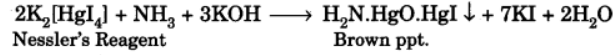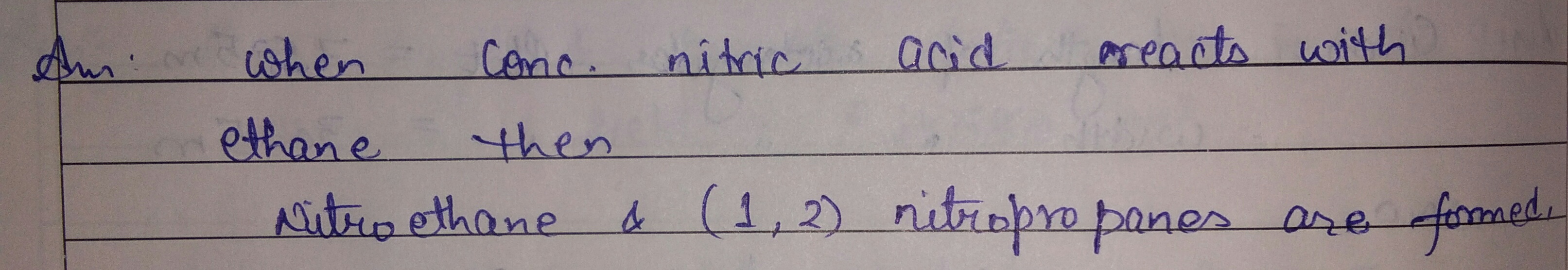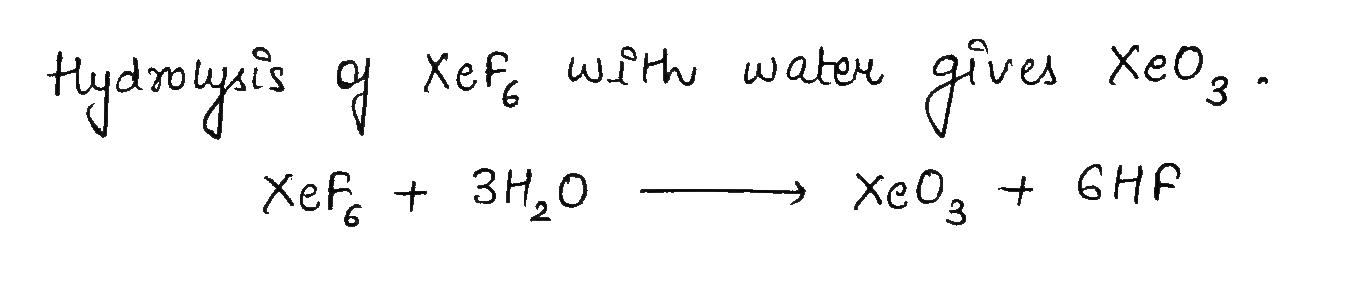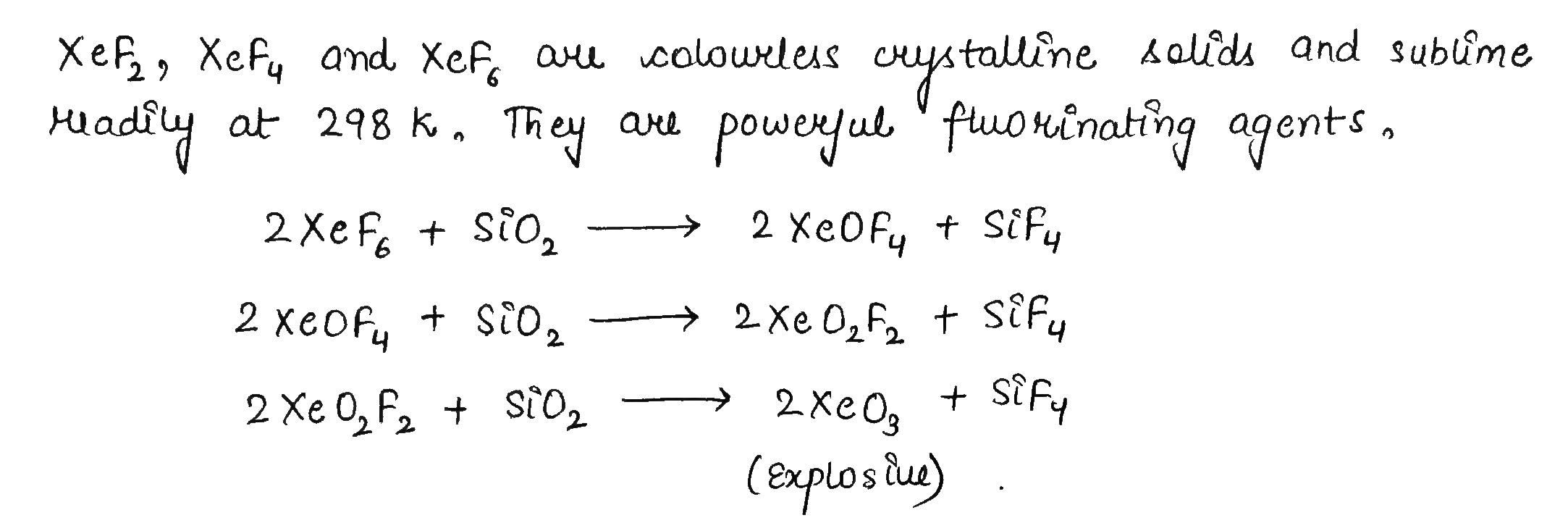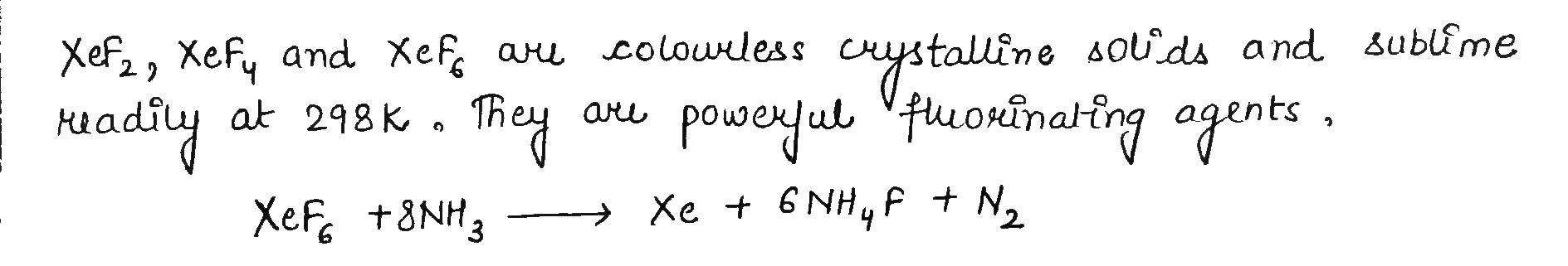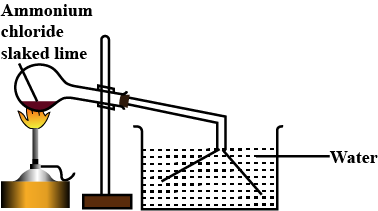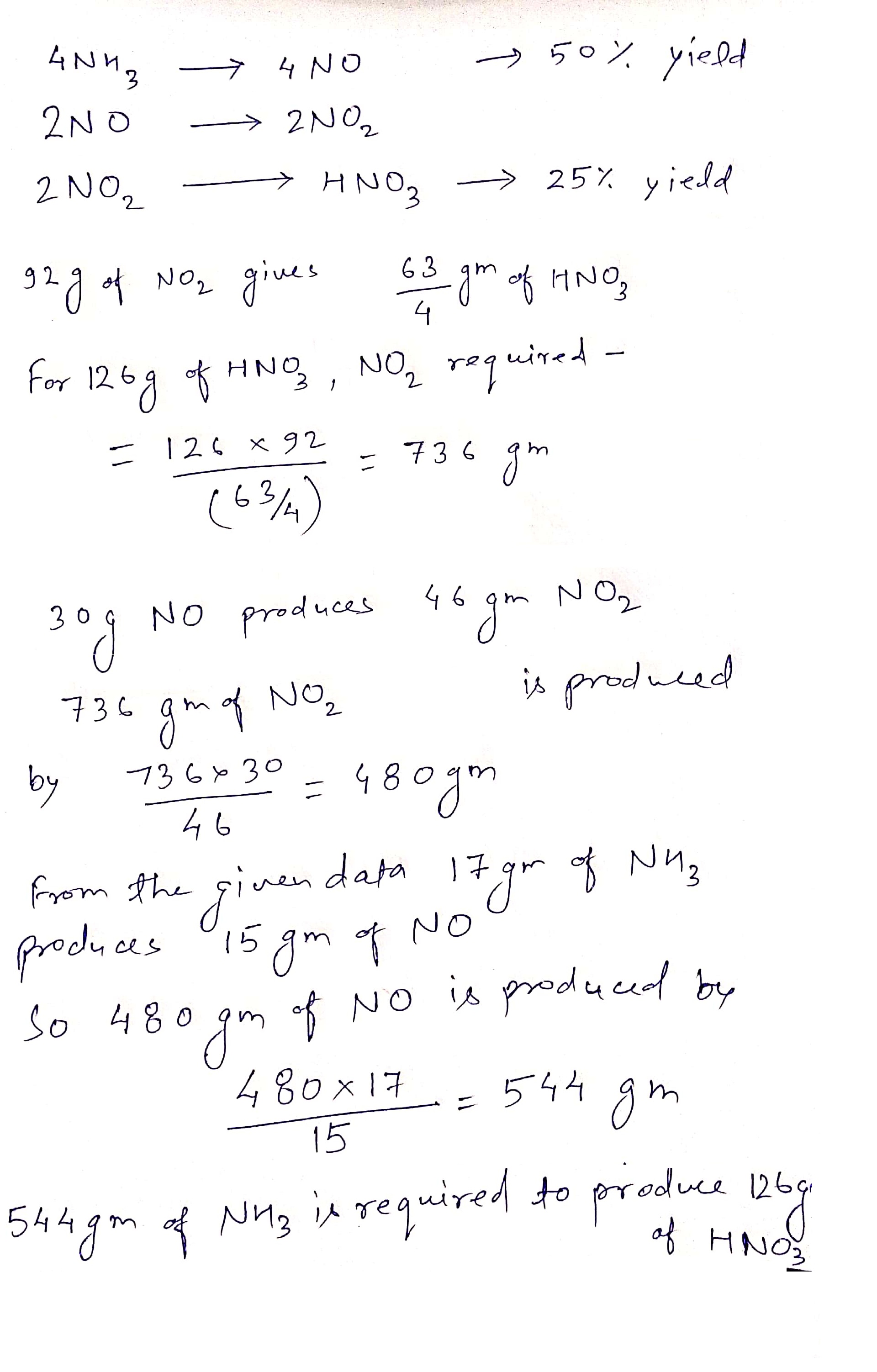The P-Block Elements - Class 12 Engineering Chemistry - Extra Questions
Fill in the blanks.
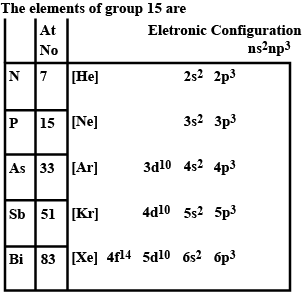
In solid state, nitrogen occurs as __________.
Nitrolium is used as fertiliser. Explain.
(a) Draw the structures of the following:
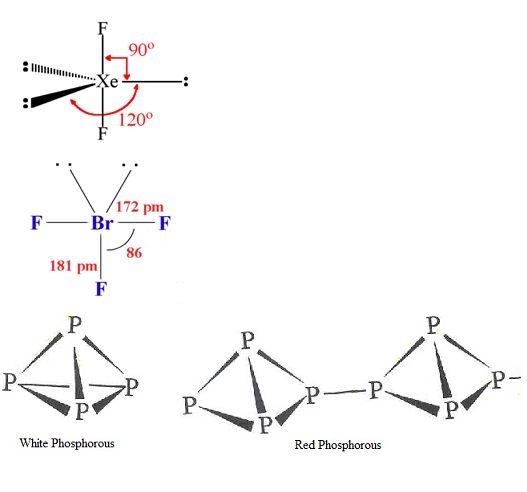
(i) XeF2 (ii) BrF3
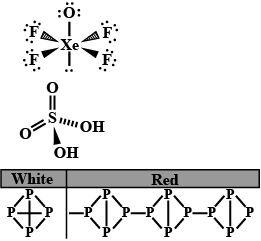
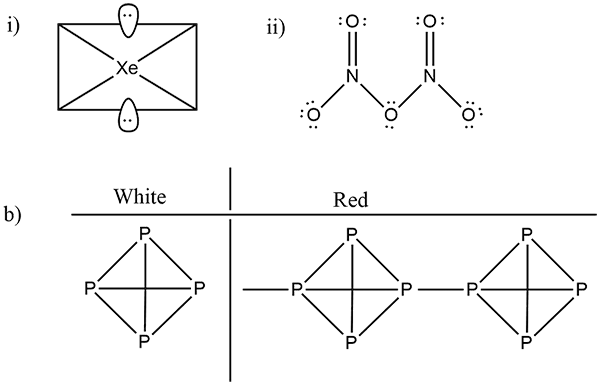
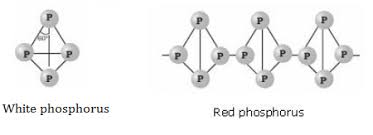
(b) Write the structural difference between white phosphorus and red phosphorus?
Name one halogen of period.
(a) Explain the Pauling scale for the determination of electronegativity. Give the disadvantages of Pauling scale.
(b) How does Fluorine differ from other halogens?
Calculate the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5.
Write the names of Inert gases.
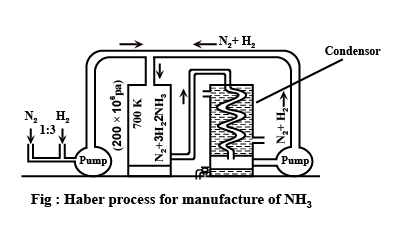
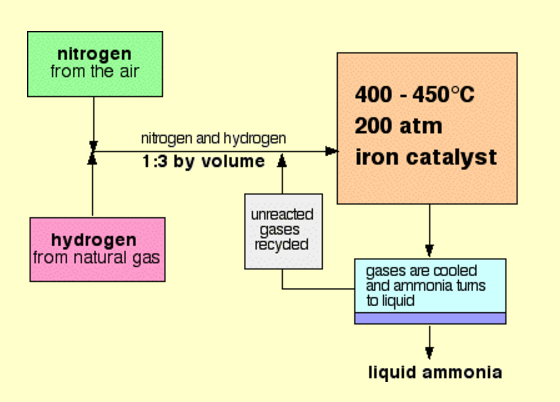
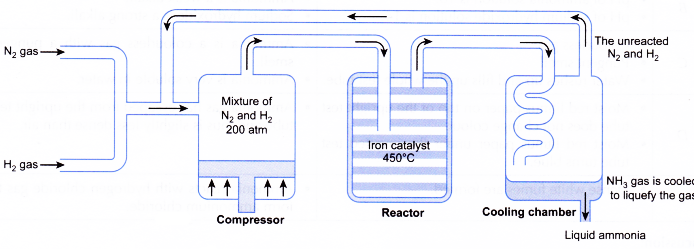
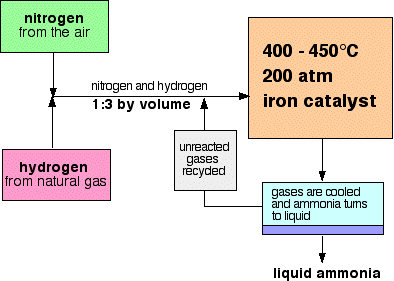
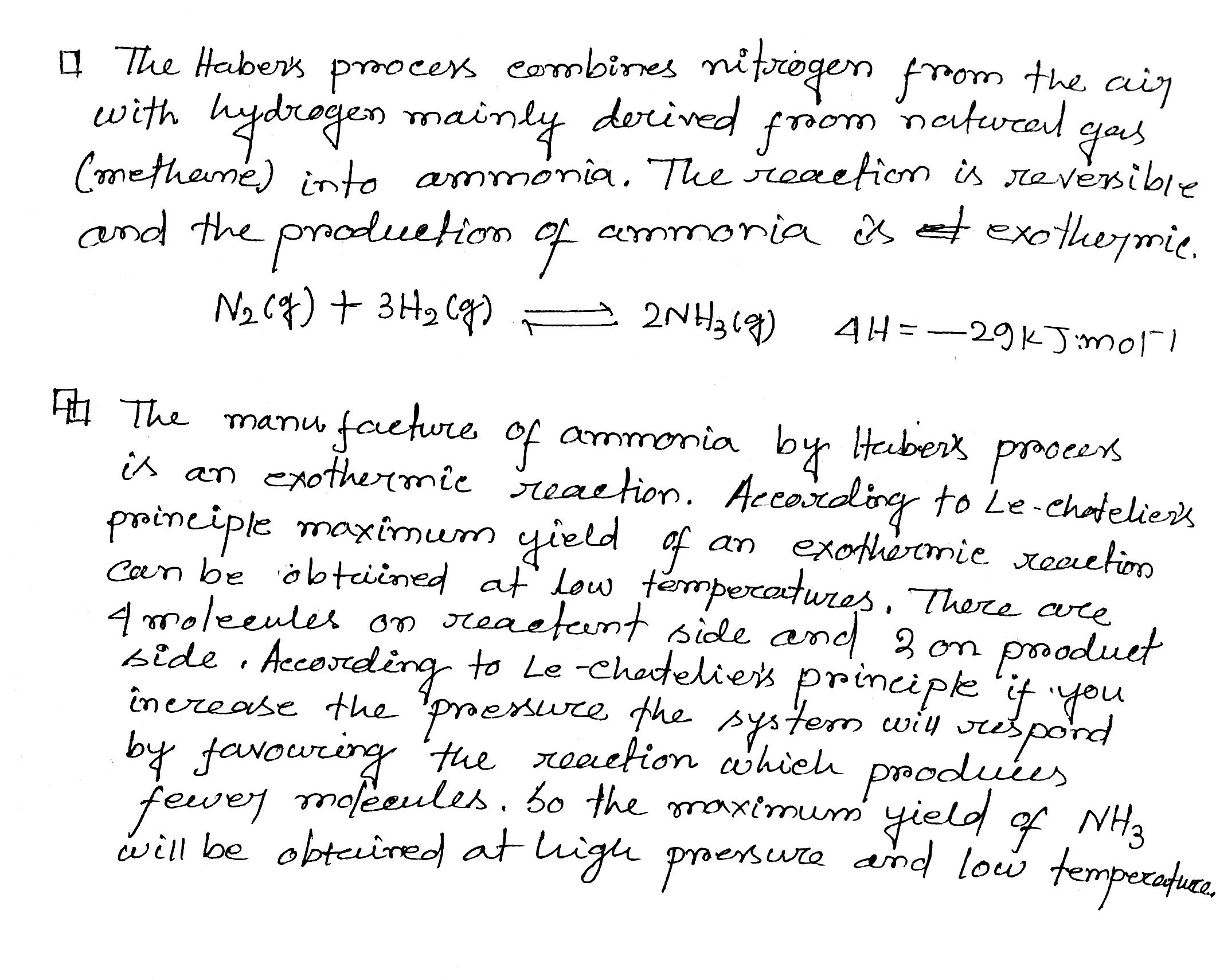
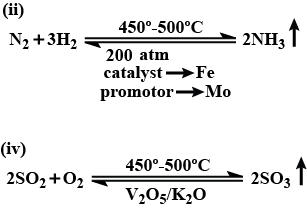
With reference to Haber's process, write the equation and the conditions required.
In the manufacture of nitric acid by Ostwald's process,___________ as a dehydrating agent is used to convert 68% by mass of HNO3 to 98% by mass of HNO3?
What are noble gases? Name all in the order of their increasing density.
LiI is soluble in water but LiF is not, why?
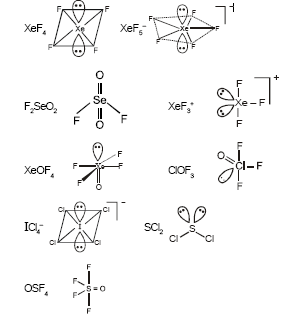
In how many of the following species the central atom has two lone pairs of electrons ?
| XeF4 | XeF5− | F2SeO2 | |
| XeF3+ | XeOF4 | ClOF3 | |
| ICl4− | SCl2 | OSF4 |
It is likely that Ar will form the anion Ar−. If true enter 1, else enter 0.
Peroxonitrous acid is an unstable intermediate formed in the oxidation of HNO2 by H2O2.
"Peroxonitrous acid has the same formula as nitric acid, HNO3."
Answer whether the above statement is true or false.If true enter 1, else 0.
The tendency to form clathrates decreases down the group.
Maximum acidic character is shown by the oxyacids of halogens with oxidation number of halogens as ____________ .
Arrange the following in the increasing order of bond energy: F2<Cl2<O2<N2
If true enter 1, else enter 0.
In the following reaction XeF2+BrO3−+H2O⟶Xe+BrO4−+2HF
equivalentmassofBrO3−=molarmass..........
In the following reaction
XeF2+BrO3−+H2O⟶Xe+BrO4−+2HF
equivalentmassofBrO3−=molarmass..........
Number of phases in the following equilibrium is :
Ra(s)⟶Rn(g)+He(g)
For inert monatomic gases, CPCV=5x. The value of x is _____ .
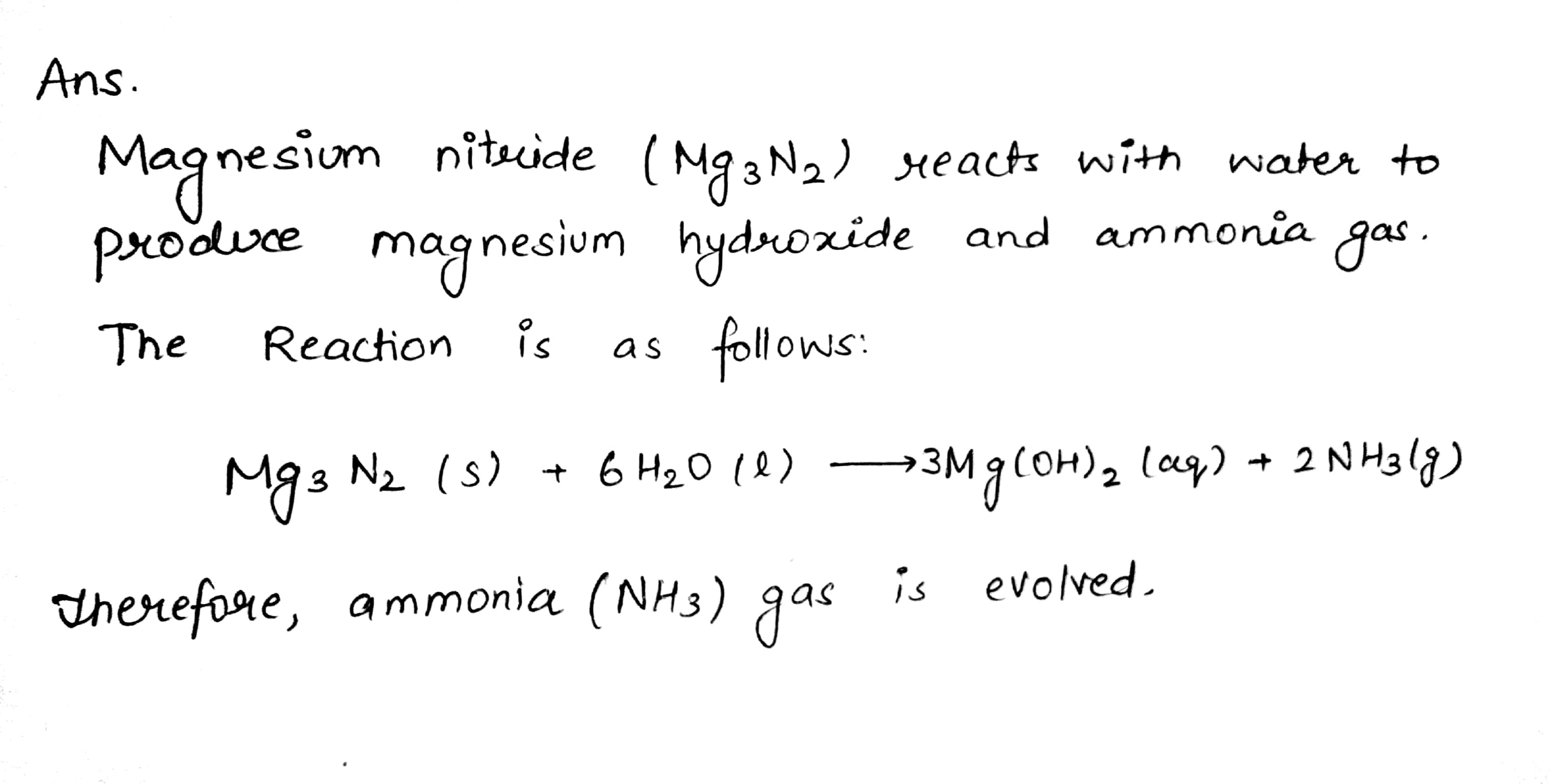
When Mg3N2 react with H2O, then how many type of gaseous product(s) is/are formed ?
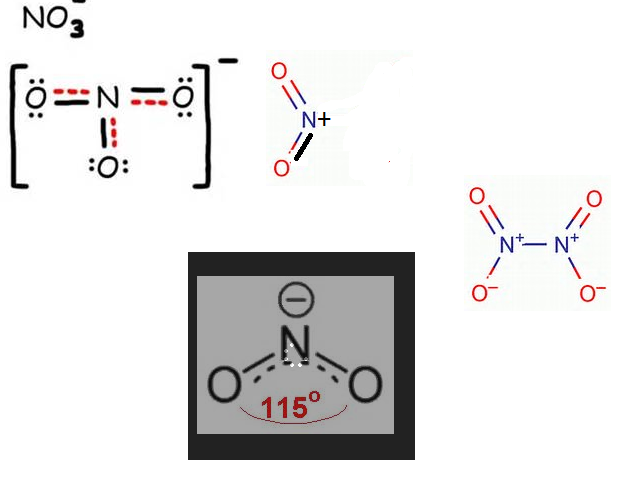
In NO−3 ion, find the number of bond pairs and lone pairs of electrons on nitrogen atoms.
[If the answer is 2 and 4, represent as 24]
Find the sum of atomicity of nitrogen and phosphorous.
Rank the bond length of the bond below in the molecules on the left column with longest as 1 and shortest as 4
Match the following.
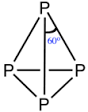
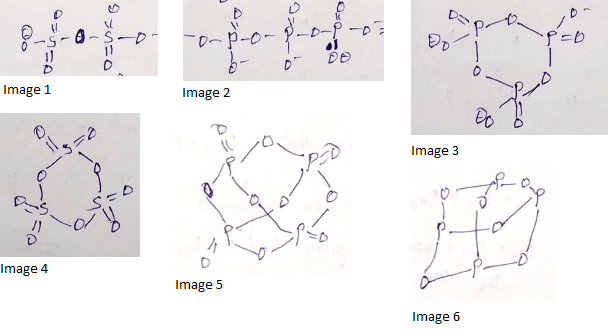
How many number of faces are there in P4 molecule?
The electronic configuration of an element is 1s2,2s2,2p6,3s23p3. What is the atomic number (X) of the element which is just below the above given element in the periodic table. Enter the value of X.
The atomicity of phosphorus is X and the P-P-P bond angle is Y. What are X and Y?
[If answers are 2 and 90, represent as 290]
Observe the chemical reaction:
P4+3NaOH+3H2O→ 3NaH2PO2+PH3
If the reaction is true enter 1 else 0
P4+3NaOH+3H2O→ 3NaH2PO2+PH3
If the reaction is true enter 1 else 0
In Nessler's reagent for the detection of ammonia, the active species is ______.H2Cl2HgI2−4
Write balanced chemical equations for the reaction of a burning of aluminum in the air.
Why is helium used in weather observation balloons?
Helium is the most abundant Noble gas in the universe
If this is true enter 1, if false enter 0.
How is ammonia manufactured industrially?
Explain why fluorine forms only one oxoacid, HOF.
Give reasons for the following:
R3P=O exists but R3N=O does not, R is an alkyl group?
How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Two elements 'P' and 'Q' belong to the same period of the modern periodic table and are in Group 1 and Group 2, respectively. Compare their following characteristics in tabular form.
(a) The number of electrons in their atoms
(b) The sizes of their atoms
(c) Their metallic character
(d) Their tendencies to lose electrons
(e) The formula of their oxides
(f) The formula of their chlorides
(a)Account for the following:
(i)Acidic character increase from HF to HI.
(ii)There is a large difference between the melting and boiling points of oxygen and sulphur.
(iii) Nitrogen does not form pentahalide.
(b) Draw the structures of the following:
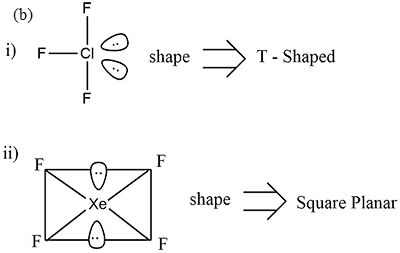
(i) ClF3
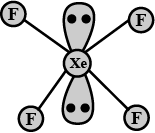
(ii) XeF4
Phosphorus has valencies of 3 and 5.
What is the formula of the two oxides of phosphorus?
Draw the structures of the following molecules:
(i) N2O5 (ii) XeF2
Account for the following:
(i) Bi(V) is a stronger oxidizing agent than Sb(V).
(ii) N−N single bond is weaker than P−P single bond.
(iii) Noble gases have very low boiling points.
Draw the structures of the following molecules:
(i) XeF6 (ii) H2S2O7
(a) Draw the structures of the following molecules:
(i) XeOF4
(ii) H2SO4
(b) Write the structural difference between white phosphorus and red phosphorus.
(a) Draw structure of the following compounds:
(i) XeF4 (ii) N2O5
(b) Write the structural difference between white phosphorus and red phosphorus.
Name a compound of chlorine that is used as:
(A) An disinfectant (B) An anesthetic (C) A bleaching agent
Chlorine does not exist in free state. Give reason.
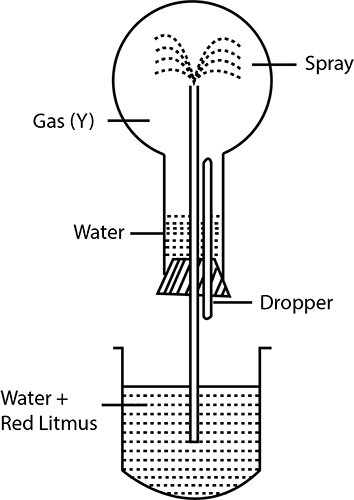
Ammonia gas is collected by ______.(an upward displacement of air, a downward displacement of water, a downward displacement of air)
Some word/ words are missing in the following statements. You are required to rewrite the statements in the correct form using the appropriate word/ words:
Magnesium nitride reacts with water to liberate ammonia.
Study the figure given below and answer the questions that follow:
Name another gas which has the same property and can be demonstrated through this experiment.
Copy and complete the following table relating to important industrial process:
| Name of the process | Temperature | Catalyst | Equation for the catalyzed reaction |
| Haber's process |
The following questions are based on the preparation of ammonia gas in the laboratory:
Explain why ammonium nitrate is not used in the preparation of ammonia?
The following questions are based on the preparation of ammonia gas in the laboratory:
Name the compound normally used as a drying agent during the process.
The following questions are based on the preparation of ammonia gas in the laboratory:
How is ammonia gas collected?
The following questions are based on the preparation of ammonia gas in the laboratory:
Explain why it is not collected over water?
Explain how does nitrogen exhibit anomalous behaviour amongst group 15 elements.
Describe anomalous behaviour of fluorine with the other elements of group 17 with reference to:
Hydrogen bonding.
Why nitrogen exists as diatomic molecule (N2) and Phosphorus as P4?
Give balanced equations and principle involved in the manufacture of nitric acid (HNO3) by Ostwald's process.
Describe anomalous behaviour of fluorine with the other elements of group 17 with reference to:
Polyhalide ions.
a) Write a chemical equation to prepare ammonia from ammonium chloride?
b) Write a chemical equation to identify Cu+2 & Ag+ ions with the application of NH3?
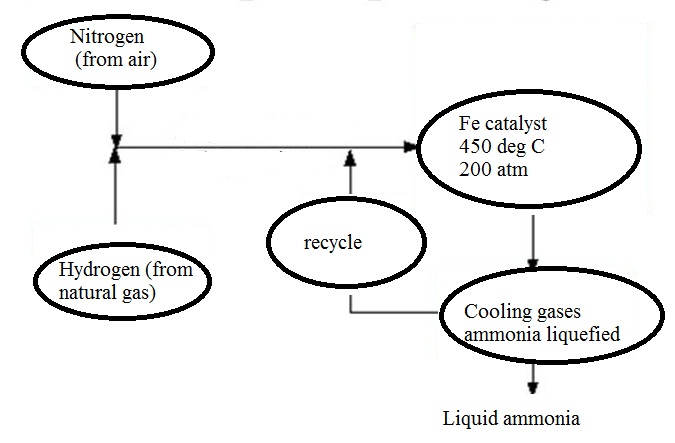
c) Draw a labeled diagram showing flowchart for the manufacture of ammonia?
Write the chemical formulae of four oxy acids of chlorine.
Describe anomalous behaviour of fluorine with the other elements of group 17 with reference to:
Oxidation state.
How are XeF2 and XeF4 prepared? Give their structures.
Write the balanced Chemical equation with condition involved in manufacture of nitric acid by ostwald's process.
Write the equations involved in the preparation of nitric acid by Ostwald's process by maintaining the reaction conditions.
Nitrogen does not form pentahalides. Give reason.
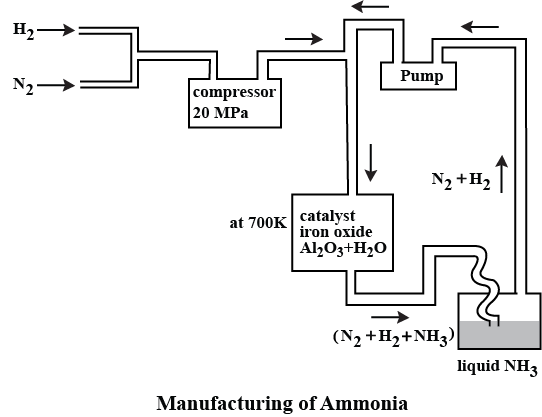
For the manufacture of Ammonia by Haber's process, write the equation and optimum conditions for maximum yield of ammonia.
Discuss Haber's process for industrial manufacture of ammonia.
Write the name of radioactive halogen?
Draw labelled diagram of Haber process for the manufacture of ammonia and write chemical equation?
The diagram showing the laboratory preparation of NH3 is given.
(a) What are the reactants used?
(b) Why CaO is used?
(c) What is the reason behind not keeping the jar in the upward direction?
(d) Write an experiment to identify presence of NH3 in the gas jar.
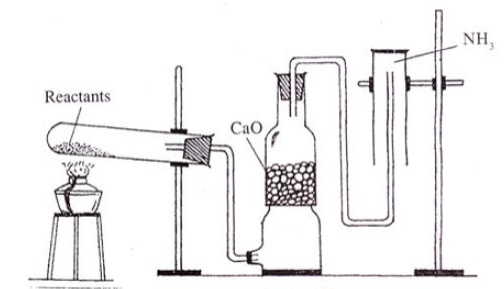
Describe Haber process for the manufacture of ammonia?
Fluorine always shows-1 oxidation state. Why?
Some elements in p-block shows allotropy.
(a) What are the allotropic forms of sulphur?
(b) (i) How will you manufacture Sulphuric Acid by contact process?
(ii) What are inter halogen compounds?
(a) Account for the following:
(i) H2O is a liquid while H2S is a gas.
(ii) Noble gases have very low boiling points.
(iii) NO2 dimerises to N2O4.
(b) (i) What are interhalogen compounds?
(ii) Suggest any two examples on interhalogen compounds.
Why the elements of group 17 are coloured?
Explain the industrial preparation of ammonia by Haber's process. Also, write two physical properties of ammonia.
What are inter-halogen compounds?
Give a reason for the following.
Inert gases do not form ions.
What are allotropes? Write the names of allotropes of phosphorus.
Describe industrial manufacture of ammonia by Haber's process with labelled diagram.
Explain why:
Nitrogen does not form pentahalides.
Explain why:
Hydride of sulphur is a gas while hydride of oxygen is a liquid.
40 ml of ammonia gas was taken in a eudiometer tube and subjected to sparks till the volume did not further change. The volume was found to increase by 40 ml. 40 ml of oxygen gas then mixed and the mixture was further exploded. The gases remaining were 30 ml. Deduce the formula of ammonia. (Ammonia contains N and H only)
Why PCl5 exists but NCl5 does not ?
Mention any two reasons for the anomalous behaviour of oxygen.
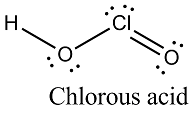
Write the structure of chlorous acid [HOClO].
Explain allotropes of phosphorous.
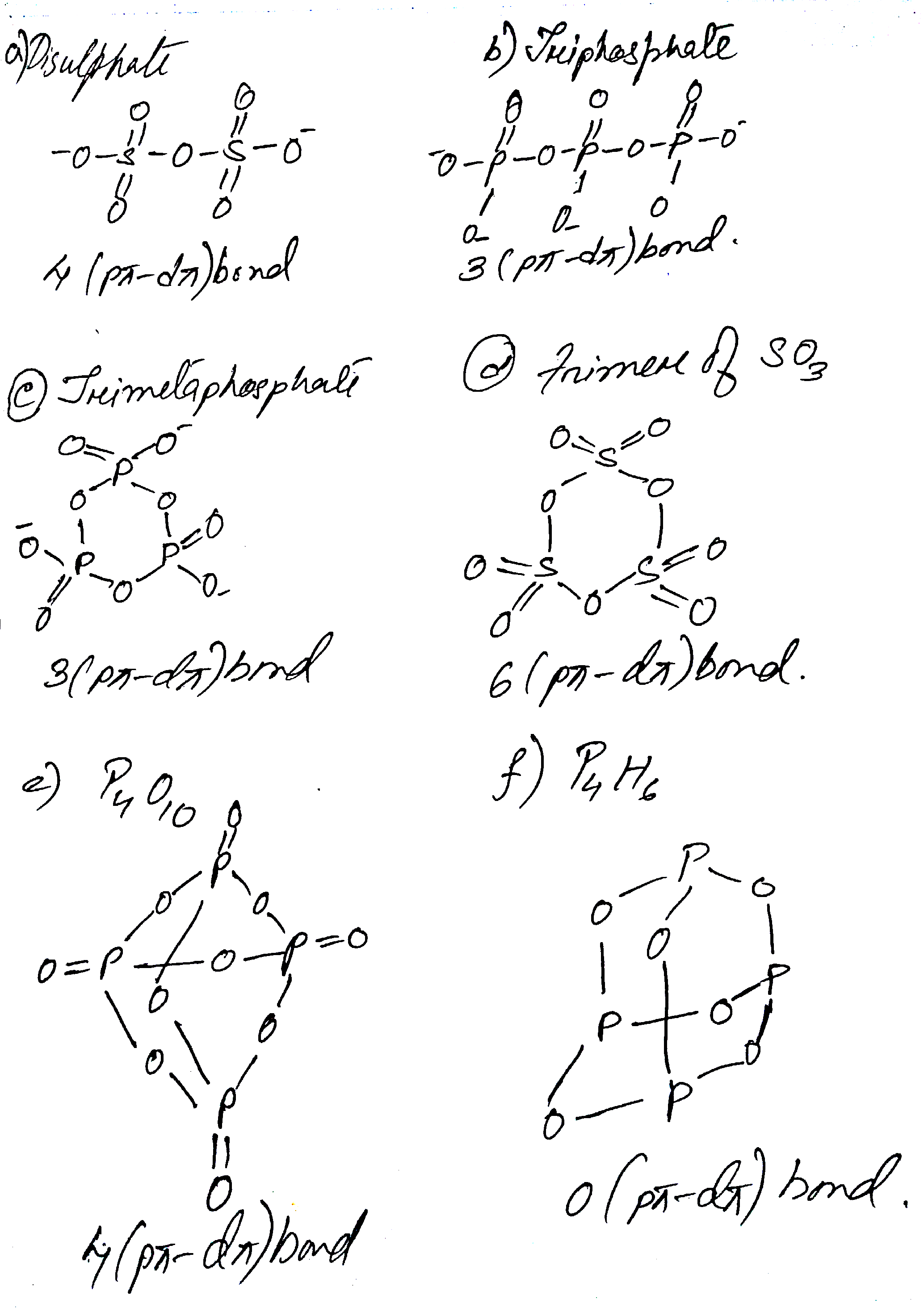
Find number of pπ−dπ bonds in
(a) Disulphate (b) triphosphate (c) trimetaphosphate (d) trimer of SO3 (e) P4O10 (f) P4O6
In Haber process 30 litres of dihydrogen and 30 litres of dinitrogen were taken for reaction which yielded only 50% of the expected product. What will be the composition of gaseous mixture under these condition in the end.
Sea is the greatest source of halogens. Comment.
What change in colour is observed when white silver chloride is left exposed in sunlight? State the type of chemical reaction and also write its chemical equation.
Which allotropic form of phosphorus has discrete tetrahedral P4 molecules.
Solid phosphorus melts and evaporates at high temperature Gaseous phosphorus effuses at a rate that is 0.567 times that of Ne in the same apparatus under the same conditions . How many atoms are in a molecule of gaseous phosphorus.
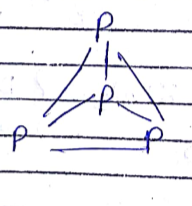
Find number of pπ−dπ bonds in:
a) Disulphate b) Triphosphate c) Trimetaphosphate
d) Trimer of SO3 e) P4O10 f) P4O6
From the artg of the periodic table given, answer the following questions
| 1 litium | 2 | 13 | 14 Carbon | 15 | 16 Oxygen | 17 | 18 neon |
| X | S | P | L | ||||
| Y | Q | ||||||
| Z | R | ||||||
| T |
Explain Haber's process and manufacture of ammonium.
Explain why nitrogen is much less reactive than phosphorous?
If we have 24g of nitrogen how much hydrogen atom is required to form ammonia?
Give reasons:
- Xenon does not form fluorides such as Xe{ F }_{ 3 } and Xe{ F }_{ 5 }
In the manufacture of nitric acid by Ostwald's process, write
The chemical equation for the dissolution of NO_2 in water.
Number of lone pairs on Xe in XeF_6 is:
Arrange following Ions in order of decreasing ionic radii { Li }^{ +2 },{ He }^{ + },{ Be }^{ +3 }?
Mg: s-block: : Nitrogen:_________
What are noble gases? Give a reason why noble gases have stable electronic configuration.
Calculate the no of moles present in 2.044 \times 10^{23} number of Helium gas ?
Write the atomic number of the element present in the third period and seventeenth group of the periodic table.
What is the action of ammonia on the ethyl chloride ?
The highest percentage of nitrogen is found in which among the following fertilizers among the given options?
Which of the following is a non-metal that remains liquid at room temperature?
Br, Cl, He, P
Why the elements of group 18 are also known as noble gases?
Why S2 is bigger than O2
.Nitrogen in soil is an example for_______.
Name :
The most abundant element in the earth's crust.
The most abundant element in the earth's crust.
How is Nitric Acid manufactured by Ostwald's Process?
How XeF_2 and XeF_4 are prepared? What are their structure?
Why does fluorine shows anomalous behaviour?
What is the action of
conc nitnic acid on ethane
State the use of aluminum
Given reasons for the following:
Dioxygen is a gas but sulphur a solid.
Which of the following is a non metal that remains liquid at room temperature ?
Give the chemical name and formula of the substance formed as a brown ring in the test for nitrate radical.
What is the third most common gas found in the air we breathe ?
One method of producing NH_{3} is by heating ammonium chloride, NH_{4}Cl, with CaO.
2NH_{4} Cl + CaO \rightarrow 2NH_{3} + CaCl_{2} + H_{2}O
Explain why the reaction of NH_{4}Cl with CaO produces ammonia.
What is the action of NaOH on ammonium salts?
Answer the following:
Name two chalcogens
Answer the following:
Which allotropic form of sulphur is more stable at room temperature?
The following statement is true under certain conditions. Mention the condition in each case in a few words.
Hydrogen reacts with nitrogen to form ammonia.
Name the element of group 17, which has the following properties:
Radioactive
In the known interahalogen compounds AB_n what is maximum value of n ?
Answer the following:
(i) Write the general electronic configuration of halogens in the valence shell.
Answer the following:
Name the halogens.
Answer the following:
Name four oxoacids of chlorine. Give their molecular formulae.
Answer the following:
Name two interhalogens of AB_3 type.
Complete the following reactions:
XeF_6 + H_2O\rightarrow
Name the noble gas which is not adsorbed in coconut charcoal
Name the noble gas which is radioactive
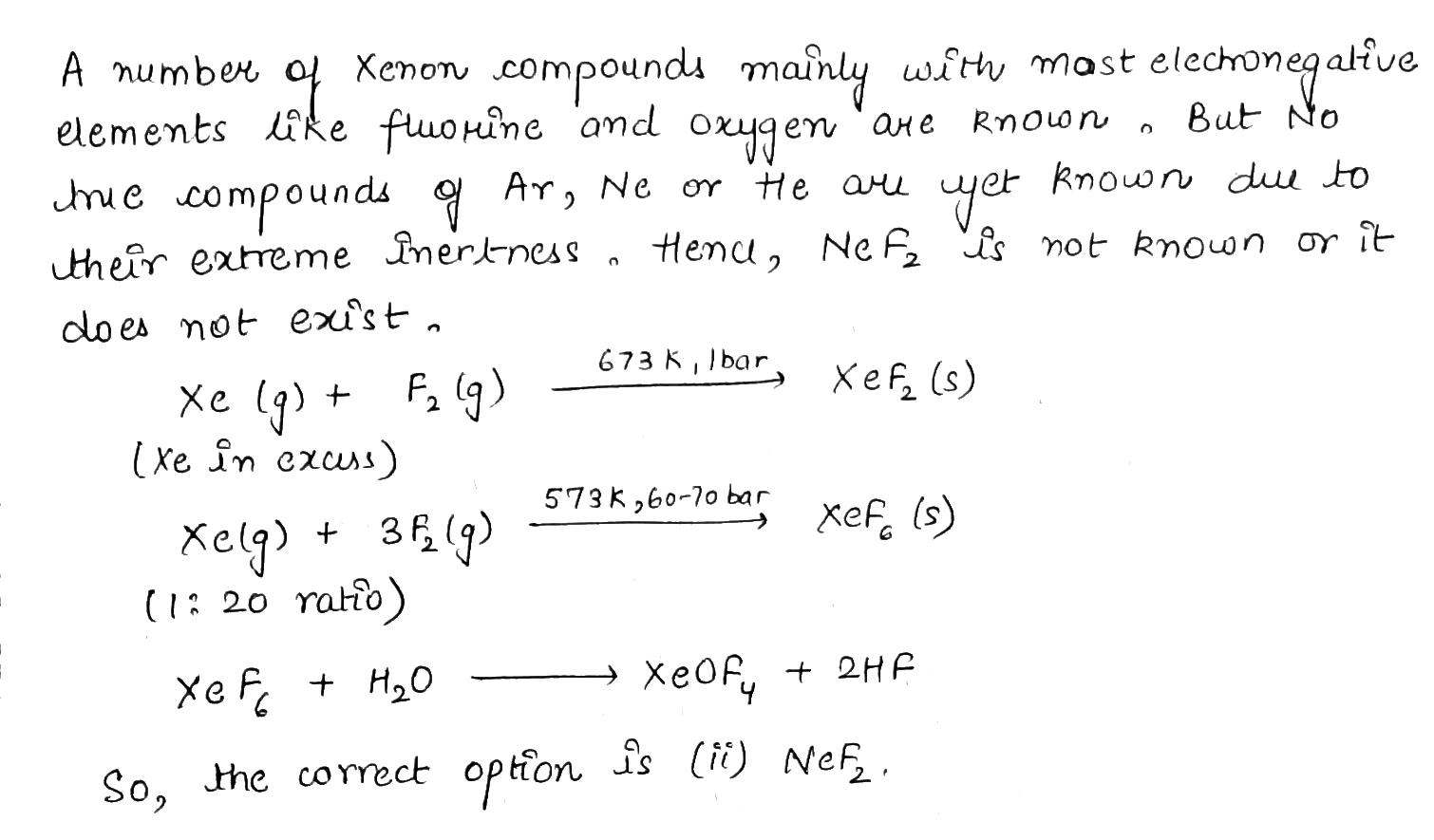
Which one of the following does not exist?
(i) XeOF_4
(ii) NeF_2
(iii) XeF_2
(iv) XeF_6
Complete the following reactions:
XeF_6 + SiO_2\rightarrow
Answer the following:
Name two poisonous gases of chlorine used in the warfare.
Complete the following reactions:
XeF_6 + NH_3\rightarrow
Write short notes on:
(i) Interhalogen compounds.
Answer the following:
Name the halogen which gives ozone with water.
Write down the two compounds of Vth or 15th group elements which are covalent in vapour state but ionic in solid state.
Answer the following questions is a single digit integer.
How many noble gases are radioactive in nature?
What happens when water reacts with the following compounds?
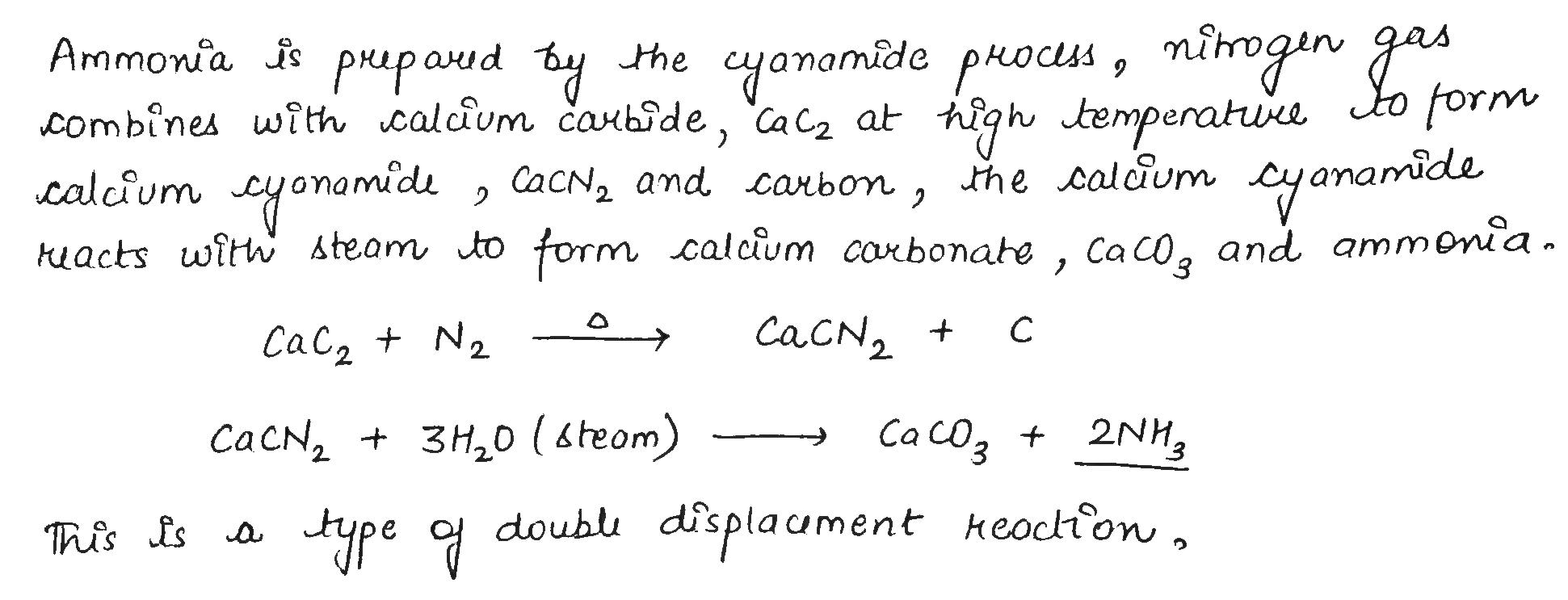
Calcium cyanamide, CaCN_2
Answer the following with relevant reason.
Why the group in which inert gases are accommodated was called zero group?
Phosphorus has three allotropic forms- (A) White phosphorus (B) red phosphorus and (C) black phosphorus, Write the difference between white and red phosphorus on the basis of their structure and reactivity.
How will you bring about the following conversions?
Oxygen from nitre.
Oxygen from nitre.
How will you bring about the following conversions?
Ammonia from nitre.
Ammonia from nitre.
Draw the structure of the following:
XeF_{4}.
State reasons for the following:
SF_{6} is kinetically an inert substance.
Can FCl_{3} exist? Comment.
Complete the following equations:
XeF_{4} + O_{2}F_{2} \rightarrow
Why He, Ne and Ar called inert gas?
What are the two forms of oxygen found in the atmosphere?
Iodine form I_{3}^{-} but F_{2} does not form F_{3}^{-} ions. Why?
Give the symbols for:
Most reactive non-metal of group 17
Account for the following:
PF_{5} is known but NF_{5} is not known.
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(a) Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773K to form ammonia gas.
Account for the following:
Both NO and ClO_{2} are odd electron species but NO dimerises while ClO_{2} does not.
Explain the following observations:
Helium forms no real chemical compound.
Answer the following question:
What is the structural difference between white phosphorus and red phosphorus.
First member of each group of representative elements (i.e., s and p-block elements) shows anomalous behavior. Illustrate with two examples.
Name:
Two substances from which oxygen can be obtained at a large scale.
Two substances from which oxygen can be obtained at a large scale.
In electron dot structure, the valence shell electrons are represented by crosses or dots.
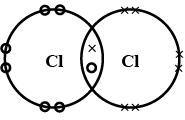
(a) the atomic number of chlorine isWrite its electronic configuration
(b) Draw the electron dot structure of chlorine molecule.
Give reasons for the following:
Neon is generally used for warning signals.
How would you account for the following:
Fluorine never acts as the central atom in polyatomic interhalogen compounds.
Give balanced equations to obtain oxygen from:
Lead dioxide
Lead dioxide
Name the type of elements , which have their
One electron short of octet - ...................
An element X belong to 4^{th} period and 17^{th} group , state name of the element.
Fluorine , chlorine and Bromine are put in one group of their similar properties .
What is the common name of this group or family.
Name two elements in each case :
Halogens
This question refers to the elements of the periodic table with atomic number from 3 to 18 . Some of the elements are shown by letters , but the letters are not the usual symbols of the elements.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | B | C | D | E | F | G | H |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| I | J | K | L | M | N | O | P |
- Which of these is a halogen ?
Which element has :
Two shells, both of which are completely filled with electrons ?
An element X belong to 4^{th} period and 17^{th} group , name the family to which it belongs.
Name the type of elements , which have their
Outermost shell complete ...............
Name two elements in each case :
Inert gas
Name :
An alkali metal in period 3 and halogen in period 2 .
Name:
a nitride of a divalent metal which reacts with warm water liberating ammonia.
Ammonia is used in the Ostwald process,
1) Name the catalyst used in the process.
2) Name the oxidizing agent used in this process.
Ammonia is used in the Ostwald process,
What is the ratio of ammonia and air taken in this process ?
Ammonia is used in the Ostwald process,
Name the oxidizing agent used in this process.
Give balanced equations for the following conversation:
A nitride of a trivalent metal to ammonia.
Ammonia is used in the Ostwald process,
Give the sources of reactants used in this process.
Name :
The noble gas with 3 shells .
Correct the following:
Ammonium salts on heating decompose to give ammonia.
Complete the following equation.
Mg_3N_2 + 6H_2O \rightarrow
Write the equations for the following reactions which result in the formation of ammonia.
A mixture of ammonium chloride and slaked lime is heated.
Write a balanced chemical equation for the lab preparation of ammonia.
Ammonia is used in the Ostwald process,
Why is quartz used in this process ?
Write the equations for the following reactions which result in the formation of ammonia.
Aluminium nitride and water.
Why is ammonia not collected over water ?
Write equation for the following:
Aluminium nitride and water.
Write the equation for the formation ammonia by the action of water on magnesium nitride.
Which compound is normally used as a drying agent for ammonia ?
Ammonia gas can be prepared by warming an ammonium salt with caustic alkali. Give two equations.
How is ammonia collected ?
How will you prepare the following from nitric acid?
Sodium nitrate
Ammonia cannot be collected over water. Give reason.
How is ammonia dried and collected in the laboratory ?
Correct the following, if required:
Constant boiling nitric acid contains 80\% nitric acid by weight.
Give reasons for the following:
In the laboratory preparation of nitric acid, the mixture of concentrated sulphuric acid and sodium nitrate should not be heated very strongly above 200^\circ \ C.
Give reasons:
Ammonium nitrate is not used in the preparation of ammonia.
A substance 'A' was heated with slaked lime and a gas 'B' with a pungent smell was obtained. Name the substances A and B and give a balanced equation.
Explain with a diagram the preparation of aqueous ammonia.
In the preparation of nitric acid from KNO_3 concentrated hydrochloric acid is not used in place of concentrated sulphuric acid. Explain why?
Give reasons:
Conc H_2SO_4 is a good drying agent, yet it is not used to dry NH_3.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
Write the equation for the reaction in which copper is oxidized by concentrated nitric acid
Name the minerals of chlorine.
How will you prepare the following from nitric acid?
Magnesium nitrate
What are the three allotropic forms of phosphorous.
The figure given above illustrates the apparatus used in the laboratory preparation of nitric acid.
Name A (a liquid), B (a solid) and C (a liquid). ( Do not give the formulae)

Explain why:
Nitric acid is kept in a reagent bottle for a long time.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
Write an equation to show how nitric acid undergoes decomposition.
How will you prepare the following from nitric acid?
Copper nitrate
Name the common element present in bleaching powder and common salt ?
Name the compound of chlorine which is disinfectant.
Arrange the apparatus as shown in figure
What do you observe ?
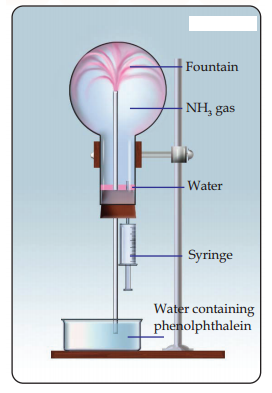
Write the equation of the reaction for the formation of ammonia.
Name the industrial production of ammonia.
In the manufacture of ammonia, the reaction in which direction results in the decrease in the number of molecules is
What is covalency of chlorine atom in second excited state ?
Discuss the conditions required in the Haber process for the manufacture of ammonia.
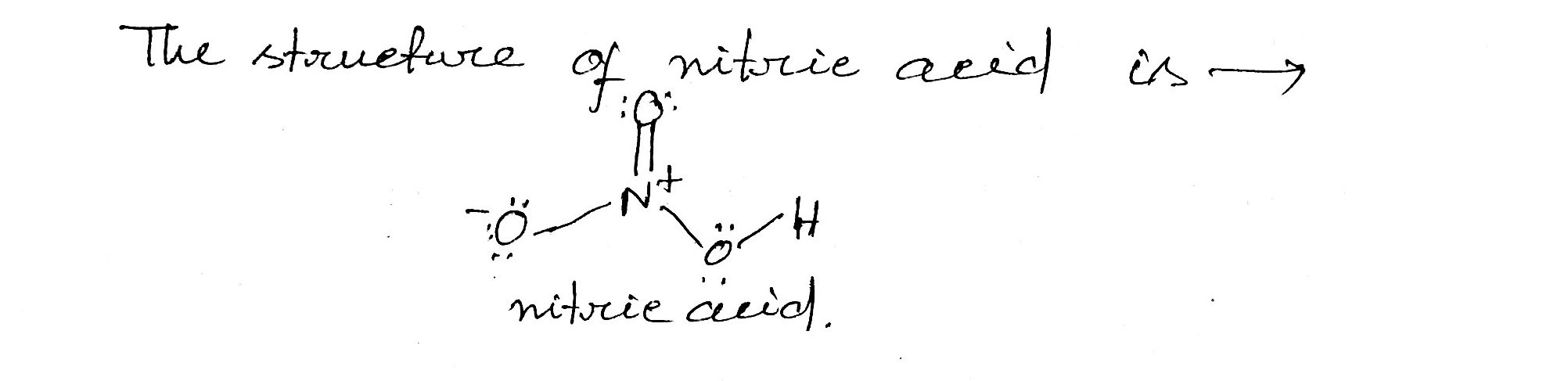
What is the structure of nitric acid ?
How is nitric acid manufactured from ammonia ?
Consider following compounds A to E.
(A) XeF_{n} (B) XeF_{(n + 1)}^{+} (C) XeF_{(n + 1)}^{-} (D) XeF_{(n + 2)} (E) XeF_{(n + 4)}^{2-}
If value of n is 4, then calculate value of "p + q" here, 'p' is total number of bond pair and 'q' is total number of lone pair on central atoms of compound A to E.
Write balanced equation(s) for the manufacture of nitric acid by the oxidation of ammonia.
Match List-I with List-II.
Find the sum of the total number of P - \hat{P} - P angle and P - P sides in P_4 molecule.
All the compounds listed in column-I react with water. Match the result of the respective reactions with appropriate options listed in column-II.
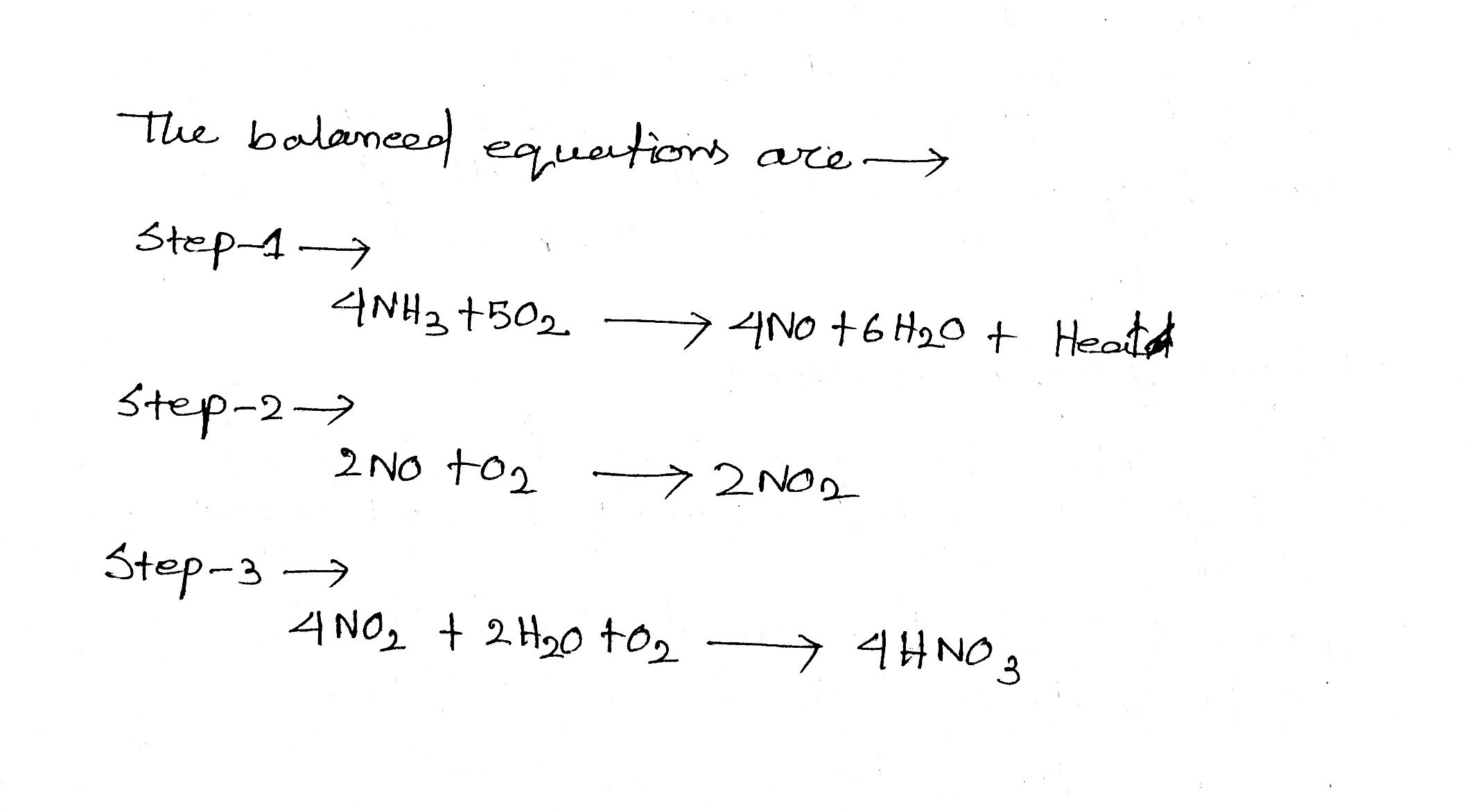
4NH_{3} (g) + 5O_{2}(g) \rightarrow 4NO(g) + 6H_{2}O(l)
2NO(g) + O_{2}(g) \rightarrow 2NO_{2}(g)
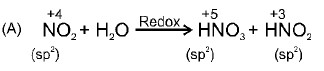
2NO_{2}(g) + H_{2}O(l)\rightarrow HNO_{3}(aq) + HNO_{2}(aq)
Consider the reactions of the Ostwald Process for the production of nitric acid above.
The first reactions have 50% yields and the last reaction has 25% yield.
Mass of NH_{3} would be needed to produce 126\ g of HNO_{3} is __________ gm.
Explain the following giving an appropriate reason in each case.
(i) O_2 and F_2 both stabilize higher oxidation states of metals but O_2 exceeds F_2 in doing so.
(ii) Structures of xenon fluorides cannot be explained by valence bond approach.
With what neutral molecule is ClO^{-} isoelectronic? Is that molecule a Lewis base?
Arrange the following in order of property indicated for each set:
a. F_{2}, Cl_{2}, Br_{2}, I_{2} - increasing bond dissociation enthalpy.
b. HF, HCl, HBr, HI - increasing acid strength.
c. NH_{3}, PH_{3}, AsH_{3}, SbH_{3}, BiH_{3} - increasing base strength.
In an experiment, the solubility of iodine in diethyl ether, n-hexane, Carbon Tetrachloride and toluene was measured. The solubilities were: 337g/kg, 182g/kg,19 g/kg and 13 g/kg. Correlate the solubilities with the solvents
| Solvent | Solubility | Solvent | Solubility |
| Carbon tetrachloride | A | n-Hexane | B |
| Diethyl ether | C | Toluene | D |
How to do lab preparation of ammonia using an ammonium salt?
Give reasons for the following:
(i) Zn^{+2} salts are while but Cu^{2+} salts are blue in colour.
(ii) Fluorine gives only one oxide but chlorine gives a series of oxides.
(a) Name two oxoacids of sulphur.
(b) (i) How will you manufacture ammonia by Haber process?
(ii) Write any two uses of inert gases.
Describe with chemical equation the Laboratory method for the preparation of ammonia. Write its reaction with the following:
(i) Oxygen in presence of platinum at {800}^{o}C.
(ii) Red hot copper oxide.
How will you obtain the following (Give only chemical equations)?
(i) { N }_{ 2 } from { NH }_{ 3 }
(ii) { NH }_{ 3 } from { NH }_{ 4 }Cl
Write only the types of hybridisation of central atom present in XeF_{2} and XeF_{4}.
Describe with suitable diagram the Deacon process of manufacture of chlorine. Write its reaction with ammonia.
40% of a mixture of 0.2 \,mol of N_2 and 0.6 \,mol of H_2reacts to give NH_3 according to the equation:
N_2(g) + 3H_2(g) \rightleftharpoons 2NH_3(g)
What happens to the following properties of group 15 hydrides as we move down the group?- Basic Character
- Thermal stability
- Reducing nature
- Dipole moment
- Bond angle
- Basic Character
- Thermal stability
- Reducing nature
- Dipole moment
- Bond angle
Write the equations involved in the manufacture of nitric acid by Ostwald's process by maintaining reaction conditions.
34 gm of a mixture containing { N }_{ 2 } and { H }_{ 2 } in 1:3 by mole is partially converted into { NH }_{ 3 }. Calculate the vapour density of the mixture (containing remaining { N }_{ 2 },{ H }_{ 2 } and { NH }_{ 3 } formed) after reaction if it has been found that the { NH }_{ 3 } formed required 0.5 moles of { H }_{ 3 }{ PO }_{ 4 } for complete neutralization.
In the manufacture of ammonia by Haber's process, write the flow chart and chemical equations with optimum conditions.
Answer the following question:
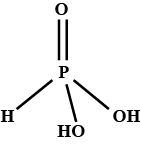
a. What is the basicity of H_{3}PO_{3} and why?
b. Why does fluorine not play the role of a central atom in interhalogen compound?
c. Why do noble gases have very low boiling points?
Write the formula of the compound of phosphorous which is obtained when conc. HNO_{3} oxidises P_{4}.
How is ammonia manufactured by Haber's process ? Explain.
Describe the manufacture of nitric acid Ostwald's process.
Why fluorine is a stronger oxidizing agent than chlorine?
What is the action of water on the following?
Calcium cyanamide
What is the action of water on the following?
Calcium nitride
Why fluorine shows anomalous behaviour as compared to other halogens?
Which gases are evolved when Mg_3N_2 reacts with water?
Describe the action of heat on ammonium chloride.
Describe the principle of manufacture with conditions of the following compounds:
Ammonia by Haber process.
How will you prepare ammonia from calcium carbide?
How many compound liberate NH_3 on heating from the following?
(NH_4)_2SO_4, (NH_4)_2CO_3, NH_4Cl,NH_4NO_3,(NH_4)_2Cr_2O_7
A compound (A) on heating with an excess of caustic soda solution liberates a gas (B) which gives white fumes on exposure to HCl. Heating it continued to expel the gas completely. The resultant alkaline solution again liberates the same gas (B) with zinc powder. However, the compound (A) when heated alone, does not give nitrogen. Identify (A) and (B).
Write short notes on:
(i) Available chlorine.
Describe the principle of manufacture with conditions of the following compounds:
Nitric acid by Ostwald's process.
Describe the principle of manufacture with conditions of the following compounds:
White phosphorous from bone ash by electrothermic method.
Complete the following reactions:
XeF_6 + SbF_5\rightarrow
Match LIst-I with List-II:
The following flow diagram represents the industrial preparation of nitric acid from ammonia.
Which of the following options describes the reagents, products, and reaction conditions?
| Option | (p) | (q) | (r) | (s) |
| (A) | Catalyst and high pressure | Cool | NO_2 | H_2O and O_2 |
| (B) | Catalyst | Cool | N_2O | HNO_3 and O_2 |
| (C) | Catalyst and high pressure | High pressure | NO_2 | H_2O and O_2 |
| (D) | High pressure | catalyst | N_2O_3 | HNO_3 |
[Enter the correct option e.g. if the answer is option C enter C]
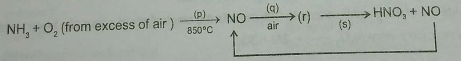
Account for the following:
Noble gases have comparatively large atomic sizes.
Nitric acid forms an oxide of nitrogen on reaction with P_{4}O_{10}.Write the resonating structures of the oxide of nitrogen formed.
Under what conditions can hydrogen be made to combine with nitrogen?
Name the product in the following case and write the equation for it.
Of the two gases, ammonia and hydrogen chloride which is more dense ? Name the method of collection of this gas.
X, Y and Z are three crystalline solids which are soluble in water and have a common anion. To help you to identify X, Y and Z you are provided with the following experimental observations. Copy and complete the corresponding inferences.
Write the equations for the following reactions:
(1) X and concentrated sulphuric acid (below 200^\circ)
(2) Action of heat on Y.
(3) Concentrated nitric acid is added to copper turnings kept in a beaker.
Name a drying agent for ammonia. Why are other drying agents such as P_2O_5 and CaCl_2 are not used ?
Give two examples to show the anamolus behaviour of fluorine.
Name any five periods properties .
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with —ic suffix.
B. If product is oxy acid with —ous suffix.
C. If product are two oxy acids one with —ic suffix and another one with —ous suffix.
D. If product is not oxy acid, neither with —ic suffix nor with —ous suffix.
H\underline NO_4+H_2O \longrightarrow HNO_3+H_2O_2
Copy and complete the following table which refers to the industrial method for the preparation of ammonia and sulphuric acid :
| Name of the compound | Name of the process | Catalytic equations (with the catalyst) |
| Ammonia | (i) ________ | (ii) _______ |
| Sulphuric acid | (iii) _______ | (iv) _______ |
Study the flow chart given and give balanced equations to represent the reactions A, B and C.
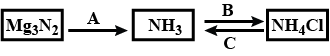
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with —ic suffix.
B. If product is oxy acid with —ous suffix.
C. If product are two oxy acids one with —ic suffix and another one with —ous suffix.
D. If product is not oxy acid, neither with —ic suffix nor with —ous suffix.
\underline N_2O_4+H_2O \longrightarrow HNO_3+HNO_2
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with —ic suffix.
B. If product is oxy acid with —ous suffix.
C. If product are two oxy acids one with —ic suffix and another one with —ous suffix.
D. If product is not oxy acid, neither with —ic suffix nor with —ous suffix.
\underline N_2O_5+H_2O \longrightarrow HNO_3
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with ic suffix.
B. If product is oxy acid with ous suffix.
C. If product are two oxy acids one with ic suffix and otherone with ous suffix.
D. If product is not oxy acid, neither with ic suffix nor with ous suffix.
\underline N_2O_3+H_2O \longrightarrow HNO_2
Class 12 Engineering Chemistry Extra Questions
- Alcohols,Phenols And Ethers Extra Questions
- Aldehydes,Ketones And Carboxylic Acids Extra Questions
- Amines Extra Questions
- Biomolecules Extra Questions
- Chemical Kinetics Extra Questions
- Chemistry In Everyday Life Extra Questions
- Coordination Compounds Extra Questions
- Electrochemistry Extra Questions
- General Principles And Processes Of Isolation Of Elements Extra Questions
- Haloalkanes And Haloarenes Extra Questions
- Polymers Extra Questions
- Solutions Extra Questions
- Surface Chemistry Extra Questions
- The D-And F-Block Elements Extra Questions
- The P-Block Elements Extra Questions
- The Solid State Extra Questions