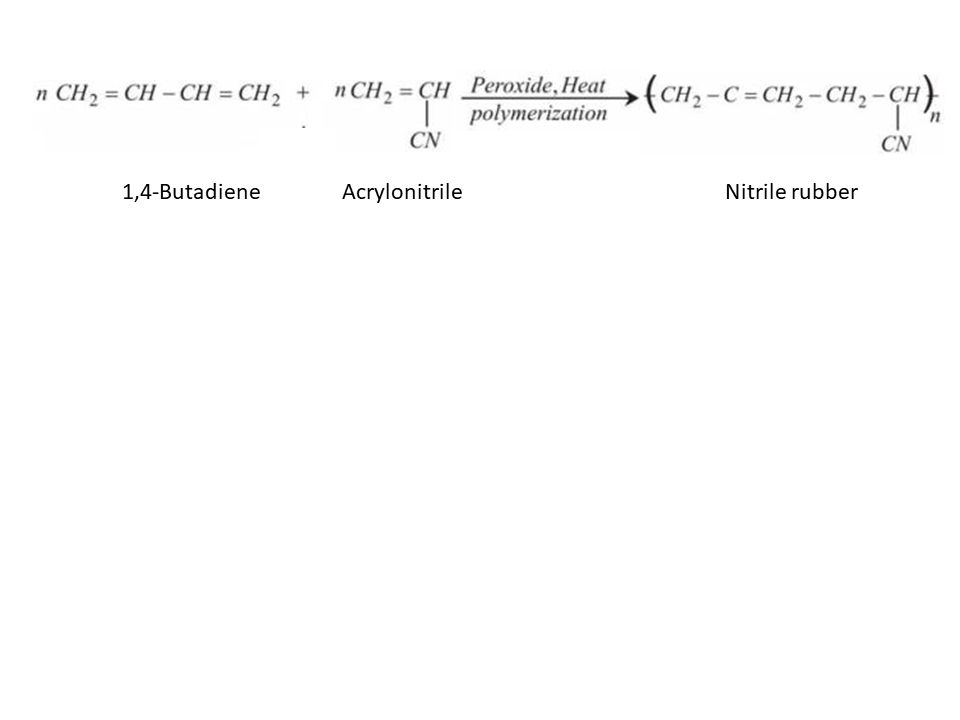
a) i) Explain the preparation of Buna-N.
ii) Give an example for thermosetting polymer.
b) Name the monomers used in the preparation of polythene and natural rubber.
What is Polymerisation? Write the names of any three polymers and their one main use.
What are the monomers of the following?
(i) Neoprene
(ii) Nylon-$$6$$
(a) Complete the reaction?
(b) Name the product formed if a large number of $$CH_2 = CH_2$$ molecules are added instead of $$H - H$$?
What are Polymers? Give the name of two polymers and their uses.
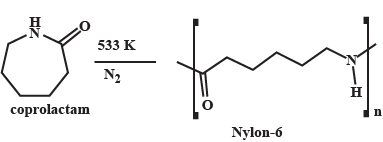
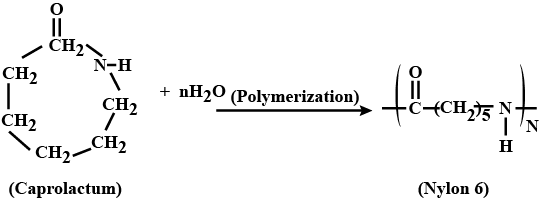
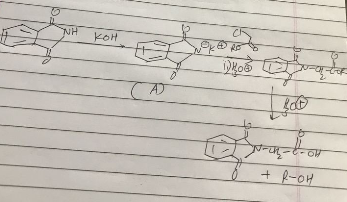
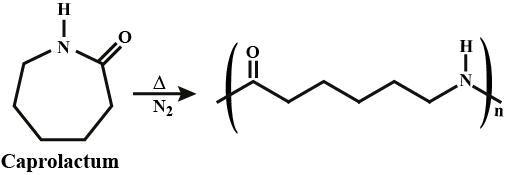
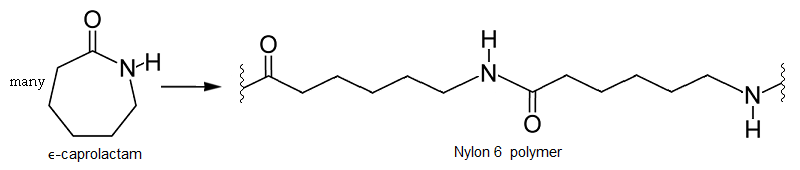
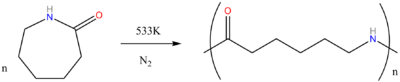
What is the common name of the polymer obtained by the polymerization of caprolactum? Is it addition polymer or condensation polymer?
Give two examples of bio-degradable polymers?
Why sulphur is added to the natural rubber?
Write the values(at least two) shown by Shyam.
Write the difference between terylene fibers and Buna-s rubber (clastomers).
Write one structural difference between low-density polythene and high-density polythene.
Write the names of the monomers for each of the following polymers:
(a) Bakelite
(b) Nylon-2-nylon-6
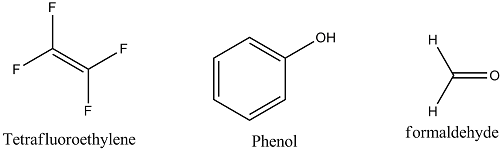
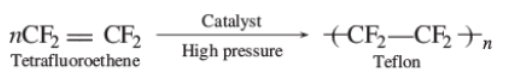
Consider the following reaction involved in the preparation of teflon polymer $$(-CF_{2} - CF_{2}-)_{n}$$
$$XeF_{2} + (-CH_{2}-CH_{2}-)_{n} \rightarrow (-CF_{2}-CF_{2}-)_{n} + HF + XeF_{a^{-}}$$.
Determine the moles of $$XeF_{5}$$ required for preparation of $$100\ g$$ Teflon.
What are the differences between Buna-N and Buna-S ?
Very short answer type.
Name the monomer(s) of the following dimers/polymers starch
Name the monomer present in the following polymer
i) Polyvinyl chloride.
ii) Natural rubber.
Give an example of bio-degradable polymer.
Explain the preparation of Buna-N with equation.
Given the chemical reaction for the preparation of Nitrile rubber. Also mention its two properties and two uses.
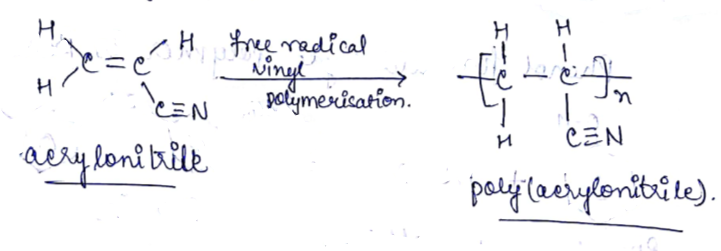
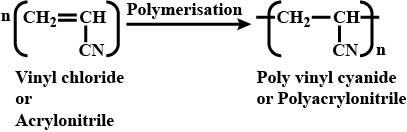
Name the polymer which is obtained from acrylonitrile.
What does term $$SBR$$ stand for ? write its application.
Answer the following in brief:
Name of the process of formation of polymer form its monomer.
Write uses of Bakelite.
(1)Name the material prepared by using wood pulp.
(2)Name the natural polymer.
Explain the difference between Buna-$$N$$ and Buna$$-S$$.
Write differences between low and high density polymers.
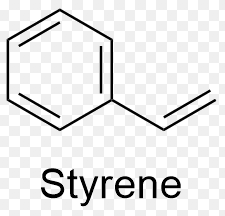
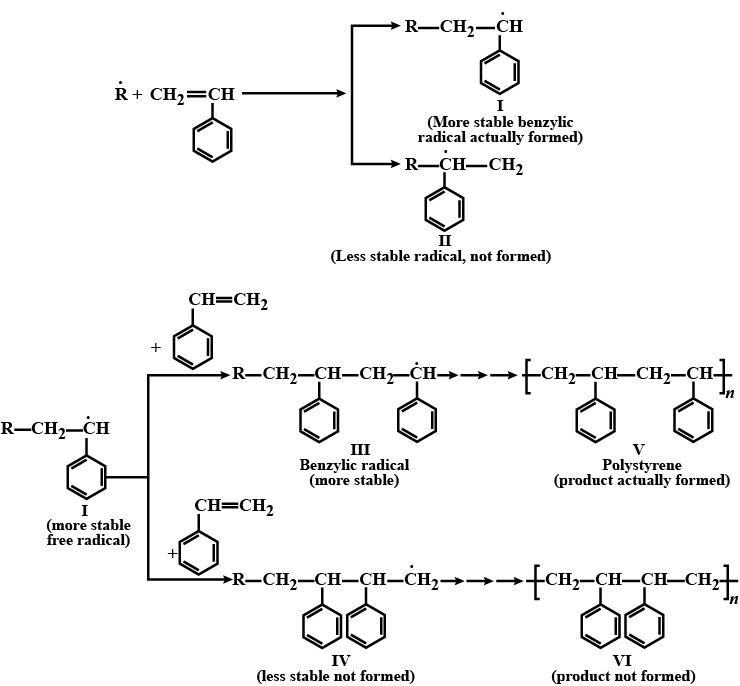
Explain why free radical polymerisation of styrene gives a product in which phenyl groups are on alternate carbon atoms rather than on adjacent carbon atoms.
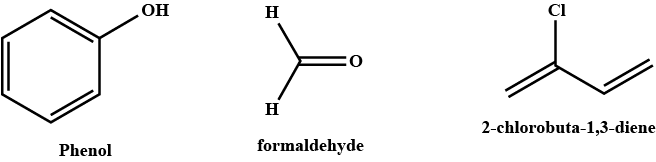
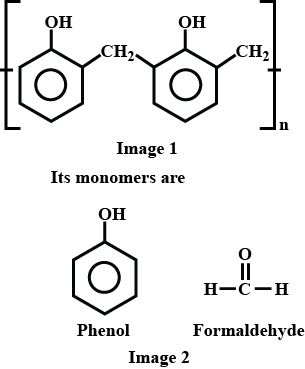
Name the phenol-formaldehyde polymers.
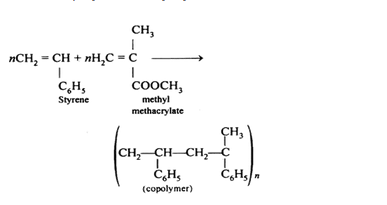
What is the commercial name of PMMA? What is its use?
Explain the term with example:Monomer
classify the following as addition and condensation polymers:
Terylene, Bakelite, Polyvinyl chloride,Polythene
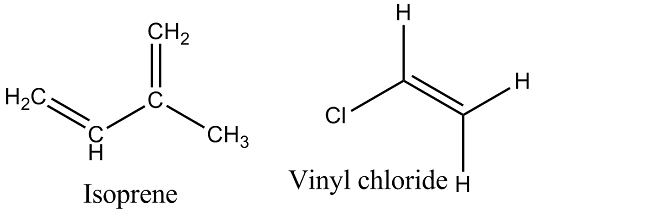
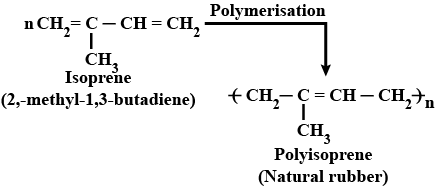
Which polymer is obtained from $$2-$$chlorobuta$$-1, 3-$$diene?
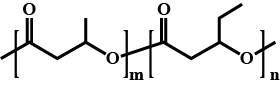
Write the structure of monomer and name the given polymer.
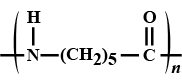
Predict missing product
Difference between homopolymer and co-polymer.
Name the monomers present in the following polymers:
(a) Bakelite
(b) Buna- N
What is biodegradable polymer ? Give one example.
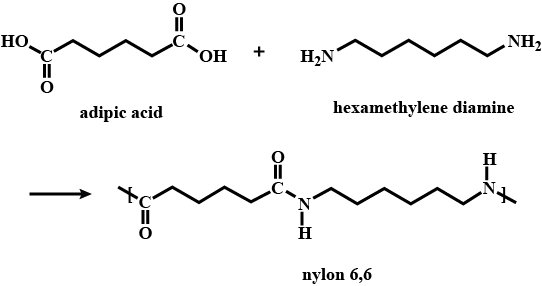
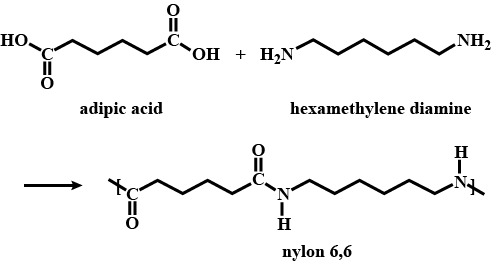
Write the names of the monomers of the following polymers:
(a) Bakelite
(b) Nylon - 6,6
Thermocol is produced from which material?
Is $$[ \mathrm { CH } _ { 2 } - \mathrm { CH } \left( \mathrm { C } _ { 6 } \mathrm { H } _ { 5 } \right) ]_{ \mathrm { n } }$$ to a homopolymer or a copolymer?
Explain the purpose of vulcanization of rubber.
What is the structural difference between high density polythene (HDP) and low density polythene (LDP)?
What are biodegradable polymers ? Give chemical equation for the preparation of any one biodegradable polymer.
Write the structures of monomers used to obtain the following polymers:
(a) Neoprene
(b) PHBV
(c) Bakelite
Write down difference between terylene fibre and Buna -S- rubber (elastomers).
Give an example of each of homopolymer and copolymer.
Write chemical reactions to prepare the Nylon-6 polymers:
Give an example of elastomers.
Write the name of monomer unit of polymer used in non-stick surface coated utensils.
Write chemical reactions to prepare the Teflon polymers:
Fill in the blanks :
A plastic bakelite is a compound of $$HCHO$$ with
Fill in the banks.
| Monomers | Polymers | Other important use |
| ............. | Teflon | ............... |
Fill in the blanks.
Bakelite is made by the action of ...... and ......
Fill in the banks.
| Monomers | Polymers | Other important use |
| Formaldehyde, phenol | ........... | ............... |
Fill in the banks.
| Monomers | Polymers | Other important use |
| .............. | ........... | For making crockery |
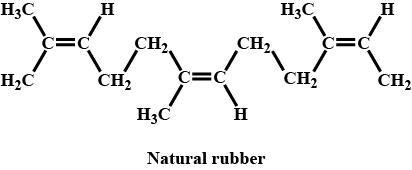
Write the name and structure of the monomer of natural rubber polymers.
Write the names and structures of the monomers of Bakelite.
Describe how Bakelite is synthesised?
Write the names and structures of the monomers of synthetic rubber.
Fill in the banks.
| Monomers | Polymers | Other important use |
| ............... | PMMA | ............... |
Can enzyme be called a polymer?
Why are rubbers called elastomers?
Name a synthetic polymer which is an amide.
Write equations for the synthesis of Teflon.
Differentiate the following pair of polymers based on the property mentioned against each.
Q. Novolac and bakelite (structure)
What are LDPE and HDPE? How are they prepared?
Why should the monomers used in addition polymerisation through free radical pathway be very pure?
Give the structure and name of the polymer which is used for making non-stick utensils.
Write names of monomer/s of the following polymers and classify them as addition or condensation polymers.
i. Teflon
ii. Bakelite
iii. Natural Rubber
Which type of biomolecules have some structural similarity with synthetic polyamides? What is this similarity?
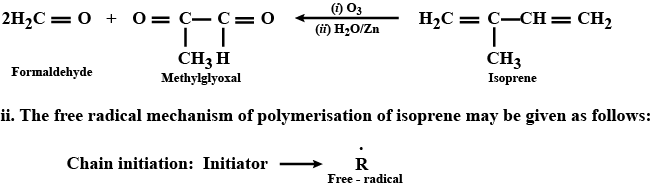
A monomer of a polymer upon ozonolysis gives one mole of methylglyoxal and two moles of formaldehyde.
i. Identify the monomer of the polymer.
ii. Give its free radical mode of addition polymerisation.
How does vulcanisation change the properties of natural rubber?
Define step growth polymerisation.
Identify the type of polymer given in the following figure.
What is the role of sulphur in the vulcanisation of rubber?
Why are rubbers called elastomers?
Why does cis-polyisoprene possess elastic property?
What are polymer ?
Thermocol melts at .............. $$^{ o }{ C }.$$
Write short notes on the following:
Natural rubber
Can nucleic acids, proteins and starch be considered as step growth polymers?
Define polymers?
Select an appropriate chemical bond among ester bond, glycosidic bond, peptide bond and hydrogen bond and write against of the following.
Water
Polymers are macromolecules formed by the combination of many monomers.
a. How are polymers classified?
b. Classify the following: Cotton, Wool, Nylon, Silk, Terylene, Jute, Polyester
Write short notes on the following:
Polyacrylo nitrile
Separate natural and artificial polymer from the list Rubber, wool, Pvc, Bakelite, nylon, rayon cellulose, silk, polythene, polyester
If we heat polyethene cover can we convert into earlier stage? Justify.
Find out odd one and give reason. Bakelite, polyester, polythene, melamine formaldehyde.
Give four difference between natural rubber and synthetic rubber.
What is teflon? What is it used for?
Filling in the blanks.
Natural polymer is __________.
Filling in the blanks.
Natural polymer which has elastic nature is ______.
Filling in the blanks.
Polymers of plant origin are made up of ________.
Match list I with list II. Matching can be one or more than one.
Match the plastic given in list I with its corresponding uses given in list II.
Explain the term copolymerisation and give two examples.
How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Match the List 1 with List 2 and choose the correct option.
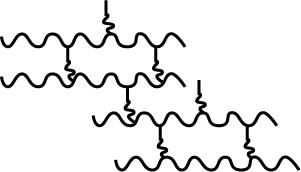
Study the given flowchart carefully. $$K,\,L,\,P\;and\;Q$$ plastics are
$$\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;K\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;L\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;P\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;Q$$
(A)$$\;\;\;\;$$Polystyrene,$$\;\;\;\;\;$$Bakelite,$$\;\;\;\;\;\;\;\;\;\;\;$$Teflon,$$\;\;\;\;\;\;\;\;\;\;\;$$PVC
(B)$$\;\;\;\;\;$$Polythene,$$\;\;\;\;\;$$Polystyrene,$$\;\;\;\;\;\;\;$$Bakelite,$$\;\;\;\;\;\;\;\;$$PVC
(C)$$\;\;\;\;$$PVC,$$\;\;\;\;\;\;\;\;\;\;\;\;\;\;$$Polythene,$$\;\;\;\;\;\;\;\;\;\;$$Polystyrene,$$\;\;\;$$Bakelite
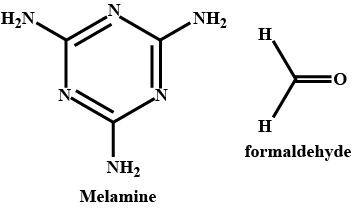
(D)$$\;\;\;\;$$PVC,$$\;\;\;\;\;\;\;\;\;\;\;\;\;\;$$Teflon,$$\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;$$Melamine,$$\;\;\;\;\;$$Bakelite
Choose your answer from A, B, C and D and enter accordingly.
Identify the monomer in the above polymeric structures.
How is dacron obtained from ethylene glycol and terephthalic acid ?
Explain the following terms giving a suitable example for each.(i) Elastomers (ii) Condensation polymer (iii) Addition polymers
Polymers are macro molecules formed by union of monomers.
(a) Name natural polymer and synthetic polymer.
(b) Distinguish between thermoplastic and thermosetting polymers with examples.
What are carbohydrates? Write the reaction for the preparation of Nylon-$$6$$.
a) Write partial structure of:
i) Neoprene
ii) Terylene (Dacron)
iii) Nylon - 6
b) Explain the preparation of Buna-N with equation
Why bakelite does not become soft on heating?
Why bakelite does not become soft on heating?
Write down the chemical equation for the preparation of Bakelite from formaldehyde?
How is Teflon prepared? Give chemical equation?
Give chemical equations for the following conversions:
(i) Ethylene into polythene
(ii) Ethyl alcohol into iodoform
Very short answer type.
Name the monomer(s) of the following dimers/polymers proteins
Why did Shyam refuse to accept the items in polythene bags?
Very short answer type.
Name the monomer(s) of the following dimers/polymers sugar
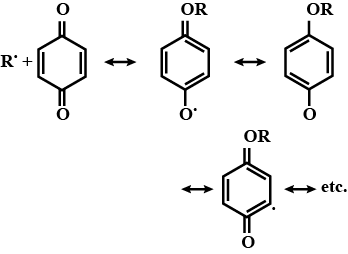
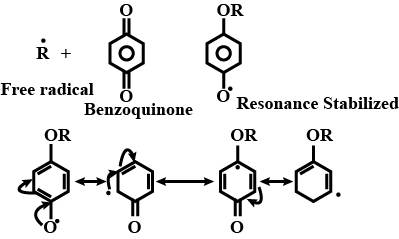
How does the presence of benzoquinone inhibit the free radical polymerisation of a vinyl derivative?
Write a note on vulcanization of rubber.
How is Orlon prepared?
Name the polymer which is used for making non-stick utensils.
Identify the monomers in the following polymers :
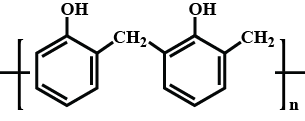
Write the short notes on the following:
(a) Co-polymer,
(b) Synthetic rubber,
(c) Thermoplastic,
(d) Thermosetting,
(e) Elastomer,
(f) Vulcanization of rubber
Describe how Nylon-6 is synthesised?
Match the following column-I with column-II
Name the sub-groups into which polymers are classified on the basis of magnitude of intermolecular forces.
Explain why free radical polymerisation of styrene gives a product in which phenyl groups are on alternate carbon atoms rather than on adjacent carbon atoms.
To have practical applications why are cross links required in rubber?
What is the structural difference between $$HDP$$ and $$LDP$$? How does the structure account for different behavior and nature, hence the use of a polymer?
Why has the use of methods like Teflon coating become more common ?
Fill in the blanks :
The chemical name of Teflon is ......
In free radical polymerization, where do we use hydroquinone? Explain.
Write down properties of teflon.
Write some properties and use of melamine .
Low-density polythene and high-density polyethylene, both are polymers of ethane but there is marked difference in their properties. Explain.
What does PMMA stand for ?
How many methods of chain termination are there in the free radical mechanism?
What do LDPE and HOPE indicate? How are they formed?
What is PHBV?
Uses of some important organic compounds are given. Pick out the suitable compounds from the box.
Power alcohol, Teflon, Polythene, Ethanoic acid, Ethanol
A) Solvent in paint industry
Power alcohol, Teflon, Polythene, Ethanoic acid, Ethanol
A) Solvent in paint industry
What is an addition polymerization? Explain the free radical addition polymerization mechanism by taking an example.
Write the method of polymerization and uses of bakelite.
How natural rubber is obtained? Write its composition and structure.
Write a short note on biopolymers and biodegradable polymers.
When we talk of functional macromolecules (e.g. proteins as enzymes, hormones, receptors, antibodies, etc), towards what are they evolving?
Class 12 Engineering Chemistry Extra Questions
- Alcohols,Phenols And Ethers Extra Questions
- Aldehydes,Ketones And Carboxylic Acids Extra Questions
- Amines Extra Questions
- Biomolecules Extra Questions
- Chemical Kinetics Extra Questions
- Chemistry In Everyday Life Extra Questions
- Coordination Compounds Extra Questions
- Electrochemistry Extra Questions
- General Principles And Processes Of Isolation Of Elements Extra Questions
- Haloalkanes And Haloarenes Extra Questions
- Polymers Extra Questions
- Solutions Extra Questions
- Surface Chemistry Extra Questions
- The D-And F-Block Elements Extra Questions
- The P-Block Elements Extra Questions
- The Solid State Extra Questions