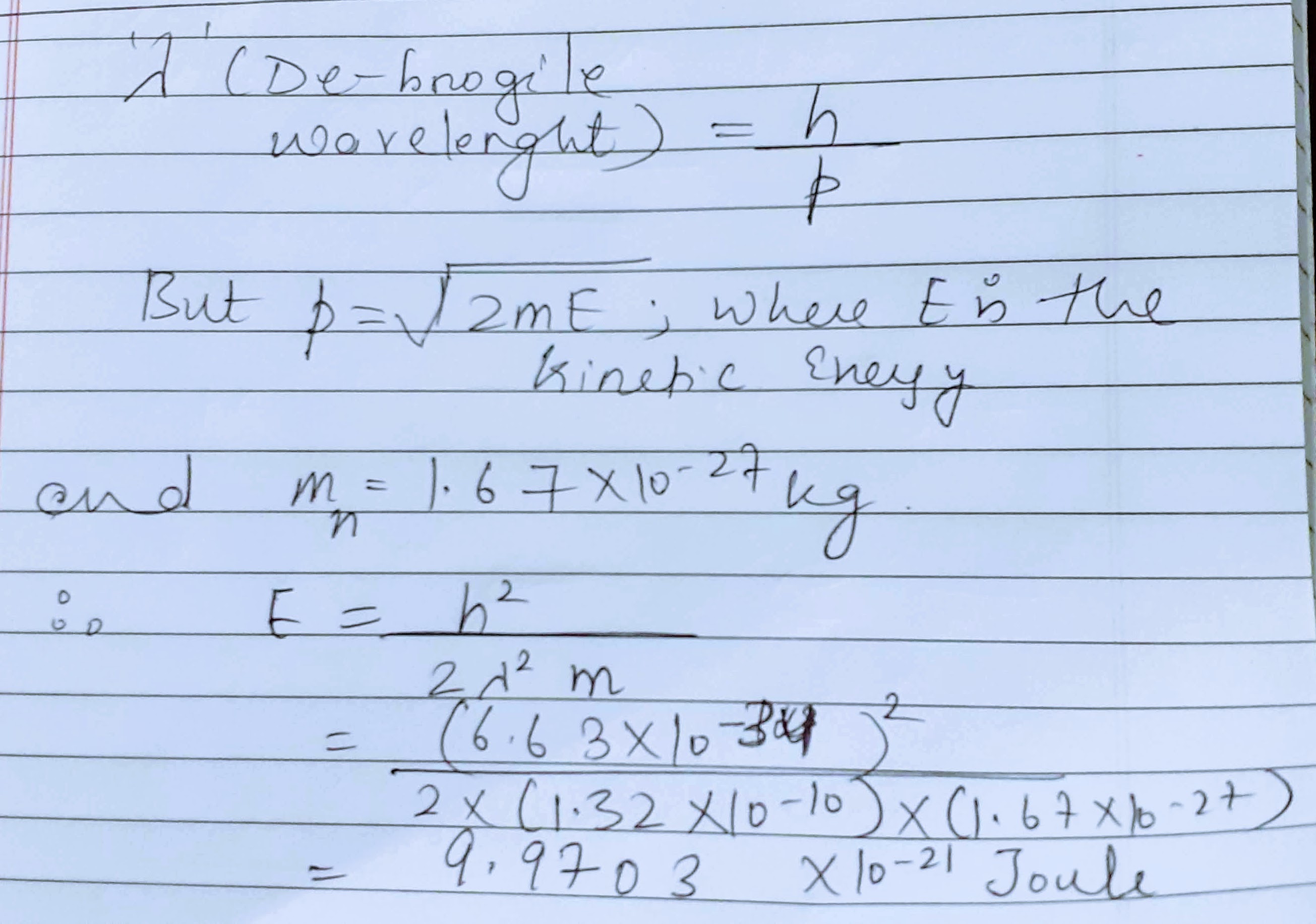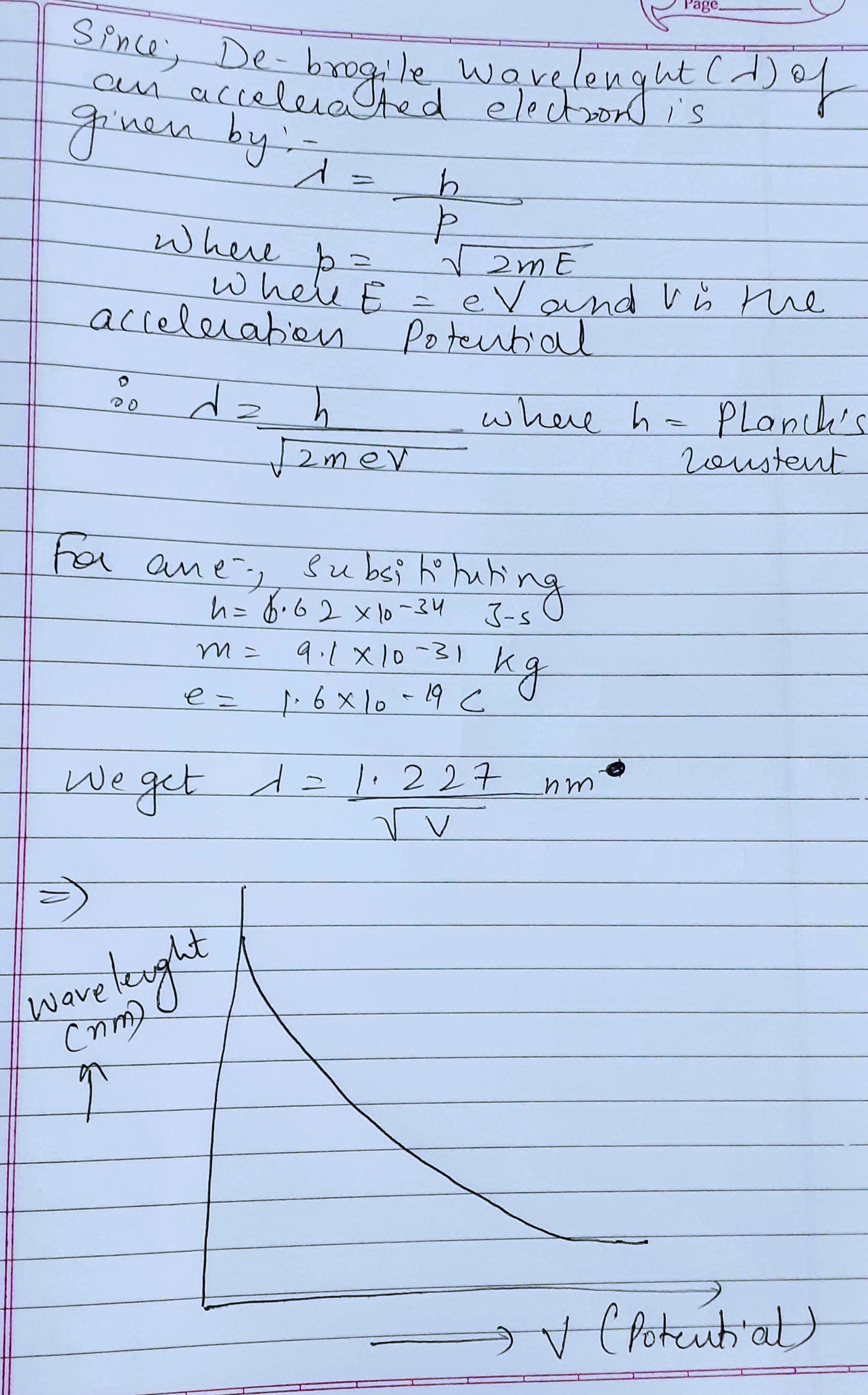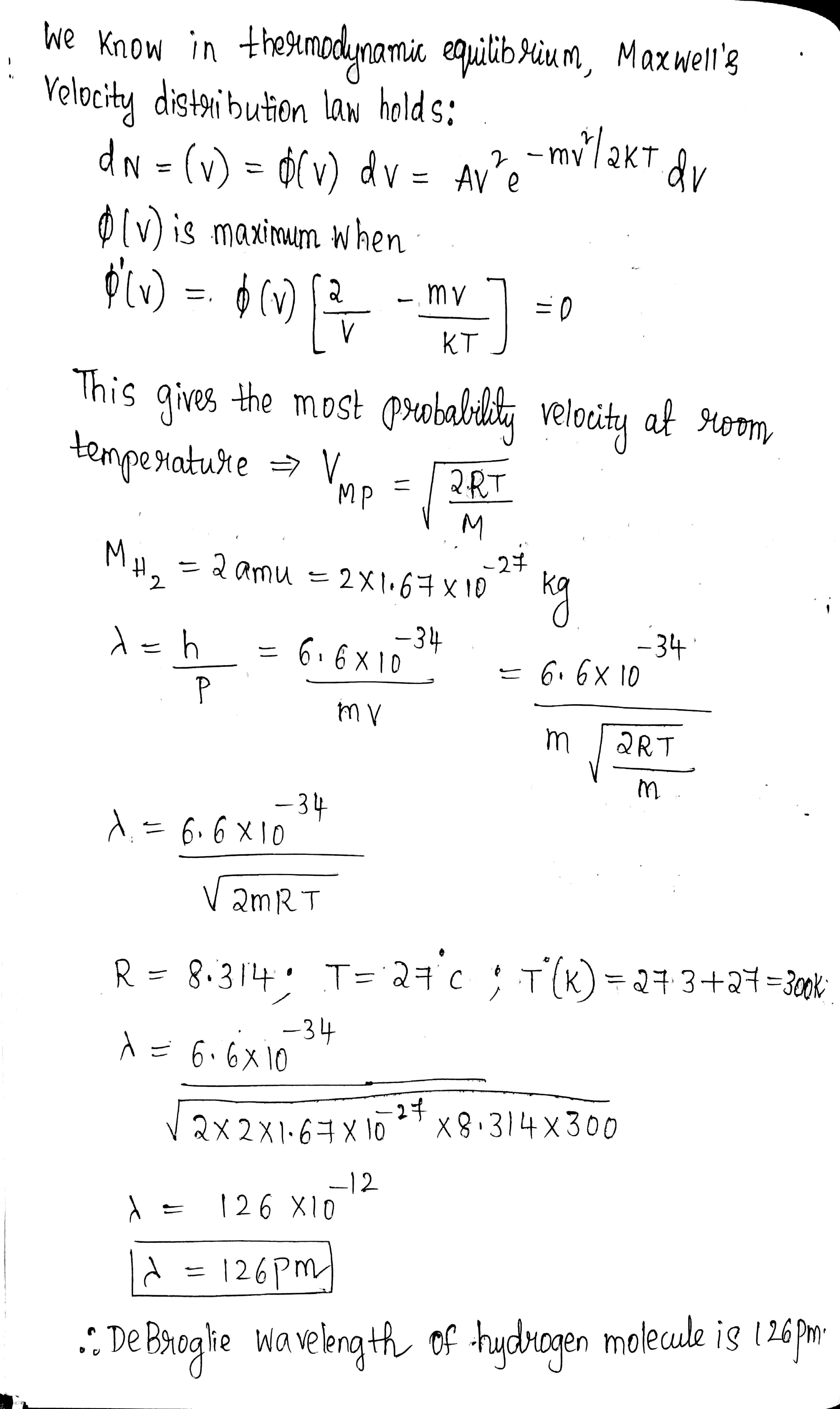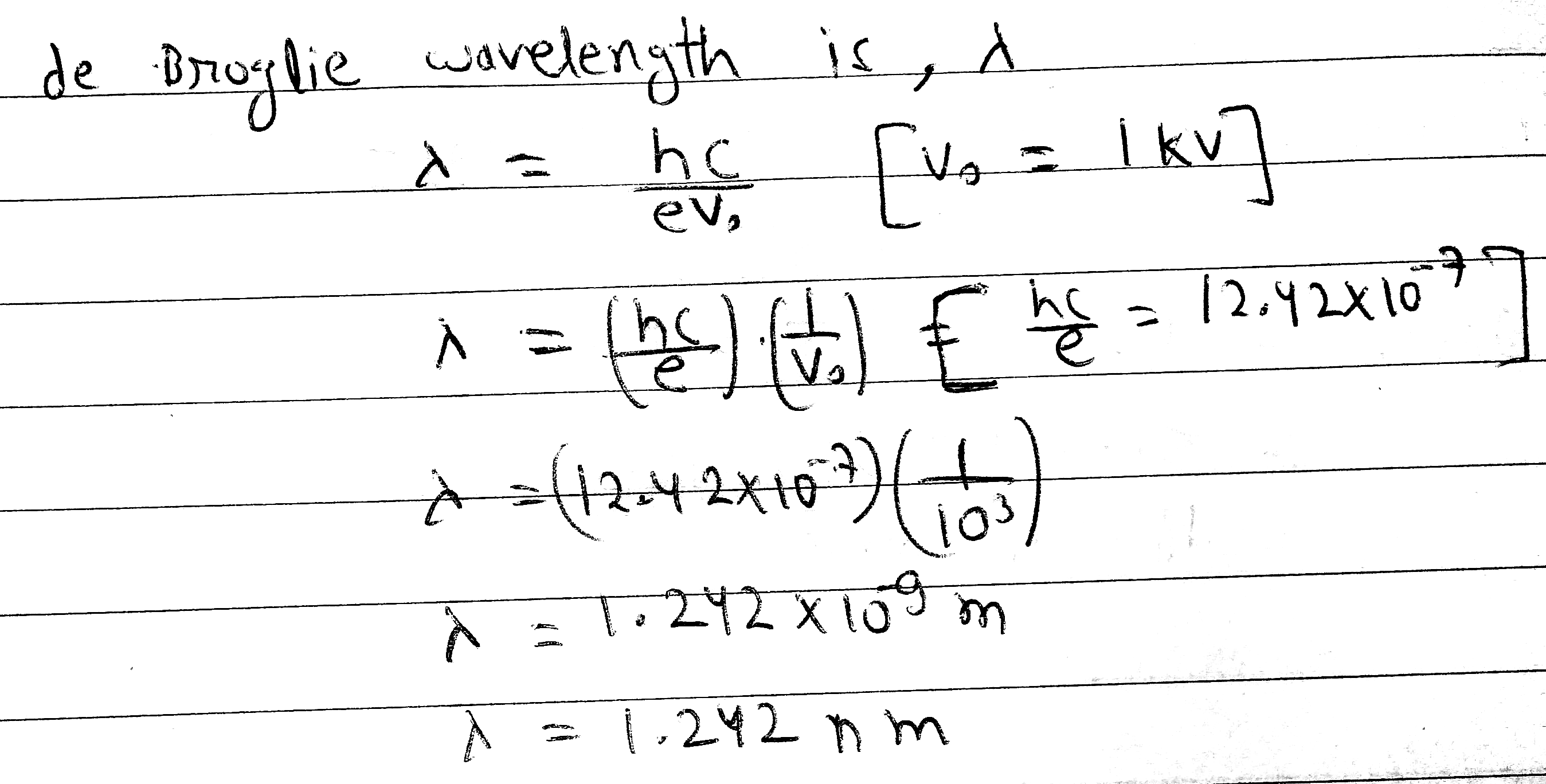Dual Nature Of Radiation And Matter - Class 12 Medical Physics - Extra Questions
An electron in an atom absorbs radiation of wavelength 392.n.m and later on it emits energy in the from of photons of two different wavelengths. If one of the wavelength is 712 n.m. Calculate the other.
The energy of the photons causing the photoelectrons emission
How many photons are emitted per second by $$a\ 5\ mW$$ laser source operating at $$632.8\ nm$$?
If the kinetic energy of the particle is increased to $$16$$ times previous the percentage change in the deBrogille wavelength of the particle is
The electron with The electron with de Broglie wavelength $$\lambda $$ is bombarded on a metal target. Found that photons are emitted through de Broglie wavelength.
The stopping potential in an experiment of photon is 2eV. What is the maximum kinetic energy of photoelectrons emitted ?
Electrons with de Broglie wavelength $$\lambda$$ are bombarded on a metal target. It is found that photons are emitted from the metal target. The minimum wavelength of emitted photons is [m = mass of eletron]
A nucleus $$X$$ is in the higher energy state where its energy is $$450 \mathrm { keV }$$ . If it comes to ground state (assuming ground state energy to be zero), what is the wavelength of the photon emitted?
Visible light has wavelengths in the range of $$400\, nm$$ to $$780\, nm$$. Calculate the range of energy of the photons of visible light.
Two neutral particles are kept 1 m apart. Suppose by some mechanism some charge is transferred from one particle to the other and the electric potential energy lost is completely converted into a photon. Calculate the longest and the next smaller wavelength of the photon possible.
A 100 W light bulb is placed at the center of a spherical chamber of radius 20 cm. Assume that 60% of the energy supplied to the bulb is converted into light and that the surface of the chamber is perfectly absorbing. Find the pressure exerted by the light on the surface of the chamber.
Find the maximum magnitude of the linear momentum of a photoelectron emitted when light of wavelength 400 nm falls on a metal having work function 2.5eV.
In a photoelectric experiment, the collector plate is at 2.0 V with respect to the emitter plate made of copper $$(\varphi =4.5eV)$$. The emitter is illuminated by a source of monochromatic light of wavelength 200nm. Find the minimum and maximum kinectic energy of the photoelectrons reaching the collector.
Calculate de Broglie wavelength for an average helium atom in a furnace at $$400\,K$$. Given the mass of helium $$= 4.002\,amu$$.
At what rate does the Sun emit photons? For simplicity, assume that the Sun's entire emission at the rate of $$3.9 \times 10^{26} \mathrm{W}$$ is at the single wavelength of $$550 \mathrm{nm}$$
Light of wavelength $$2.40 \mathrm{pm}$$ is directed onto a target containing free electrons. (a) Find the wavelength of light scattered at $$30.0^{\circ}$$ from the incident direction. (b) Do the same for a scattering angle of $$120^{\circ}$$
What percentage increase in wavelength leads to a $$75 \%$$ loss of photon energy in a photon-free electron collision?
The PE of a particle of mass m is given by $$V_{(x)} = \left\{\begin{matrix}E_0 \\0 \end{matrix}\right.$$ $$\begin{matrix}0 \le x \le 1 \\ x > 1 \end{matrix}$$
$$\lambda_1$$ and $$\lambda_2$$ are the de-Broglie wavelengths of the particle, when $$0 \le x \le 1$$ and $$x > 1$$ respectively. The total energy of the particle is $$42E_0$$. If the ratio $$\dfrac{\lambda_1}{\lambda_2}$$ is $$\sqrt{x} $$, find $$x$$.
What amount of energy should be added to an electron to reduce its de Broglie wavelength from 100 to 50 pm?
The radius of an $$\alpha$$-particle moving in a circle in a constant magnetic field is half of the radius of an electron moving in circular path in the same field. The de Broglie wavelength of $$\alpha$$-particle is $$x$$ times that of the electrons. Find $$x$$ (an integer).
An $$\alpha$$-particle and a proton are accelerated from rest by a potential difference of $$100\ V$$. After this, their de Broglie wavelengths are $$\lambda_{\alpha}$$ and $$\lambda_p$$, respectively. The ratio $$\lambda_p/\lambda_{\alpha}$$, to the nearest integer, is:
A proton is fired from very far away towards a nucleus with charge $$Q=120e$$, where $$e$$ is the electronic charge. It makes a closest approach of $$10 fm$$ to the nucleus. The de-Broglie wavelength (in unit of $$fm$$) of the proton at its start is: (take the proton mass, $$m_p=(5/3)\times 10^{-27} kg; h/e=4.2\times 10^{-15}Js/C; $$ $$\dfrac {1}{4\pi \epsilon_0}=9\times 10^9m/F; 1fm=10^{-15} m)$$
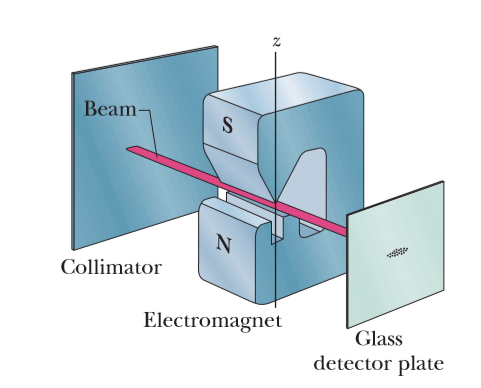
Calculate the (a) smaller and (b) larger value of the semiclassical angle between the electron spin angular momentum vector and the magnetic field in a SternGerlach experiment. Bear in
mind that the orbital angular momentum of the valence electron in the silver atom is zero.
An electron and photon have same energy 100 eV. Which has greater associated wavelength?
If an electron has a wavelength does it also have a colour?
What is the momentum of a photon if the
wavelength of X-rays is 1 angstrom?
What is the momentum of a photon if the wavelength of X-rays is 1 angstrom?
A parallel beam of monochromatic light of wavelength $$500\ nm$$ is incident normally on a perfectly absorbing surface. The power through any cross-section of the beam is $$10 W$$. The number of photons absorbed per second by the surface is $$k\times 10^{19}$$. what is a value of k?
De Broglie wavelength for an average helium atom in a furnace at 400 K is $$k\times 10^{-10} m$$. Find the value of k. Here, Given the mass of helium $$=4.002 amu$$
If the wavelength of electromagnetic radiation is doubled, what happens to the energy of photon?
Give an example where energy is converted into matter
Why we do not observe de Broglie wave in daily life?
An insulated metal plate yields photoelectrons when first illumminated by ultraviolet light but then after some time photoelectron emission stops. Explain
Explain the concept of de Broglie waves (matter wave).
Answer the following questions:
(a) Quarks inside protons and neutrons are thought to carry fractional charges $$[(+2/3)e ; (1/3)e]$$. Why do they not show up in Millikan's oil-drop experiment ?
(b) What is so special about the combination e/m? Why do we not simply talk of e and m separately?
(c) Why should gases be insulators at ordinary pressures and start conducting at very low pressures?
(d) Every metal has a definite work function. Why do all photoelectrons not come out with the same energy if incident radiation is monochromatic? Why is there an energy distribution of photoelectrons?
(e) The energy and momentum of an electron are related to the frequency and wavelength of the associated matter wave by the relations: $$E = h \nu$$, p = $$\displaystyle\frac{h}{\lambda }$$.But while the value of $$\displaystyle\lambda$$ is physically significant, the value of $$\nu$$ (and therefore, the value of the phase speed n $$\displaystyle\lambda)$$ has no physical significance. Why?
(a) Quarks inside protons and neutrons are thought to carry fractional charges $$[(+2/3)e ; (1/3)e]$$. Why do they not show up in Millikan's oil-drop experiment ?
(b) What is so special about the combination e/m? Why do we not simply talk of e and m separately?
(c) Why should gases be insulators at ordinary pressures and start conducting at very low pressures?
(d) Every metal has a definite work function. Why do all photoelectrons not come out with the same energy if incident radiation is monochromatic? Why is there an energy distribution of photoelectrons?
(e) The energy and momentum of an electron are related to the frequency and wavelength of the associated matter wave by the relations: $$E = h \nu$$, p = $$\displaystyle\frac{h}{\lambda }$$.
But while the value of $$\displaystyle\lambda$$ is physically significant, the value of $$\nu$$ (and therefore, the value of the phase speed n $$\displaystyle\lambda)$$ has no physical significance. Why?
(a) An X-ray tube produces a continuous spectrum of radiation with its short wavelength end at 0.45 . What is the maximum energy of a photon in the radiation?(b) From your answer to (a), guess what order of accelerating voltage(for electrons) is required in such a tube?
Fill in the blanks with suitable answer
The energy of a photon is given by the formula _____________
You might have seen mercury street lamps. The light blue glow given by these lamps has a wavelength of = 436nm. What is its frequency?
State how de-Broglie wavelength $$(\lambda )$$ of moving particles varies with their linear momentum (p).
Derive an expression for de Broglie wavelength of matter waves.
A ball of mass 0.12 kg is moving with a speed 20 m/s. Calculate the de Broglie wavelength. (Planck constant $$h = 6.62 \times 10^{-34} J.s$$)
Show with the help of a labelled graph how their wavelength $$(\lambda)$$ varies with their linear momentum (p).
Find the de Broglie wavelength of electrons moving with a speed of $$7\times 10^6ms^{-1}$$.
Calculate the momentum of a photo of energy $$6\times 10^{-19}$$J.
Assume that a 100 W sodium vapour lamp rediates its energy uniformly in all directions in the form of photons with an associated wavelength of 589 nm.
At what rate are photons emitted from the lamp?
S is isotropic monochromatic source of constant power P. r is very large and v is frequency of radiation .The number of photons crossing per unit time the given surface is in $$n=\dfrac{P\Delta S cos \theta}{k \pi vr^2h}$$ .Find k if $$\Delta S$$ is the area of small section.

Assume that a 100 W sodium vapour lamp rediates its energy uniformly in all directions in the form of photons with an associated wavelength of 589 nm.
At what distance from the lamp will the average flux of photons be 1.00 photons /$$(cm^2s)$$ ?
An electron, an alpha $$(\alpha)$$ particle and a proton have same kinetic energies. Which one of these particles has largest de Broglie wavelength?
A light beam of wavelength $$400\ nm$$ is incident on a metal plate of work function $$2.2 \ eV$$. A particular electron absorbs a photon and makes some collisions before coming out of the metal. Assuming that $$10\%$$ of the instantaneous energy is lost to the metal in each collision, find the minimum number of collisions the electron can suffer before it becomes unable to come out of metal. (Use $$hc=12400 \ eV \ \dot{A}$$)
An $$\alpha$$ particle moves in circular path of radius $$0.83cm$$. In the presence of a magnetic field of $$0.25Wb/{m}^{2}$$. Find the De Broglie wavelength associated with the particle
Find the energy of photon in each of the following:
A. Microwaves of wavelength $$1.5\ cm$$B. Red light of wavelength $$660\ nm$$C. Radiowaves of frequency $$96\ MHz$$D. $$X-$$rays of wavelength $$0.17\ nm$$
When light of frequency $$2.2\times 10^{15}Hz$$ is incident on a metal surface, photoelectrons emitted can be stopped by a retarding potential of $$6.6\ V$$. For light of frequency $$4.6\times 10^{15}Hz$$, the reverse potential is $$16.5\ V$$. Find $$h$$
The energy and momentum of an electron are related to the frequency and wavelength of the associated matter wave by the relations:
$$E = h v, p = \dfrac{h}{\lambda}$$
But while the value of $$\lambda$$ is physically significant, the value of $$v$$ (and therefore, the value of the phase speed $$v$$ $$\lambda$$) has no physical significations. Why?
In an experiment of photoelectric effect, the slope of the cut-off voltage versus frequency of incident light is found to be $$4.2\times 10^{-15} V s$$. Calculate the value of Plank's constant.
What kinetic energy of a neuron will be associated by the de-broglie wavelength $$1.32 \times 10^{-10}m$$?
An atom absorbs a photon of wavelength $$500 nm $$ emits another photon of wavelength $$700nm$$ find the net energy absorbed by the atom in the process
What is photoelectric effect? State and explain its characteristics.
Find the frequency of 1 MeV photon. Given wavelength of a 1 keV photon is $$1.24 \times {10^{ - 9}}m$$
Hint: $$E = h\nu $$
The energy of a photo is equal to the kinetic energy of proton. Let $$ \lambda_1 $$ be the de-Broglie wavelength of the photon and $$ \lambda_2 $$ be the wavelength of the radiation and the de-Broglie wavelength of a photon of that radiation?
A $$1\ MeV$$ positron and a $$1\ MeV$$ electron meet each moving in opposite directions. They annihilate each other by emitting two photons. If the rest mass energy of an electron is $$0.51\ MeV$$, the wavelength of each photon is ?
What is the de-Broglie wavelength of an electron beam accelerated through a potential difference of $$25 \,V$$?
Find the de-Broglie wave length related to an election accelerated by $$10^4$$ volt
Find the typical de-Broglie wavelength of an electron in a metal at $${27}^{o}C$$ and compare it with the mean separation between two electrons in a metal which is given to be about $$2\times{10}^{-10}m$$
The radius of two bohr radius of a hydrogen like atom are $$r_1$$ and $$r_2$$. Find the wave length of photon when electron jumps from $$r_2$$ to $$r_1$$.
An electron and a proton have the same kinetic energy. Which one of the two has the larger de Broglie wavelength and why?
What will be the energy of each photon in monochromatic light of frequency $$5\times10^{14}Hz$$?
Find out the ratio between de-Broglie wavelength of proton and a-particle of equal energy.
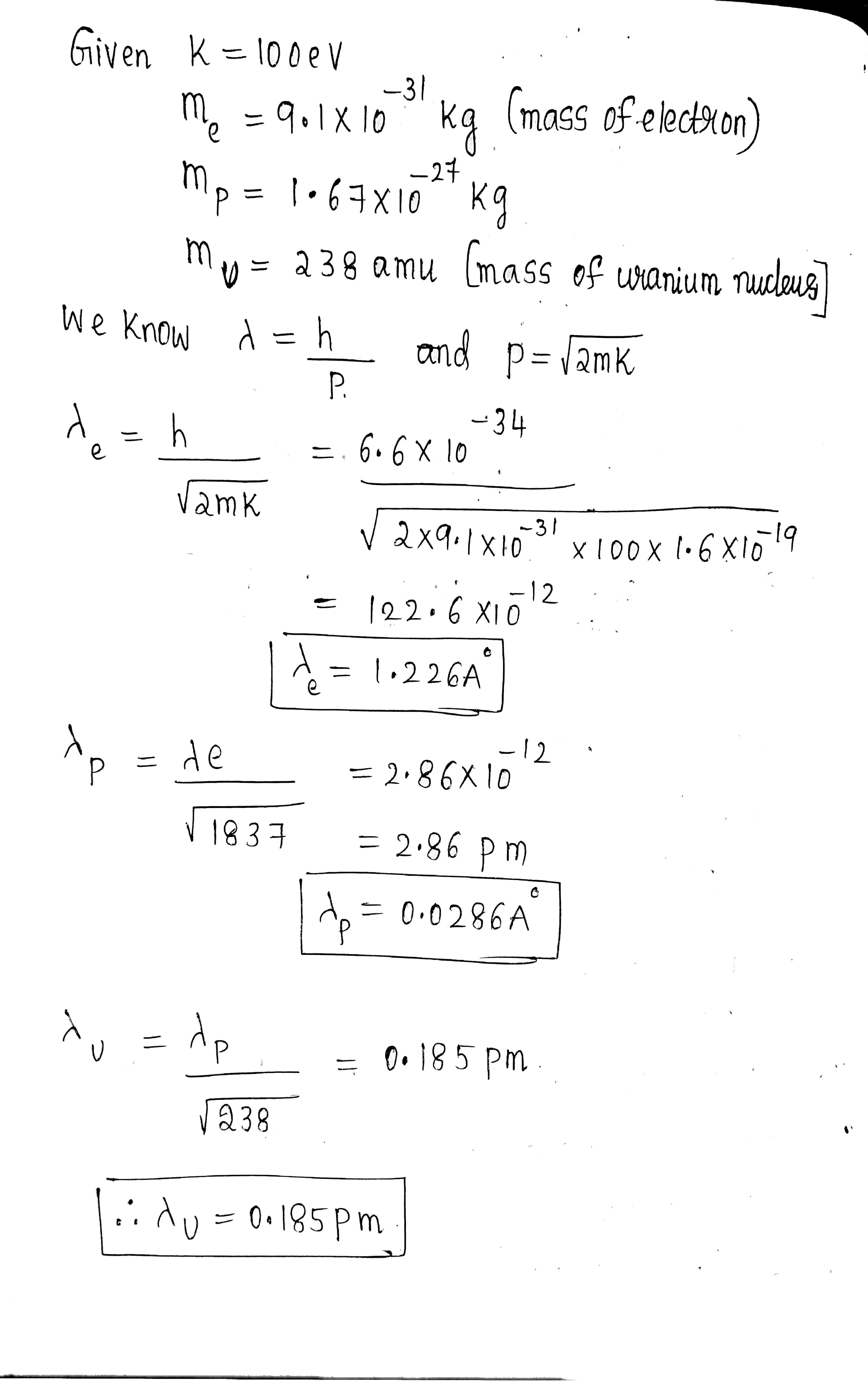
Calculate the de Broglie wavelengths of an electron, proton, and uranium atom, all having the same kinetic energy $$100\ eV $$.
At room temperature $$ (T = 300\,K)$$ neutron are in thermal equilibrium. Calculate its de-Broglie wavelength.
According to de-Broglie hypothesis, write formula of wavelength of matter waves.
Derive the expression for a de Broglie wavelength $$ \lambda $$ of a relativistic particle moving with kinetic energy T. At what values of T does the error in determining $$ \lambda $$ using the non-relativistic formula not exceed $$1\% $$ for an electron and a proton ?
The potential energy of a particle varies as
$$V(x)=E_{o} ; 0\leq x \leq 1$$
$$=0; x >1$$
For $$0\leq x \leq 1$$, de Broglie wavelength is $$\gamma_{1}$$ and for $$x >1$$ the de Broglie wavelength is $$\gamma_{2}$$. Total energy of the particle is $$2E_{o}$$. If $$\gamma_{1}/\gamma_{2}=\sqrt x$$. Find $$x$$
Give reasons for the following
Laser is used in cutting metals.
State the factors on which the following depend
i) Kinetic energy of photo electrons.
ii) Number of photo electrons.
Distinguish between ordinary light and laser light.
Give reasons for the following
Ordinary key chain laser light should not be directly viewed.
The de Broglie wavelength of an electron moving with a velocity of $$1.5\times 10^8 ms^{-1}$$ is equal to that of a photon. Find the ratio of the kinetic energy of the photon to that of the electron.
A monochromatic source of light operating at 200 W emits $$4\times 10^{20}$$ photons per second. Find the wavelength of the light $$(in \times 10^{-7}m)$$
A parallel beam of monochromatic light of wavelength 663 nm is incident on a totally reflecting plane mirror. The angle of incident is $$60^o$$ and the number of photons striking the mirror per second is $$1.0\times 10^{19}$$. If the force exerted by light beam on the mirror is $$Y\times10^{-8}N$$. Find Y.
The potential energy of a particle of mass m is given by $$\displaystyle V\left( x \right) =\left\{ \begin{matrix} { E }_{ 0 } \\ 0 \end{matrix}\begin{matrix} 0\le x\le 1 \\ x>1 \end{matrix} \right\} $$
$$\displaystyle { \lambda }_{ 1 }$$ and $$\displaystyle { \lambda }_{ 2 }$$ are the de-Broglie wavelengths of the particle, when $$\displaystyle 0\le x\le 1$$ and $$\displaystyle x>1$$ respectively.
If the total energy of particle is $$\displaystyle { 2E }_{ 0 }$$, find $$\displaystyle \left( { { \lambda }_{ 1 } }/{ { \lambda }_{ 2 } } \right) $$.
The ratio of energy of photon of $$\lambda$$ = 2000 A$$^{o}$$ to that of $$\lambda$$ = 4000 A$$^{o}$$
The ratio of the energy of a photon of 2000 nm wavelength radiation to that of 4000 nm radiation is :
Find the typical de Broglie wavelength associated with a He atom in helium gas at room temperature ($$27 °C$$) and 1 atm pressure; and compare it with the mean separation between two atoms under these conditions.
The terminology of different parts of the electromagnetic spectrum is given in the text. Use the formula $$E = h\nu$$ (for energy of a quantum of radiation: photon) and obtain the photon energy in units of eV for different parts of the electromagnetic spectrum. In what way are the different scales of photon energies that you obtain related to the sources of electromagnetic radiation?
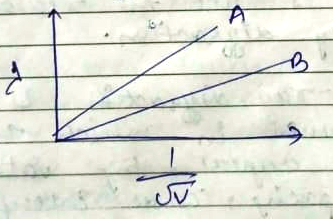
Plot a graph showing variation of de-broglie wavelength $$\lambda $$ versus $$\frac { 1 }{ \sqrt { V } } $$ where v is acclerating potential for two particles A and B carrying same charge but of masses $${ m }_{ 1 },{ m }_{ 2 }({ m }_{ 1 }>{ m }_{ 2 })$$. Which one of the two represents a particle of smaller mass and why?
(a) Why photoelectric effect cannot be explained on the basis of wave nature of light? Give reasons.
(b) Write the basic features of photon picture of electromagnetic radiation on which Einstein's photoelectric equation is based.
Given the ground state energy $$E_0 = - 13.6 \,eV$$ and Bohr radius $$a_0 = 0.53 \, \overset{o}{A}$$ . Find out how the de Broglie wavelength associated with the electron orbiting in the ground state would change when it jumps into the first excited state.
Yellow colours seen in fireworks are due to the emission of light with wavelength around 588 nm when sodium salts are burned. Calculate the frequency of yellow light of wave length 588 nm.
What is photoelectric effect? Give Einsteins explanation of the phenomenon.
List any three applications of laser.
Draw a neat diagram of Helium -Neon gas laser tube.
Derive an expression for de-Brogile wavelength of matter waves.
Write down de Broglie's relation and explain the terms therein.
All photo electrons are not emitted with the same energy as the incident photons. Why?
Determine De-Broglie Wave equation.
What is deBroglie's hypothesis? Write the expression for the wavelength associated with a moving particle.
Find energy of photon of wavelength $$4000\overset{o}{A}$$.
What are basic processes involved in the working of a LASER?
An electron, an $$\alpha$$ particle and a proton have the same kinetic energy. Which of these particles has the shortest de Broglie wavelength?
Write the formula for de Broglie wavelength. An electron is moving with speed of $$0.5\times { 10 }^{ 3 }m/s$$. Find the de Broglie wavelength associated with it.
Find the initial momentum of electron if the momentum of electron is changed by $$Pm$$ and the de Broglie wavelegth associated with it changes by $$0.50$$%.
The work function for the surface of aluminium is 4.2 eV. How much potential difference will be required to stop the emission of maximum energy electrons emitted by light of 2000 $$\dot{A}$$ wavelength? What will be the wavelength of that incident light for which stopping potential will be $$0$$ ?
A beam of light has three wavelengths 4121 A, 4972 A and 6216 A with a total intensity of $$3.6 \times 10^{-3} \, Wm^{-2}$$ equally distributed amongst the three wavelengths .The beam falls normally on an area $$ 1.0 cm^2$$ of a clean metallic surface of work function 2.3 eV .Assume that there is no loss of lighy by reflection and that each energetically oapable photon eject one electron .Calculate the number of photoelectrons liberated in two seconds
Give an expression for de-Broglie's wavelength of matter waves?
Calculate the de-Broglie wavelength of an electron moving with one fifth of the speed of light. Neglect relativistic effects. ($$h = 6.63 \times 10^{-34}\ J.s., c = 3 \times 10^8\ m/s$$, mass of electron $$= 9 \times 10^{-31}\ kg$$)
State de Broglie hypothesis.
How does one explain the emission of electrons from a photosensitive surface with the help of Einstein's photoelectric equation?
Intensity of a LASER beam $$(\lambda =6600\overset{o}{A})$$ is $$6$$ mW. Calculate no. of photons passing along path of beam in per second.
Write any three experimental observations of photoelectric effect.
An electron, in a hydrogen-like atom, is in an excited state. It has a total energy of $$-3.4\ eV$$. Calculate the kinetic energy.(in ev)
Write the expression for the de-Broglie wavelength of a particle.
Find the maximum kinetic energy of photo-electron liberated from the surface of lithium ($$\phi =2.39eV$$) by electromagnetic radiation whose electric component varies with time as $$F=a(1+\cos \omega t) \cos \omega_{o}t$$, where $$'a'$$ is a constant, $$\omega=6\times 10^{14}\ rad/sec$$
and $$\omega_{o}=3.60\times 10^{15}\ rad/s$$.
A sample of monoatomic hydrogen gas contains n atoms all the third excited state. Find the minimum value of n so that on subsequent de-excitation all possible different energy photons are emitted.
Show that it is not possible for a photon to be completely absorbed by a free electron.
An electron microscope uses eletrons accelerated by a voltage of $$50kV$$. Determine the deBroglie wavelength associated with the electrons. If other factors (such as numerical aperture, etc) are taken to be roughly the same, how does the resolving power of an electron microscope compare with that of an optical microscope which uses yellow light?
A TV station is operated at $$100MW$$ with a signal frequency of $$10MHz$$. Calculate the number of photons radiated per second by its antenna.
A hydrogen atom in a state having a binding energy 0.85eV makes a transiton to a state of excitation energy 10.2eV.The wave length of emitted photon is _____________nm.
A particle moving with velocity that is three times that of velocity of electron. If ratio of de-Brogile wave length of particle to that of the electron is $$1.8 \times 10^{-4}$$. Find mass of particle ($$m_e = 9.1 \times 10^{-31}$$ kg )
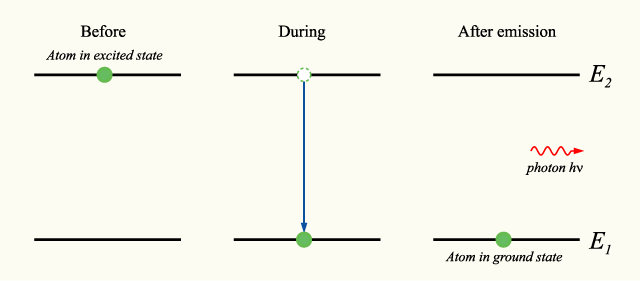
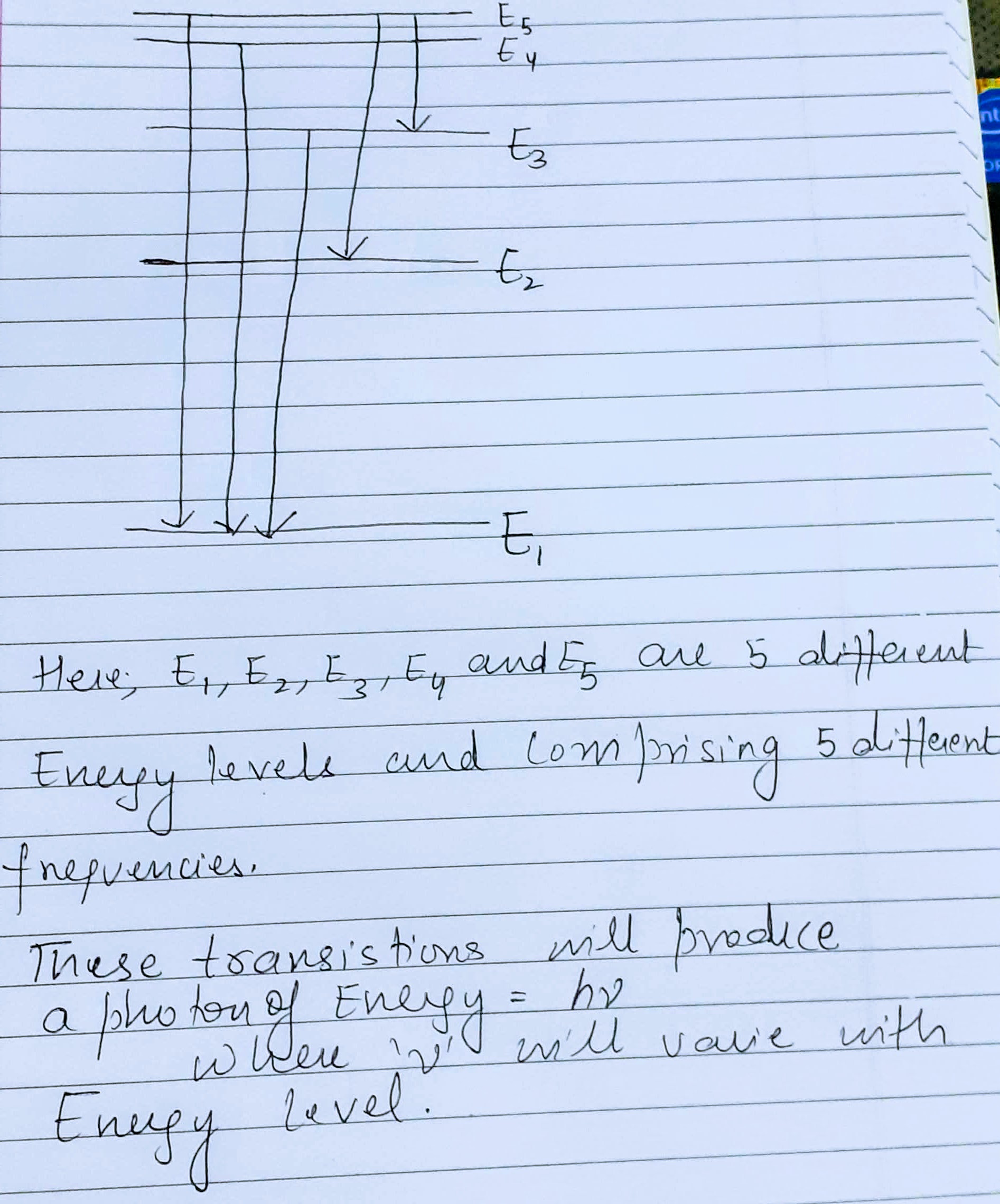
Show five energy levels of an atom, one being much lower than the other four. Five transitions between the levels are indicated, each of which produces a photon of definite energy and frequency.
Find out velocity of electron so that its momentum is equal to that of photon with a wavelength $$\lambda = 52000 \mathring{A}$$
How many photons per second enter one eye if you look directly at a 100 W light bulb 2.00 m away? Assume a pupil diameter of 4.00 mm and a wavelength of 600 nm. How many photons per second enter your eye if a 1.00 m W laser beam is directed into your eye? $$ \lambda = 633 nm ) $$
If the velocity of a particle is increased three times then the percentage decreases in its de Broglie wavelength will be
Show graphically, the variation of the de Broglie wavelength $$\left( \lambda \right)$$ with the potential $$\left( V \right)$$ through which an electron is accelerated from rest.
An electron, an $$\alpha-particle$$ and a proton have the same de Broglie wavelength. Which of these particles has (i) the minimum kinetic energy (ii) the maximum kinetic energy and why? Is what why has the wave nature of electron been exploited in electron microscope?
An electron and alpha particle have the same de Broglie wavelength associated with them. How are their kinetic energies related to each other?
A hydrogen atom initially in ground level absorbs a photon,which excites it to the n=4 level.Determine the wavelength and frequency of the photon.
A photon and electron have got the same de Broglie wavelength. Explain which has greater total energy.
Why is the de Broglie wavelength associated with macroscopic objects (cricket ball, car etc) is not served in daily life?
Monochromatic light of frequency $$6 \times 10^{14}$$ Hz is produced by a laser. The power emitted is $$2 \times 10^{-3}$$ W
(i) What is the energy of the photon in the light beam?
(ii) How many photons per second, on an average are emitted by the source ?
A particle of mass M at rest decay into two particles of masses $$ m_1$$ and $$m_2 $$ having velocities $$ v_1$$ and $$v_2 $$ respectively. Find the ratio of de-Broglie wavelength of the two particles.
The energy of a photon is given by E= hv,where'v' is the frequency of radiation, Use this equation to get the dimensional formula of 'h'.
An electron of mass m$$_{e}$$ and a proton of mass m$$_{p}$$ are moving with the same speed . The ratio of their de-Broglie wavelengths$$\lambda _{e}/\lambda _{p}$$ is
Monochromatic light of frequency $$6.0 \times {10^{14}}Hz$$ is produced by a laser$$.$$ What is the energy of a photon in the light beam$$?$$
The work function of a metal is l eV. On making light of wavelength $$3000A$$ incident on this metals, the velocity of photoelectrons emitted from the metal, in m/s will be-
Find the frequency of $$1$$ MeV photon. Given wavelength of a $$1$$ kev photon is $$1.24\times { 10 }^{ -9 } m$$.
Proton and alpha particle have same $$K.E$$. Which one has greater de-Broglie wavelength $$?$$
The velocity of an electron of de-Broglie wavelength $$\lambda $$ increases four times. Find the new wavelength.
A proton and electron have same velocity. Which one has greater de Broglie wavelength and why?
An $$\alpha$$- particle and a proton are accelerated from rest by the same potential. Find the ratio of their de- Broglie wavelength.
A deutron and an alpha particle have speeds in the ratio $$2 : 3$$. What is the ratio of the de Broglie wavelengths associated with the particles ?
A parallel beam of monochromatic light of wavelength 500 nm is incident normally on a perfectly absorbing surface. The power through any cross section of the beam is 10 W. Find the number of photon absorbed by the surface per second.
Calculate the de Broglie wavelength of a neutron of kinetic energy $$150eV$$.
Write De-Broglie's relation.
The ratio of potential applied to accelerate the $$\alpha $$- particle and proton in order to have the same de Broglie wavelength is
State the conditions required to achieve laser action.
Calculate the Compton wavelength for (a) an electron and(b) a proton. What is the photon energy for an electromagnetic wave with a wavelength equal to the Compton wavelength of(c) the electron and (d) the proton?
(a) $$\mathrm{In} \mathrm{MeV} / \mathrm{c},$$ what is the magnitude of the momentum associated with a photon having an energy equal to the electron rest energy? What are the (b) wavelength and (c) frequency of the corresponding radiation?
The meter was once defined as 1650763.73 wavelengths of the orange light emitted by a source containing krypton- 86 atoms. What is the photon energy of that light?
Under ideal conditions, a visual sensation can occur in the human visual system if the light of wavelength 550 nm is absorbed by the eyes retina at a rate as low as 100 photons per second. What is the corresponding rate at which energy is absorbed by the retina?
Calculate the de Broglie wavelength of (a) a 1.00 keV electron,(b) a 1.00 keV photon, and (c) a 1.00 keV neutron.
What is the maximum wavelength shift for a Compton collision between a photon and a free proton?
What is the photon energy for yellow light from a highway sodium lamp at a wavelength of 589 nm?
Calculate the percentage change in photon energy during a collision like that in Fig. $$38-5$$ for $$\phi=90^{\circ}$$ and for radiation in (a) the microwave range, with $$\lambda=3.0 \mathrm{cm} ;$$ (b) the visible range, with $$\lambda=500 \mathrm{nm} ;(\mathrm{c})$$ the x-ray range, with $$\lambda=25 \mathrm{pm} ;$$ and (d) the gamma-ray range, with a gamma photon energy of $$1.0 \mathrm{MeV}$$(e) What are your conclusions about the feasibility of detecting the Compton shift in these various regions of the electromagnetic spectrum, judging solely by the criterion of energy loss in a single photon-electron encounter?
The highest achievable resolving power of a microscope is limited only by the wavelength used; that is, the smallest item that can be distinguished has dimensions about equal to the wavelength. Suppose one wishes to see inside an atom. Assuming the atom to have a diameter of 100 pm, this means that one must be able to resolve a width of, say, 10 pm. (a) If an electron microscope is used, what minimum electron energy is required? (b) If a light microscope is used, what minimum photon energy is required? (c) Which microscope seems more practical? Why?
In an old-fashioned television set, electrons are accelerated through a potential difference of $$25.0 \mathrm{kV}$$. What is the de Broglie wavelength of such electrons? (Relativity is not needed.)
An electron, an alpha particle and a proton have the same kinetic energy. Which one of these particles has (i) the shortest and (ii) the largest, de Broglie wavelength?
Define intensity of radiation on the basis of photon picture of light. Write its SI unit.
State de-Broglie hypothesis.
What is the wavelength of (a) a photon with energy $$1.00 \mathrm{eV}$$(b) an electron with energy $$1.00 \mathrm{eV}$$(c) a photon of energy $$1.00 \mathrm{GeV},$$ and (d) an electron with energy $$1.00 \mathrm{GeV} ?$$
Using the classical equations for momentum and kinetic energy, show that an electron's de Broglie wavelength in nanometers can be written as $$\lambda=1.226 / \sqrt{K},$$ in which $$K$$ is the electron's kinetic energy in electron-volts.
A bullet of mass $$40 \mathrm{g }$$ travels at $$1000 \mathrm{m} / \mathrm{s}$$. Although the bullet is clearly too large to be treated as a matter-wave, determine what Eq. $$38-17$$ predicts for the de Broglie wavelength of the bullet at that speed.
What are (a) the energy of a photon corresponding to wavelength $$1.00 \mathrm{nm}$$(b) the kinetic energy of an electron with de Broglie wavelength $$1.00 \mathrm{nm},(\mathrm{c})$$ the energy of a photon corresponding to wavelength $$1.00 \mathrm{fm},$$ and $$(\mathrm{d})$$ the kinetic energy of an electron with de Broglie wavelength $$1.00 \mathrm{fm} ?$$
A proton and a deuteron have the same velocity; what is the ratio of thier de-Broglie wavelengths?
Why are de Broglie waves associated with a moving football not visible?
The wavelength $$'\lambda'$$ of a photon and the de Broglie wavelength of an electron have the same value.Show that the energy of photon is $$\dfrac{2\lambda mc}{h}$$ times the kinetic energy of electron, where m,c,h have their usual meanings.
Determine the value of the de Broglie wavelength associated with the electron orbiting in the ground state of hydrogen atom(Given $$E_n= - (13.6/n^2)$$eV and Bohr radius $$ r0=0.53A^\circ$$). How will the de Broglie wavelength change when it is in the first excited state?
Which of the two:(i) light and (ii) moving material particle is concerned with de-Broglie hypotheses?
Which of the following moving with the same velocity has the longest de Broglie wavelength; electron,proton,deuteron, $$\alpha$$ particle?
The de-Broglie wavelength of a neutron of kinetic energy K is $$\lambda.$$ When its kinetic energy is 4 K, what is the de-Broglie wavelength of the neutron ?
An electromagnetic wave of wavelength $$\lambda_1$$ is incident on a photosensitive surface of negligible work function. If the photo-electrons emitted from this surface have the de-Broglie wavelength prove that $$\lambda=\left(\dfrac{2mc}{h}\right)\lambda_1^2$$
Do all the electrons that absorb a photon come out as photoelectrons?
An electron and a proton possess equal kinetic energy. Which of these has the greater de Broglie wavelength?
The terminology of different part of the electromagnetic spectrum is given in the text ,Use the formula E = hv (for energy of a radiation .: photon ) and obtain the photon energy in units of eV for different parts of the electromagnetic spectrum .In what way are the different scales of photon energies that you obtain related to the sources of electromagnetic radiation ?
Establish formula to find de-Broglie wavelength of electron , proton and $$ \alpha $$ particle and unchanged particles.
Work function for sodium is $$ 2.3\,eV $$. Find out the maximum wavelength of light, which can emit photoelectrons from sodium.
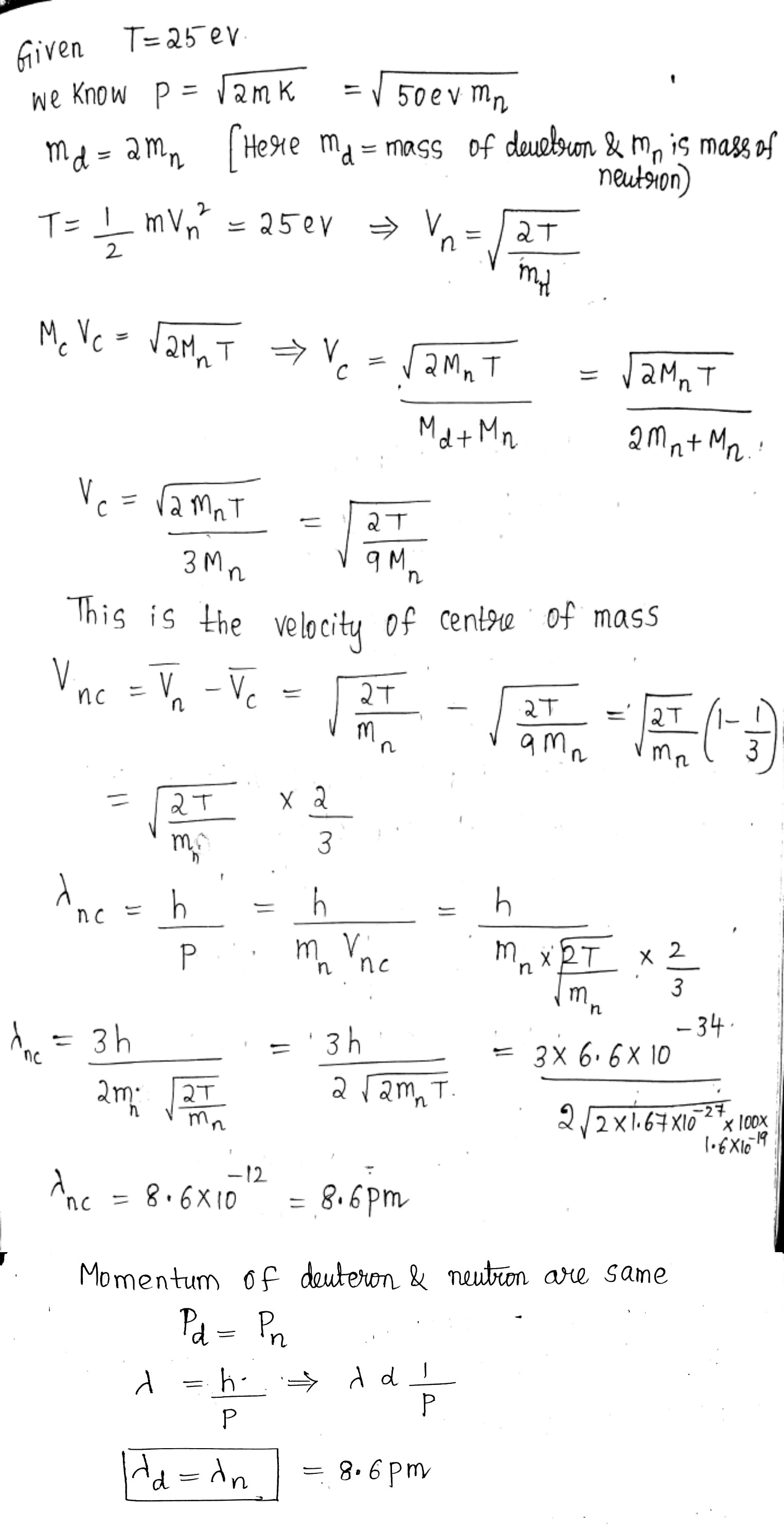
A neutron with kinetic energy $$T= 25\ eV$$ strikes a stationary deuteron (heavy hydrogen nucleus). Find the de Broglie wavelengths of both particles in the frame of their centre of inertia.
A photon with energy $$ \hbar \omega=250 \mathrm{keV} $$ is scattered at an angle $$ \theta=120^{\circ} $$ by a stationary free electron. Find the energy of the scattered photon.
Two identical non-relativistic particles move at right angles to each other, possessing de Broglie wavelengths $$ \lambda_{1}$$ and $$\lambda_2 $$. Find the de Broglie wavelength of each particle in the frame of their centre of inertia.
Calculate the most probable de Broglie wavelength of hydrogen molecules being in thermodynamic equilibrium at room temperature.
Find the energy of a photon having a wavelength of $$ 3.00 \times 10^{2} $$ nm. Express your answers in units of electron volts, noting that $$ 1 \mathrm{eV}=1.60 \times 10^{-19} \mathrm{J} $$
Find the energy of a photon having a frequency of $$\quad 5.00 \times 10^{17} \mathrm{Hz} $$
Electromagnetic radiation of wavelength $$ \lambda=0.30 \mu \mathrm{m} $$ falls on a photocell operating in the saturation mode. The corresponding spectral sensitivity of the photocell is $$ J=4.8 \mathrm{mA} / \mathrm{W} $$. Find the yield of photoelectrons, i.e. the number of photoelectrons produced by each incident photon.
Find the de Broglie wavelength of hydrogen molecules, which corresponds to their most probable velocity at room temperature.
An $$\alpha$$-particle and a proton are accelerated from rest by a potential difference of $$100 V$$. After this, their de Broglie wavelengths are $$\lambda_{\alpha}$$ and $$\lambda_{p}$$ respectively. The ratio $$\dfrac{\lambda_{p}}{\lambda_{\alpha}}$$, to the nearest integer, is:
(a) Obtain the de Broglie wavelength of a neutron of kinetic energy 150 eV. As you have seen in Exercise 11.31, an electron beam of this energy is suitable for crystal diffraction experiments. Would a neutron beam of the same energy be equally suitable? Explain.($$m_n = 1.675 \times 10^{27}$$kg)
(b) Obtain the de Broglie wavelength associated with thermal neutrons at room temperature ($$27^o C$$). Hence, explain why a fast neutron beam needs to be thermalised with the environment before it can be used for neutron diffraction experiments.
Exercise 11.31:
Crystal diffraction experiments can be performed using X-rays, or electrons accelerated through appropriate voltage. Which probe has greater energy? (For quantitative comparison, take the wave length of the probe equal to 1 A∘A∘ which is of the order of inter-atomic spacing in the lattice) ($$m_e=9.11 \times 10^{-31} kg$$)
Exercise 11.31:
Crystal diffraction experiments can be performed using X-rays, or electrons accelerated through appropriate voltage. Which probe has greater energy? (For quantitative comparison, take the wave length of the probe equal to 1 A∘A∘ which is of the order of inter-atomic spacing in the lattice) ($$m_e=9.11 \times 10^{-31} kg$$)
An electron in an excited state of $$Li^{2+}$$ ion has angular momentum 3h/2$$\pi$$. The de Broglie wavelength of the electron in this state in $$p \pi \alpha_0$$ (where $$\alpha_0$$ is the Bohr radius). The value of $$p$$ is :
Estimating the following two numbers should be interesting. The first number will tell you why radio engineers do not need to worry much about photons. The second number tells you why our eye can never count photons, even in barely detectable light.
(a) The number of photons emitted per second by a medium wave transmitter of 10 kW power, emitting radio waves of wave length 500 m.
(b) The number of photons entering the pupil of our eye per second corresponding to the minimum intensity of white light that we humans can perceive $$(~\,10^{10}\, W\, m^{2})$$. Take the area of the pupil to be about $$0.4\, cm^2$$, and the average frequency of white light to be about $$6\, \times\, 10^{14}\, Hz$$.
(b) The number of photons entering the pupil of our eye per second corresponding to the minimum intensity of white light that we humans can perceive $$(~\,10^{10}\, W\, m^{2})$$. Take the area of the pupil to be about $$0.4\, cm^2$$, and the average frequency of white light to be about $$6\, \times\, 10^{14}\, Hz$$.
An electron microscope uses electrons accelerated by a voltage of 50 kV. Determine the de-Broglie wavelength associated with the electrons. Taking other factors, such as numerical aperture etc. to be same, how does the resolving power of an electron microscope compare with that of an optical microscope which uses yellow light? $$\left( {{\lambda _y} = 5.9\; \times {{10}^{ - 7}}\;{\text{m}}} \right)$$
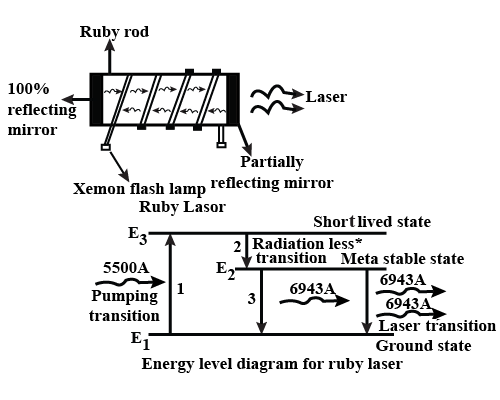
Explain the working of Ruby Laser with the help of energy level diagram.
The work function of iron is $$4.7 eV$$. Calculate the cut-off frequency and the corresponding cut off wavelength for this metal.
Draw a neat sketch of Ruby Laser. Explain its working with the help of energy level diagram.
A beam of light has three wavelengths $$4144 A^{o}$$,$$4972\mathring{A}$$ and $$6216\mathring{A} $$ with a total intensity of $$3.6\times 10^{-3} Wm^{-2}$$ equally distributed amongst the three wavelengths. The beam falls normally on an area $$1.0 cm^2$$ of a clean metallic surface of work function $$2.3 $$eV. Assume that the there is no loss of light by reflection and that each energetically capable photon ejects one electron. Calculate a number of photoelectrons liberated in two seconds.
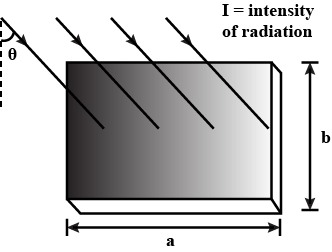
Violet light $$(\lambda=4000\ \mathring { A } )$$ of intensity $$4\ watt/{m}^{2}$$ falls normally on a surface of area $$10\ cm\times 20\ cm$$. Find
(a) the energy received by the surface per second,
(b) the number of photons hitting the surface per second,
(c) if surface is tilted such that plane of the surface makes an angle $${30}^{o}$$ with light beam, find the number of photons hitting the surface per second.
Calculate number of photons passing through a ring of unit area in unit time if light of intensity $$100W{m}^{2}$$ and of wavelength $$400nm$$ is falling normally on the ring.
Atoms of a hydrogen like gas are in a particular excited energy level. When these atoms de-excite they emits photons of different energies. Maximum and minimum energies of emitted photons are $$E_{max}=52.224\ eV$$ and $$E_{min}=1.224\ eV$$ respectively. Calculate the principle quantum number of initially excited level. (lonisation energy of hydrogen atom $$=13.6\ eV$$)
The photoelectric threshold wavelength of a metal is 230nm. Determine the maximum kinetic energy in joule and in eV of the ejected electron for the metal surface when it is exposed to a radiation of wavelength 180 nm. [Plank's constant : h = $$6.63 \times 10^{-34}$$ Js, velocity of light; c = $$3 \times 10^8$$ m/s)
X- rays of wavelength $$\lambda$$ falls on a photosensitive surface emitting electrons.Assuming that the work function of the surface can be neglected, prove that the de Broglie wavelength of electrons emitted will be $$\sqrt{\cfrac{h\lambda}{2mc}}$$
When an electron in hydrogen atom jumps from the third excited state to the ground state. how would the de broglie wavelength have associated with the electron change?
The ionisation potential of hydrogen atom is $$13.6V$$.Draw the energy level diagram showing four levels.Calculate (i) the energy of the photon emitted when an electron falls from the third orbit to the second orbit (ii) the wavelength of this photon (value of h and c as above)
what will be the minimum energy of a photon which can be absorbed by hydrogen atom at ordinary temperature ?
Calculate the de Broglie wavelength associated with an electron accelerated through a potential difference of 1 kV.
5% energy of a monochromatic light source is converted into light of wavelength 5600 $$A^0 $$. If the power of the source be 100 W, then how many photons of light will be emitted by it per second?
If the ratio of de-Broglle wavelength of a proton and an $$\alpha-particle$$ is $$1:3$$ , then
$$1.5mW$$ of $$400nm$$ light is directed at a photoelectric cell. If $$0.1\%$$ of the incident photons produce photo electrons, find the current in the cell.
An electron microscope uses electrons accelerated by a potential difference 50 kV. Calculate the de Broglie wavelength of the electrons. Compare the resolving power of an electron microscope with that of an optical microscope, which uses visible light of wavelength 550 nm. Assume the numerical aperture of the objective lens of the microscopes are the same.
Derived Einstein's Photoelectric equation. Explain photoelectric effect with help of this equation.
Find the maximum kinetic energy of the photo electrons ejected when light of wavelength 350 nm is incident on a cesium surface. Work function of cesium = 1.9eV.
The electric-field at a point associated with a light wave is $$E=(100Vm^{ -1 })$$sin$$[(3.0\times10^{ 10 }s^{ -1 })t]$$sin$$[(6.0\times10^{ 10 }s^{ -1 })t]$$ If this light falls on a 2.0 eV, what will be the maximum kinetic energy of the photoelectrons?
A totally reflecting, small plane mirror placed horizontally faces a parallel beam of light as shown in figure (42-E1). The mass of the mirror is 20 g. Assume that there is no absorption in the lens and that 30% of the light emitted by the source goes through the lens. Find the power of the source needed to support the weight of the mirror. Take $$g=10ms^{-2}$$.

A light beam of wavelength 400 nm is incident on a metal plate of work function 2-2e V. (a) A particular electron absorbs a photon and makes two collisions before coming out of the metal. Assuming that 10% of the extra energy is lost to the metal in each collision, find the kinectic energy of this electron as it comes out of the metal. (b) Under the same assumptions, find the maximum number of collisions the electron can suffer before it becomes unable to come out of the metal.
Electrons accelerated to an energy of $$50 \mathrm{GeV}$$ have a de Broglie wavelength $$\lambda$$ small enough for them to probe the structure within a target nucleus by scattering from the structure. Assume that the energy is so large that the extreme relativistic relation $$p=$$ $$E / c$$ between momentum magnitude $$p$$ and energy $$E$$ applies. (In this extreme situation, the kinetic energy of an electron is much greater than its rest energy.) (a) What is $$\lambda ?$$ (b) If the target nucleus has radius $$R=5.0 \mathrm{fm},$$ what is the ratio $$R / \lambda ?$$sodium is 590 nm.
The existence of the atomic nucleus was discovered in 1911 by Ernest Rutherford, who properly interpreted some experiments in which a beam of alpha particles was scattered from a metal foil of atoms such as gold. (a) If the alpha particles had akinetic energy of 7.5 MeV, what was their de Broglie wavelength? (b) Explain whether the wave nature of the incident alpha particles should have been taken into account in interpreting these experiments. The mass of an alpha particle is 4.00 u(atomic mass units), and its distance of closest approach to the nuclear center in these experiments was about 30 fm. (The wave nature of matter was not postulated until more than a decade after these crucial experiments were first performed.)
X rays of wavelength 0.0100 nm are directed in the positive direction of an $$x$$ axis onto a target containing loosely bound electrons. For Compton scattering from one of those electrons, at an angle of $$180^{\circ},$$ what are (a) the Compton shift, (b) the corresponding change in photon energy, (c) the kinetic energy of the recoiling electron, and (d) the angle between the positive direction of the $$x$$ axis and the electron's direction of motion?
Gamma rays of photon energy $$0.511 \mathrm{MeV}$$ are directed onto an aluminum target and are scattered in various directions by loosely bound electrons there. (a) What is the wavelength of the incident gamma rays?(b) What is the wavelength of gamma rays scattered at $$90.0^{\circ}$$ to the incident beam? (c) What is the photon energy of the rays scattered in this direction?
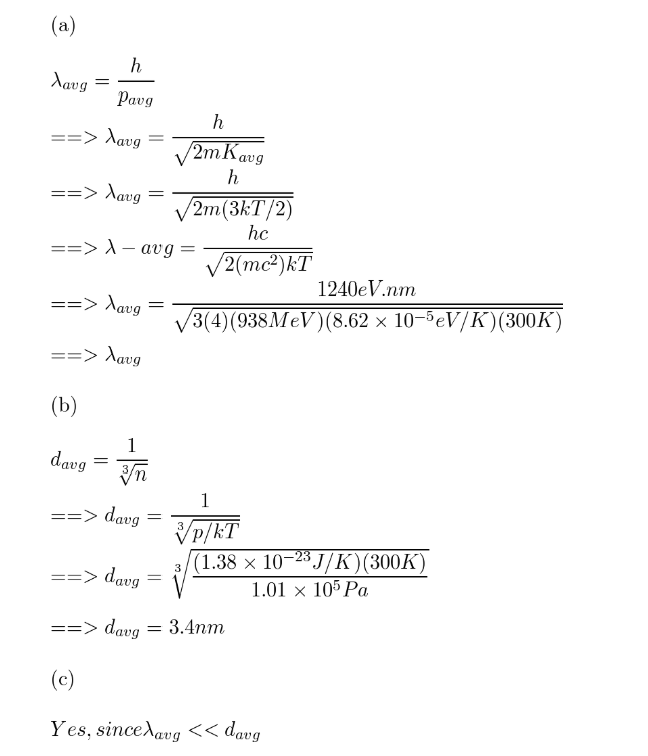
Consider a balloon filled with helium gas at room temperature and atmospheric pressure. Calculate (a) the average de Broglie wavelength of the helium atoms and (b) the average distance between atoms under these conditions. The average kinetic energy of an atom is equal to $$(3 / 2) k T,$$ where $$k$$ is the Boltzmann constant. (c) Can the atoms be treated as particles under these conditions? Explain.
A $$3.0 \mathrm{MeV}$$ proton is incident on a potential energy barrier of thickness $$10 \mathrm{fm}$$ and height $$10 \mathrm{MeV}$$. What are (a) the transmission coefficient $$T,(\mathrm{b})$$ the kinetic energy $$K_{t}$$ the proton will have on the other side of the barrier if it tunnels through the barrier, and(c) the kinetic energy $$K_{r}$$ it will have if it reflects from the barrier? A $$3.0 \mathrm{MeV}$$ deuteron (the same charge but twice the mass as a proton is incident on the same barrier. What are (d) $$T$$(e) $$K_{t},$$ and(f) $$K_{r} ?$$
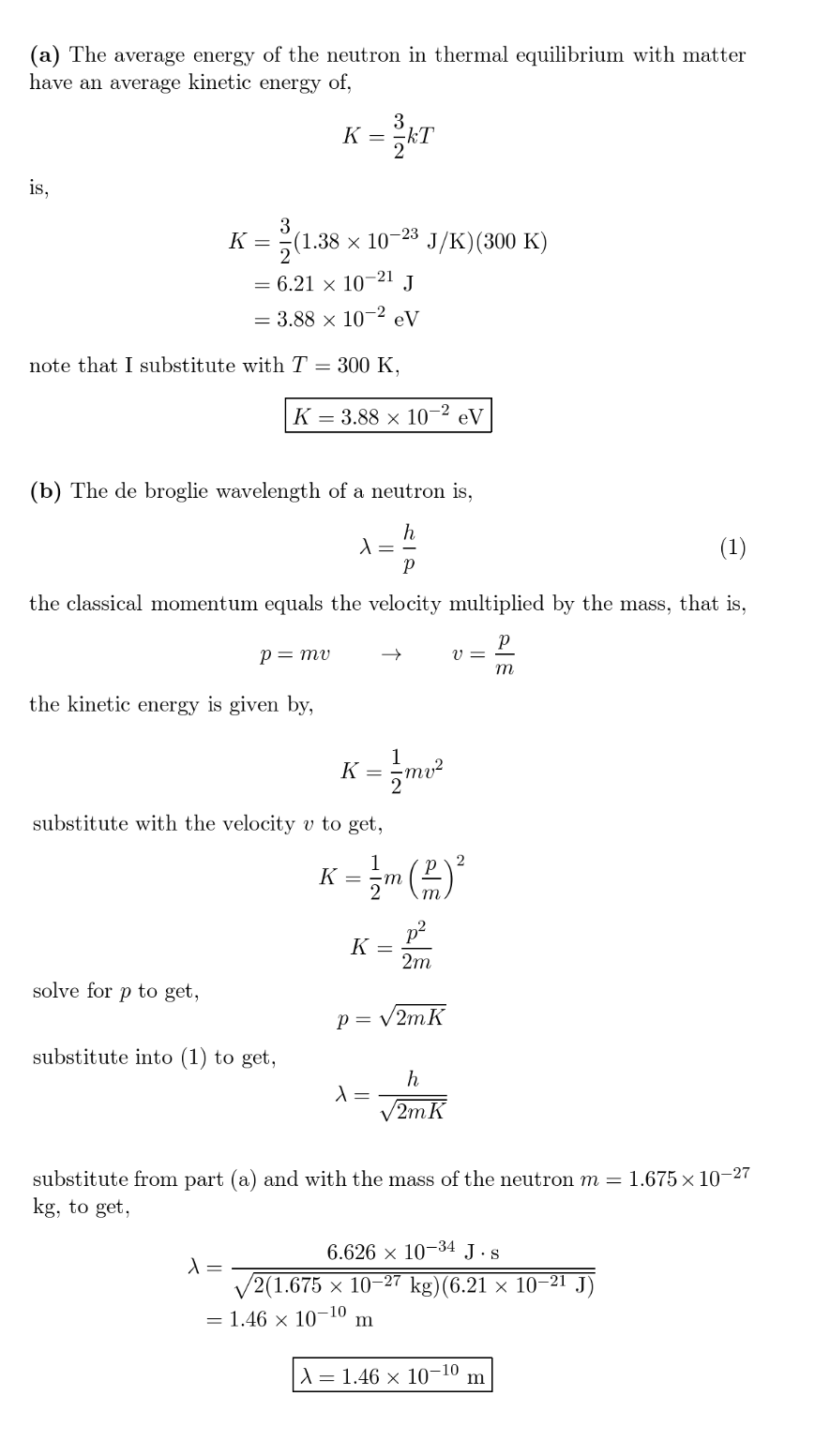
Neutrons in thermal equilibrium with matter have an average kinetic energy of $$(3 / 2) k T,$$ where $$k$$ is the Boltzmann constant and$$T,$$ which may be taken to be $$300 \mathrm{K},$$ is the temperature of the environment of the neutrons. (a) What is the average kinetic energy of such a neutron?(b) What is the corresponding de Broglie wavelength?
A spectral emission line is an electromagnetic radiation that is emitted in a wavelength range narrow enough to be taken as a single wavelength. One such emission line that is important in astronomy has a wavelength of 21 cm. What is the photon energy in the electromagnetic wave at that wavelength?
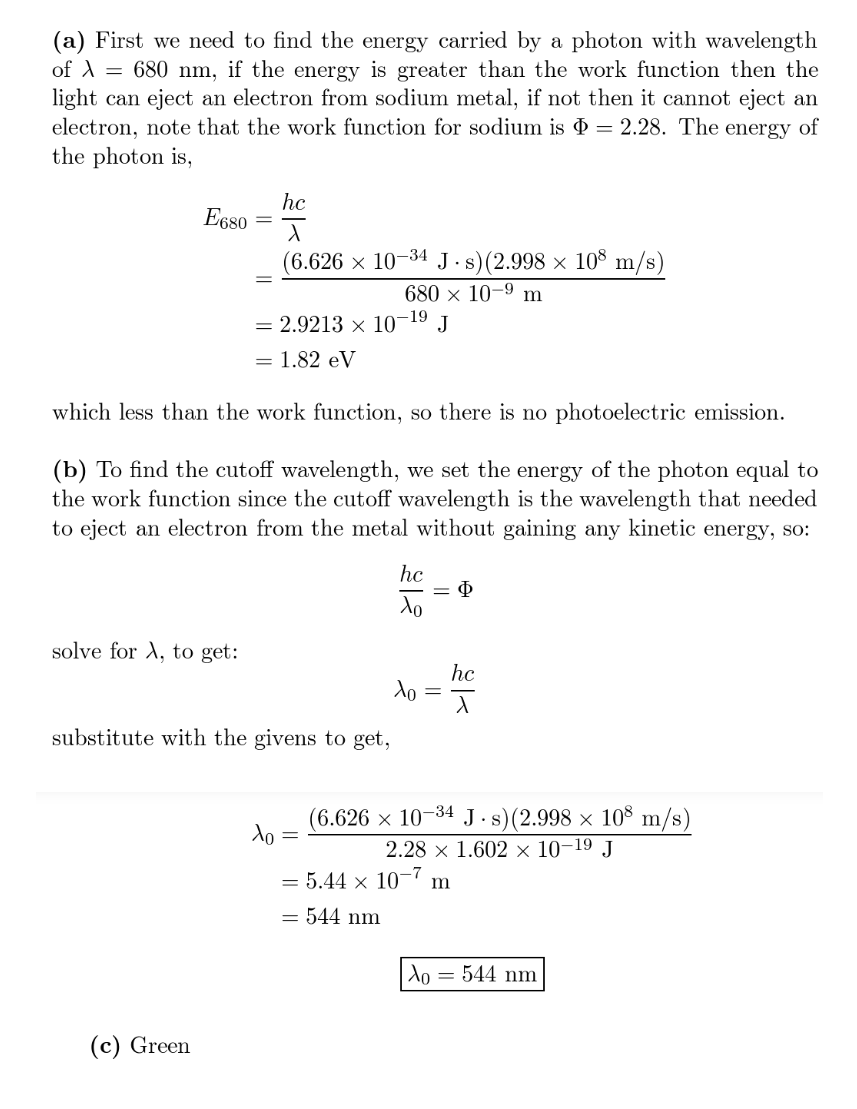
(a) The smallest amount of energy needed to eject an electron from metallic sodium is $$2.28 \mathrm{eV}$$. Does sodium show a photoelectric effect for red light, with $$\lambda=680 \mathrm{nm} ?$$ (That is, does the light cause electron emission?) (b) What is the cutoff wavelength for photoelectric emission from sodium? (c) To what color does that wavelength correspond?
Show that $$\Delta E / E$$, the fractional loss of energy of a photon during a collision with a particle of mass $$m$$, is given by
$$\dfrac{\Delta E}{E}=\dfrac{h f^{\prime}}{m c^{2}}(1-\cos \phi)\\$$
where $$E$$ is the energy of the incident photon, $$f^{\prime}$$ is the frequency of the scattered photon, and $$\phi$$ is defined as in Fig. $$38-5$$
$$(a)$$ Show that $$ Equation $$ $$N(E)=\frac{8\sqrt{2}\pi m^{3/2}}{h^3}E^{1/2}$$ can be written as $$N(E) = CE^{1/2}$$ $$(B)$$ Evaluate $$C$$ in terms of meters and electron-volts. $$(c)$$ Calculate $$ N(E)$$ for $$E = 5.00 eV.$$
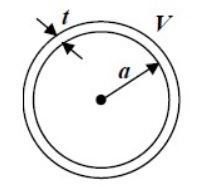
What is the acceleration of a silver atom as it passes through the deflecting magnet in the SternGerlach experiment of the above figure if the magnetic field gradient is $$1.4 \ T/mm$$?
A $$20 \ keV$$ electron is brought to rest by colliding twice with target nuclei (Assume the nuclei remain stationary.) The wavelength associated with the photon emitted in the second collision is $$130 \ pm$$ greater than that associated with the photon emitted in the first collision. (a) What is the kinetic energy of the electron after the first collision? What are (b) the wavelength $$\lambda_1$$ and (c) the energy $$E_1$$ associated with the first photon? What are (d) $$\lambda_2$$ and (e) $$E_2$$ associated with the second photon?
A certain heliumneon laser emits red light in a narrow band of wavelengths centered at $$632.8 nm$$ and with a wavelength width (such as on the scale above) of $$0.0100 nm$$. What is the corresponding frequency width for the emission?
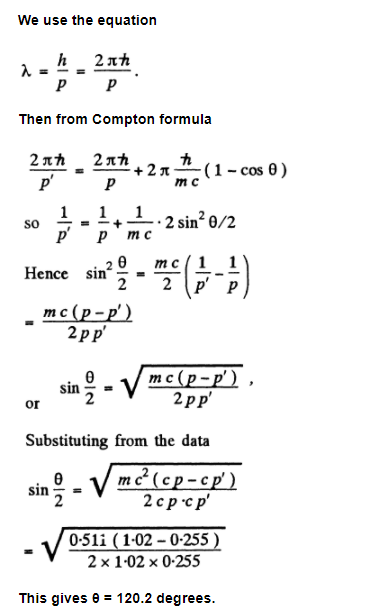
A photon with momentum $$ p=1.02 \mathrm{MeV} / \mathrm{c}, $$ where $$ c $$ is the velocity of light, is scattered by a stationary free electron, changing in the process its momentum to the value $$ p^{\prime}=0.255 \mathrm{MeV} / \mathrm{c} $$. At what angle is the photon scattered?
Zinc is a bivalent metal. Calculate $$(a)$$ the number density of conduction electrons, $$(b)$$ the Fermi energy, $$(c)$$ the Fermi speed, and $$(d)$$ the de Broglie wavelength corresponding to this electron speed. See Appendix $$F$$ for the needed data on zinc. $$m_ec^2=511\times10^3eV$$
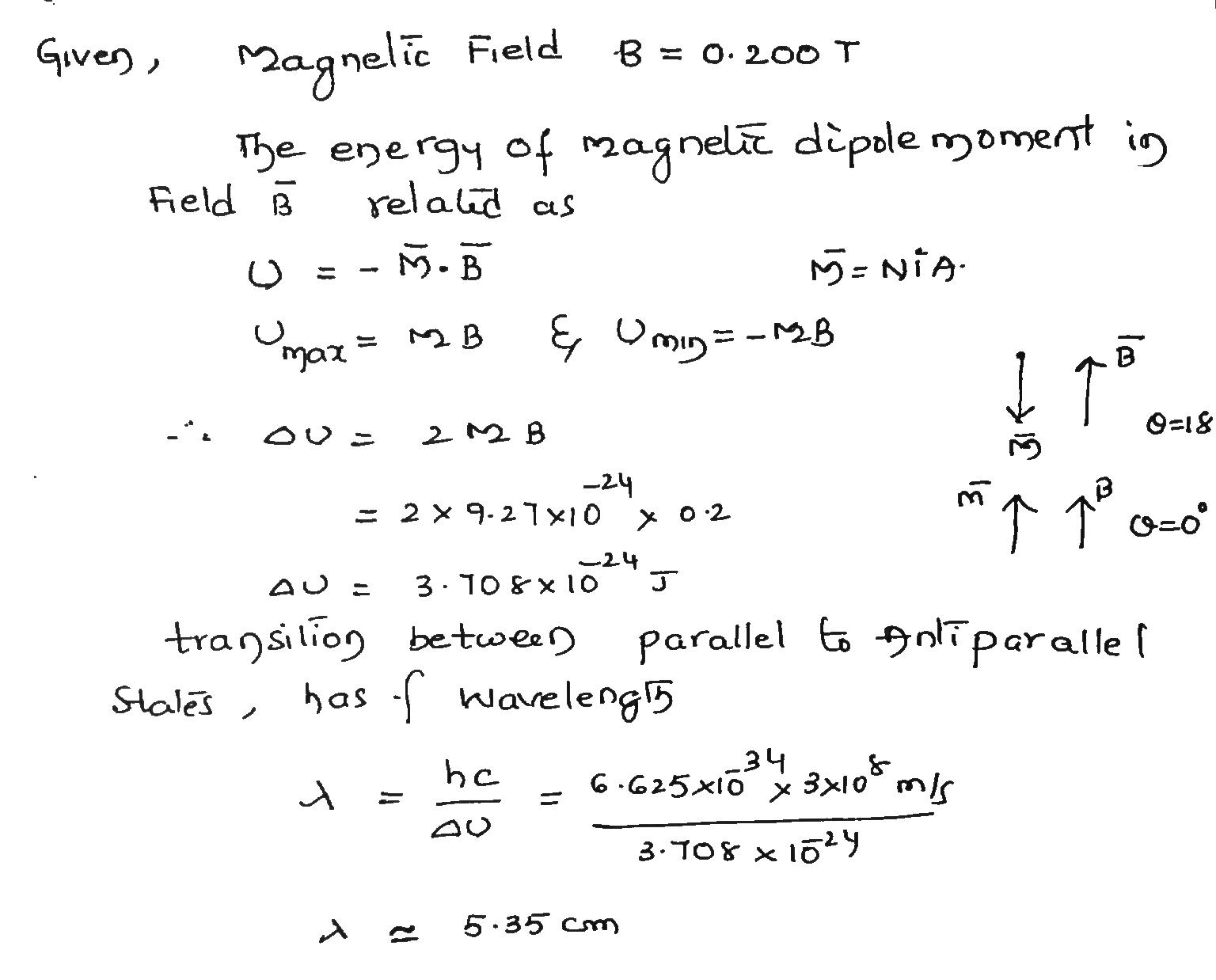
What is the wavelength associated with a photon that will induce a transition of an electron spin from parallel to antiparallel orientation in a magnetic field of magnitude 0.200T? Assume that. $$ l = 0 $$
Silver is a monovalent metal. Calculate $$(a)$$ thenumber density of conduction electrons, $$(b)$$ the Fermi energy, $$(c)$$ theFermi speed, and $$(d)$$ the de Broglie wavelength corresponding to thiselectron speed. See Appendix $$F$$ for the needed data on silver.
A photon is scattered at an angle $$ \theta=120^{\circ} $$ by a stationary free electron. As a result, the electron acquires a kinetic energy $$ T=0.45 \mathrm{MeV} . $$ Find the energy that the photon had prior to scattering.
The wavelength of light from the spectral emission line of sodium is $$589 nm$$. Find the kinetic energy at which (a) electron and (b) neutron would have the same De-Broglie wavelength. Given that mass of neutron $$= 1.66 \times 10^{-27} kg$$.
A plank of mass 'm' is lying on a rough surface having coefficient of friction $$'\mu'$$ in situation as shown in the figure. Find the acceleration of the plank assuming that it slips and surface of body exposed to radiation is black body.
Determine the De-Broglie wavelength of a proton, whose kinetic energy is equal to the rest mass energy of an electrons. Given that the mass of the electron is $$9.11 \times 10^{-31} kg$$ and mass of proton is $$1837$$ times as that of the electron.
In a photocell the plates P and Q have a separation of $$5 cm$$ which are connected through a galvanometer without any cell. Bichromatic light of wavelengths $$4000 \mathring{A}$$ and $$6000 \mathring{A}$$ are incident on plate Q whose work function is $$2.39 eV$$, If a uniform magnetic field B exists parallel to the plates, find the minimum value for B for which the galvanometer shows zero deflection.
Electron beams are accelerated to potentials $$40 kV$$ and $$150 V$$. Estimate the wavelengths of the electrons in the two beams. Given : $$h = 6.6 \times 10^{-34} Js, m = 9.1 \times 10^{-31} kg$$ and $$1eV = 1.6 \times 10^{-19} J$$.
Sun gives light at the rate of $$1400 $$ W of area perpendicular to the direction of light. Assume wavelength (sunlight) $$= 6000 \mathring{A}$$. Calculate the
(a) Number of photons per second arriving at $$1$$ squared metres at earth.
(b) Number of photons emitted from the sun per second assuming that the average radius of earth's orbit is $$1.49 m$$.
Class 12 Medical Physics Extra Questions
- Alternating Current Extra Questions
- Atoms Extra Questions
- Current Electricity Extra Questions
- Dual Nature Of Radiation And Matter Extra Questions
- Electric Charges And Fields Extra Questions
- Electromagnetic Induction Extra Questions
- Electromagnetic Waves Extra Questions
- Electrostatic Potential And Capacitance Extra Questions
- Magnetism And Matter Extra Questions
- Moving Charges And Magnetism Extra Questions
- Nuclei Extra Questions
- Ray Optics And Optical Instruments Extra Questions
- Semiconductor Electronics: Materials, Devices And Simple Circuits Extra Questions
- Wave Optics Extra Questions