- Alcohols Phenols And Ethers MCQ Quiz
- Aldehydes Ketones And Carboxylic Acids MCQ Quiz
- Analytical Chemistry MCQ Quiz
- Biomolecules MCQ Quiz
- Chemical Bonding And Molecular Structure MCQ Quiz
- Chemical Kinetics MCQ Quiz
- Chemistry In Everyday Life MCQ Quiz
- Class 11 P Block Elements MCQ Quiz
- Class 12 P Block Elements MCQ Quiz
- Classification Of Elements And Periodicity In Properties MCQ Quiz
- Coordination Compounds MCQ Quiz
- Electrochemistry MCQ Quiz
- Environmental Chemistry MCQ Quiz
- Equilibrium MCQ Quiz
- General Principles And Processes Of Isolation Of Elements MCQ Quiz
- Haloalkanes And Haloarenes MCQ Quiz
- Hydrocarbons MCQ Quiz
- Hydrogen MCQ Quiz
- Nitrogen Containing Compounds MCQ Quiz
- Nuclear Chemistry MCQ Quiz
- Organic Chemistry Some Basic Principles And Techniques MCQ Quiz
- Polymers MCQ Quiz
- Redox Reactions MCQ Quiz
- Sblock Elements MCQ Quiz
- Solutions MCQ Quiz
- Some Basic Concepts Of Chemistry MCQ Quiz
- States Of Matter MCQ Quiz
- Structure Of Atom MCQ Quiz
- Surface Chemistry MCQ Quiz
- The D And F Block Elements MCQ Quiz
- Thermodynamics MCQ Quiz
- The Solid State MCQ Quiz
Alcohols Phenols And Ethers MCQ Questions



Alcohols Phenols And Ethers Questions and Answers
| Alcohols Phenols And Ethers Quiz Question | Answer |
|---|---|
| What reaction does hydroquinone undergo most readily? | Oxidation |
| The enzymes which are used to convert starch into ethyl alcohol are | diastase, maltase, zymase |
| Ethylene can be converted into alcohol by treatment of | H2SO4 as catalyst  |
| The alcohol obtained by the hydrolysis of oils and fats is | glycerol |
| In the conversion of ethanol into methanol which of the following reagents will be used? | All of these |
| Argol, a brown crust, formed during the fermentation of grape juice contains | potassium hydrogen tartarate |
| Ketone upon treatment with Grignard reagent gives | tertiary alcohol |
| In fermentation by zymase, alcohol and CO2 are obtained from | glucose |
| Grignard reagent reacts with HCHO to produce | primary alcohol |
| Ethyl alcohol can be prepared from Grignard reagent by the reaction of | HCHO |
Aldehydes Ketones And Carboxylic Acids MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14
Quiz 15
Quiz 16



Aldehydes Ketones And Carboxylic Acids Questions and Answers
| Aldehydes Ketones And Carboxylic Acids Quiz Question | Answer |
|---|---|
| Calcium formate on distillation gives | HCHO |
| The catalyst used in Rosenmund reaction is | Pd/BaSO4  |
| Stephen's reduction is used to prepare aldehyde from | alkyl cyanides  |
| Which of the following isomerism is shown by ethyl acetoacetate? | Keto enol tautomerism |
| Ethyl benzoate reacts with PCl5 to give | C2H5Cl + C6H5COCl + POCl3 |
| Benzaldehyde reacts with methyl amine to give | C6H5CH =NCH3 |
| Which of the following gives an aldehyde on dry distillation? | Calcium formate + calcium acetate |
| Collin's reagent is used to convert | —CH2OH→CHO |
| 3-pentanol on reaction with aluminium tertiary butoxide in the presence of acetone gives | 3-pentanone |
| When CH2 = CH — O — CH2 — CH3 reacts with one mole of HI, one of the products formed is | ethanal |
Analytical Chemistry MCQ Questions



Analytical Chemistry Questions and Answers
| Analytical Chemistry Quiz Question | Answer |
|---|---|
| MnO2 and H2SO4 added to NaCl, the greenish-yellow gas liberated is | Cl2 |
| Which of the following doesn't give a ppt. with silver nitrate solution? | Ethyl bromide |
| What colour is imparted into the flame when lithium is burnt? | Crimson red |
| Which of the following radicals gives the apple green flame during flame test ? | Ba2+ |
| Borax bead test of Cr (chromium) is | blue |
| The yellow precipitate formed during the chromyl chloride test is chemically | lead chromate |
| The incorrect statement in respect of chromyl chloride test is | liberation of chlorine |
| In the brown ring test, the brown colour of the ring is due to | ferrous nitrososulphate |
| Brown ring is made for | 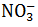 |
| Silver chloride dissolves in aqueous ammonia due to formation of | [Ag(NH3)2]+ |
Biomolecules MCQ Questions



Biomolecules Questions and Answers
| Biomolecules Quiz Question | Answer |
|---|---|
| α-maltose consists of | two α-D-glucopyranose units with 1-4 glycosidic linkage |
| Number of chiral carbon atoms in β-D-(+)- glucose is | five  |
| Sucrose on hydrolysis gives | glucose and fructose |
| In aqueous solution glucose remains as | in all three forms in equilibrium |
| How can you say that glucose is cyclic compound? | Glucose fails to react with sodium hydrogen sulphite |
| Which of the following pairs give positive Tollen's test? | Glucose, fructose |
| Which of the following compounds does not undergo mutarotation? | Sucrose  |
| If two isomers have been classified correctly as anomers, they may be also called | diastereomers |
| The carbohydrate used as storage molecule in animal is | glycogen |
| The monosaccharide constituents of lactose are | β-D-glucose and β-D-galactose |
Chemical Bonding And Molecular Structure MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14
Quiz 15
Quiz 16
Quiz 17



Chemical Bonding And Molecular Structure Questions and Answers
| Chemical Bonding And Molecular Structure Quiz Question | Answer |
|---|---|
| Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? | HF |
| Which of the following is the electron deficient molecule? | B2H6 |
| Which of the following represents the Lewis structure of N2 molecule? | 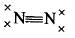 |
| A coordinate bond is a dative covalent bond. Which of the below is true ? | Two atoms form bond and one of them provides both electrons |
| Which one of the following molecules has the smallest bond angle? | H2 Se |
| The percentage s-character of the hybrid orbitals in methane, ethene and ethyne are respectively | 25, 33, 50 |
| Which of the following hydrogen bonds are strongest in vapour phase? | HF......HF |
| Which of the following is electron - deficient? | (BH3)2 |
| The molecular interactions responsible for hydrogen bonding in HF | dipole-dipole |
| The correct sequence of increasing covalent character is represented by | NaCl < LiCl < BeCl2 |
Chemical Kinetics MCQ Questions



Chemical Kinetics Questions and Answers
| Chemical Kinetics Quiz Question | Answer |
|---|---|
| Rate of reaction depends upon | All of these |
| The unit of second order reaction rate constant is | L mol-1 s-1 |
| The half-life of a reaction is halved as the initial concentration of the reactant is doubled. The order of the reaction is | 2 |
| For a first order reaction the ratio of times to complete 99.9% and half of the reaction is | 10 |
| A first order reaction is 60% complete in 20 min. How long will the reaction take to be 84% complete? | 40 min |
| A reaction proceeds by first order, 75% of this reaction was completed in 32 min. The time required for 50% completion is | 16 min |
| For a reaction, the rate constant is 2.34 s-1. The half-life period for the reaction is | 0.30 s |
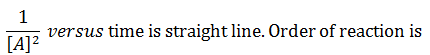 |
third |
| Inversion of cane-sugar in dilute acid is a | pseudo-unimolecular reaction |
| For which order half-life period is independent of initial concentration ? | First |
Chemistry In Everyday Life MCQ Questions



Chemistry In Everyday Life Questions and Answers
| Chemistry In Everyday Life Quiz Question | Answer |
|---|---|
| What are the substances which mimic the natural chemical messengers? | Agonists |
| Use of chemicals for therapeutic effect is called | chemotherapy |
| Which of the following is antipyretic and analgesic? | Paracetamol |
| Paracetamol is | N-acetyl p-amino phenol |
| Chloramine-T is a | antiseptic |
| Aspirin is an acetylation product of | o-hydroxybenzoic acid  |
| Tranquillisers are also known as | psychotherapeutic drugs |
| Which one among the following is not an analgesic? | Valium |
| Which among the following is not an antibiotic ? | Oxytocin |
| Which of the following is used as a morning after pill ? | Mifepristone |
Class 11 P Block Elements MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14



Class 11 P Block Elements Questions and Answers
| Class 11 P Block Elements Quiz Question | Answer |
|---|---|
| Dry ice is | solid CO2  |
| Correct formula of aluminium nitride is | AlN |
| The ratio of Fe2O3 and Al by weight in thermite is | 3:1 |
| Magnalium contains | Al + Mg |
| Aluminium reacts with caustic soda to form | sodium metaaluminate  |
| In diborane the two H—B—H angles are nearly | 97°, 120° |
| Water gas is an important fuel. It is a mixture of : | CO + H2 |
| Muddy water can be purified through coagulation by using | alums |
| Aluminium is extracted by the electrolysis of | alumina mixed with molten cryolite |
| Which of the following structure is similar to graphite? | BN |
Class 12 P Block Elements MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14
Quiz 15
Quiz 16
Quiz 17
Quiz 18



Class 12 P Block Elements Questions and Answers
| Class 12 P Block Elements Quiz Question | Answer |
|---|---|
| SO2 + H2S → Product. The final product is | H2O + S |
| Crystalline metal can be transformed into metallic glass by | Very rapid cooling of the molten metal |
| Which metal is protected by a layer of it own oxide | Al |
| The product formed in the reaction of SOCl2 with white phosphorus is | PCl3 |
| Which has the smallest size ? | P 5+ |
| Solid N2O5 is | ionic  |
| Which of the following phosphorus is the most reactive ? | White phosphorus |
| Which one of the following is least covalent in nature ? | BiF3 |
| Which of the following oxides, at the same concentration when dissolved in water, results in the most acidic solutions ? | N2 O5 |
| Which is the anhydride of nitric acid ? | N2O5 |
Classification Of Elements And Periodicity In Properties MCQ Questions



Classification Of Elements And Periodicity In Properties Questions and Answers
| Classification Of Elements And Periodicity In Properties Quiz Question | Answer |
|---|---|
| The first ionisation potential of Na is 5.1 eV. The value of electron gain enthalpy of Na+ will be | - 5.1 eV |
| Which one of the following orders presents the correct sequence of the increasing basic nature of the given oxides? | Al2O3 < MgO < Na2O < K2O |
| The telluric helix was given by | De Chancourtois |
| Number of elements presents in the fifth period of periodic table is | 18 |
| The element Z = 114 has been discovered recently.
It will belong to which of the following family/group and electronic configuration ? |
Carbon family, [Rn] 5f 14 6d10 7s2 7p2 |
| An element with atomic number 21 is a | transition element |
| The electronic configuration, 1s2, 2s2, 2p6, 3s2, 3p6, 3d9 represents a | metallic cation |
| Differentiating electron in inner transition elements enters the ________ orbital. | f |
| In the periodic table metals usually used as catalyst belong to | d-block |
| Which of the following electronic configuration represents noble gas? | ns2 np6 |
Coordination Compounds MCQ Questions



Coordination Compounds Questions and Answers
| Coordination Compounds Quiz Question | Answer |
|---|---|
| Benzoylacetonato beryllium exhibit isomerism of the type | optical |
| The oxidation state and covalency of Al in [AlCl(H2O)5 ]2+ , respectively are | +3, 6 |
| What is the oxidation number of Pt in K[PtNH3 Cl5] ? ? | +4 |
| EDTA can form complex with how many number of donor atoms ? | Six |
| Which of the following is a negatively charged bidentate ligand ? | Dimethyl glyoxime |
| Oxidation state of Fe in [Fe(H2O)5 NO+ ]SO4 is | 1 |
| A group of atoms can function as a ligand only when | it has an unshared electron pair |
| The oxidation state of Fe in the brown ring complex [Fe(H2O)5NO]SO4 is | +1 |
| The name of the ring structure complex compound formed between metal ion and polydentate ligand is | chelate complex |
| Which of the following system is most stable for a chelate ? | Five fused cyclic system |
Electrochemistry MCQ Questions



Electrochemistry Questions and Answers
| Electrochemistry Quiz Question | Answer |
|---|---|
| The specific conductance (κ) of an electrolyte of 0.1 N concentration is related to equivalent conductance (Ʌ) by the following formula | Ʌ = 10000 κ |
| The resistance of 1N solution of acetic acid is 250Ω, when measured in a cell having a cell constant of 1.15 cm-1. The equivalent conduction (in ohm-1 cm2 equiv-1) of 1N acetic acid is | 4.6 |
| The one which decreases with dilution is | specific conductance |
| Given the limiting molar conductivity as ˄0m (HCl) = 425.9Ω-1 cm2 mol-1 ˄0m (NaCl) = 126.4Ω-1 cm2 mol-1 ˄0m (CH3 COONa) = 91Ω-1 cm2 mol-1 The molar conductivity, at infinite dilution, of acetic acid (in Ω-1 cm2 mol-1 ) will be | 390.5 |
| The molar conductivities of KCl, NaCl and KNO3 are 152, 128 and 111 S cm2mo1-1 respectively. What is the molar conductivity of NaNO3 ? | 87 S cm2 mol-1 |
| Which one of the following solutions will have highest conductivity? | 0.1 M HCl |
| Which of the following electrolytic solutions has the least specific conductance? | 0.002 N |
| Which of the following does not conduct electricity? | Solid NaCl |
| For strong electrolytes the plot of molar conductance vs√C is | linear |
| The units of equivalent conductance, are | Ω-1 cm2 equiv-1 |
Environmental Chemistry MCQ Questions



Environmental Chemistry Questions and Answers
| Environmental Chemistry Quiz Question | Answer |
|---|---|
| Which compound is mainly responsible for the depletion of ozone layer? | CF2 Cl2 |
| Green house effect is caused by | CO2 |
| Among the following compounds, which one is not responsible for the depletion of ozone layer? | CH4 |
| Which of the following is secondary pollutant? | PAN |
| The gas that is not considered as a 'green house gas' is | O2 |
| Which of the following is not an air pollutant? | N2 |
| Which of the following is a measurement of water pollution ? | BOD |
| The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was | methyl isocyanate |
| Use of chlorofluorocarbons is not encouraged because | they eat away the ozone in the atmosphere |
| What is DDT among the following? | Non-biodegradable pollutant |
Equilibrium MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14



Equilibrium Questions and Answers
| Equilibrium Quiz Question | Answer |
|---|---|
| On adding ammonia to water, | 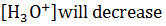 |
| Which of following base is weakest | 2)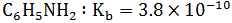 |
| In the reaction H2 (g) + Cl2 (g) ⇌ 2HCl (g) | KP = KC |
| Which of the following has highest pH ? | 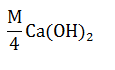 |
| Which is the best choice for weak base-strong acid titration? | Methyl red |
| The pH of 10- 4 M KOH solution will be | 10 |
| On doubling P and V with constant temperature, the equilibrium constant will | remain constant |
| What is the pH of 10-2 M H2SO4 ? | 1.6990 |
| 2HI (g) ⇌ H2 (g) + I2 (g) - QkJ For the above reaction, equilibrium constant depends upon | temperature |
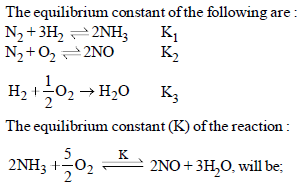 |
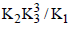 |
General Principles And Processes Of Isolation Of Elements MCQ Questions



General Principles And Processes Of Isolation Of Elements Questions and Answers
| General Principles And Processes Of Isolation Of Elements Quiz Question | Answer |
|---|---|
| Sulphide ores are common for metals | Ag, Cu and Pb |
| Which of the following is an oxide ore? | Haematite |
| In metallurgical process, for which metal carbon is used for reduction of metal oxides? | Fe |
| Argentite is an ore of | Ag |
| Thermite process is used in the reduction of | Cr2O3 |
| Hydrometallurgy is based on | reduction |
| Calamine is | ZnCO3 |
| Aluminium metal is refined by | Hoope's process |
| Identify the ore not containing iron. | Carnallite |
| Which one of the following is the chief ore of copper? | Copper pyrites |
Haloalkanes And Haloarenes MCQ Questions



Haloalkanes And Haloarenes Questions and Answers
| Haloalkanes And Haloarenes Quiz Question | Answer |
|---|---|
| Which of the following will not respond to iodoform test? | Propanol-1 |
| Which of the following will not give positive iodoform test? | CH3CH2COCH2CH3 |
| Which of the following gives iodoform test? | CH3 —CH2 (OH) |
| Tertiary butyl alcohol gives tertiary butyl chloride on treatment with | conc. HCl/anhy. ZnCl2 |
| When chloroform is treated with chlorine in the presence of sunlight, it yields | pyrene |
| Which of the following does not answer iodoform test? | n-butyl alcohol |
| Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence of dry ether? | Propane |
| Number of monochloro derivatives obtained when neo-pentane is chlorinated, is | one  |
| The catalyst used in the preparation of an alkyl chloride by the action of dry HCl on an alcohol is | anhy. ZnCl2  |
| Of the five isomeric hexanes, the isomer which can give two monochlorinated compounds is | 2,3-dimethyl butane |
Hydrocarbons MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14



Hydrocarbons Questions and Answers
| Hydrocarbons Quiz Question | Answer |
|---|---|
| What is the product formed when acetylene reacts with hypochlorous acid ? | Cl3CHCHO |
| When sodium propionate is heated with soda lime, the product formed is | ethane |
| Which of the following will yield trans product from butyne ? | Na/Liq.NH3  |
| Propene on reaction with hypochlorous acid to give | 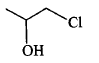 |
| Propyne on passing through red hot iron tube gives | 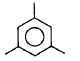 |
| Which one of the following has the minimum boiling point? | Iso-butene |
| The carbide which reacts with water to form ethyne is | CaC2 |
| Wurtz reaction involves the reduction of alkyl halide with | Na in ether |
| Thermal decomposition of alkanes in the absence of air is called | cracking |
| With respect to the conformers of ethane, which of the following statements is true ? | Both bond angles and bond length remains same |
Hydrogen MCQ Questions



Hydrogen Questions and Answers
| Hydrogen Quiz Question | Answer |
|---|---|
| Hydrogen is evolved by the action of cold dil. HNO3 on | Mn |
| The most abundant element in the universe is thought to be | hydrogen |
| Water possesses a high dielectric constant, therefore | it is universal solvent |
| Among CaH2 , NH3 NaH and B2 H6 , which are covalent hydride? | NH3 and B2 H6  |
| Which one of the following reactions does not form gaseous product ? | PbS + H2O2 → |
| Water boils and freezes exactly at 100ºC and 0ºC respectively; find the reason for it from the following | Boiling and freezing temperature of water were used to define temperature scales |
| The dielectric constant of H2O is 80. The electrostatic force of attraction between Na+ and Cl– will be | reduced to 1/80 in water than in air |
| The volume strength of 1.5 N H2 O2 solution is | 8.4 L |
| Commercial sample of H2O2 is labeled as 10 V. Its % strength is nearly | 3 |
| Calgon used as a water softener is | 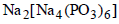 |
Nitrogen Containing Compounds MCQ Questions



Nitrogen Containing Compounds Questions and Answers
| Nitrogen Containing Compounds Quiz Question | Answer |
|---|---|
| Reduction of nitrobenzene in the presence of Zn /NH4 Cl gives | N-phenyl hydroxylamine |
| Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is diazotised and then heated with cuprous bromide. The reaction mixture so formed contains. | mixture of o and p-bromotoluenes |
| Nitrobenzene is reduced by Zn and alcoholic potash mixture to get | C6H5 —NH—NH—C6H5  |
| Nitration of nitrobenzene at 125°C with mixed acids gives | meta-dinitrobenzene |
| Which of the following compounds is soluble in benzene but almost insoluble in water? | C6H5NO2 |
| Which compound is known as alkyl carbylamine? | R ∙ NC |
| Alkyl cyanides undergo Stephen reduction to produce | aldehyde |
| Benzamide can be converted into benzonitrile with | P2O5 |
| Ethyl isocyanide on hydrolysis in acidic medium generates | ethylamine salt and methanoic acid |
| Aliphatic nitrites are prepared by the treatment of alkyl halides with | sodium cyanide |
Nuclear Chemistry MCQ Questions



Nuclear Chemistry Questions and Answers
| Nuclear Chemistry Quiz Question | Answer |
|---|---|
| The half-life of Ra 226 is 1620 yr, the decay constant (k) is | 0.0004278 |
| A radioactive isotope has a half-life of 27 days. Starting with 4 g of the isotope, what will be mass remaining after 75 days ? | 0.58 g |
| In a radioactive decay, an emitted electron comes from | the nucleus of atom |
| If half-life period is 100 yr, average life is nearly | 144 yr |
| What is the fuel of atomic pile ? | Uranium |
| Which of the following has magic number of neutrons? | 83Bi209 |
| The cause of instability of nucleus is | low proton, neutron ratio |
| If the mass defect of 5B11 is 0.081 u, its average binding energy (in MeV) is | 6.85 |
| In terms of energy 1 u is equal to | 931.1 MeV |
| The energy released in an atom bomb explosion is mainly due to | lesser mass of products than initial material |
Organic Chemistry Some Basic Principles And Techniques MCQ Questions



Organic Chemistry Some Basic Principles And Techniques Questions and Answers
| Organic Chemistry Some Basic Principles And Techniques Quiz Question | Answer |
|---|---|
| Which type of isomerism is shown by 2, 3-dichlorobutane ? | Optical |
| Among the following compounds, the most acidic is | o-hydroxybenzoic acid |
| Orbital interaction between the a-bonds of a substituent group and a neighbouring it-orbital is known as | hyperconjugation |
| +I-effect is shown by | -CH3 |
The IUPAC name of the following compound is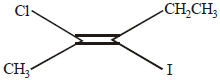 |
trans-2-chloro-3-iodo-2-pentene |
| The reaction intermediate produced by homolytic cleavage of a bond is called | free radical |
| Which kind of fission is favoured by sunlight? | Homolytic fission |
| The correct order for homolytic bond dissociation energies (AH in kcal/mol) for CH4 (A), C2 H6 (B) and CH3 Br (C), under identical experimental conditions, is | A > B > C |
| Chlorobenzene is o, p -directing in electrophilic substitution reaction. The directing influence is explained by | +M of Cl |
The state of hybridization of C2, C3, C5 and C6 of the hydrocarbon,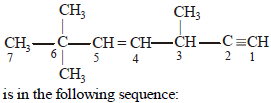 |
sp, sp3, sp2 and sp3 |
Polymers MCQ Questions



Polymers Questions and Answers
| Polymers Quiz Question | Answer |
|---|---|
| Which one is classified as a condensation polymer? | Dacron  |
| A copolymer of ethene and vinyl chloride contains alternate monomers of each type. What is the mass percentage of vinyl chloride in this copolymer? | 69%  |
| Which of the following has an ester linkage? | Dacron  |
| Terylene is not a | chain growth polymer |
| Cellulose, protein and starch are classified as | natural polymers |
| The process of formation of polymers from respective monomers is called | polymerization |
| Polycondensation products of dicarboxylic acids and diols are | polyesters |
| PDI for natural polymers is generally close to | 1 |
| Which one of the following is not a condensation polymer? | Buna-S |
| Which type of polymer is bakelite? | Condensation polymer |
Redox Reactions MCQ Questions



Redox Reactions Questions and Answers
| Redox Reactions Quiz Question | Answer |
|---|---|
| HNO3 acts as | both 1 and 2 |
| Which of the following involves a redox reaction? | Production of nitrogen oxides from nitrogen and oxygen in the atmosphere by lightning |
| In Br3O8 compound, oxidation number of bromine is | 16/3 |
| Oxidation state of oxygen in H2O2 is | -1 |
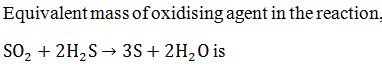 |
16 |
| Which one of the following oxidation states is not possible in metal carbonyls ? | +2 |
| What is the oxidation number of Pt in K [Pt NH3 Cl5] ? | +4 |
| Among the following, identify the species with an atom in +6 oxidation state | 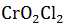 |
| The oxidation number of iron in Fe3O4 is | 8/3 |
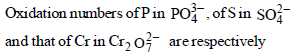 |
+ 5, + 6 and + 6 |
Sblock Elements MCQ Questions



Sblock Elements Questions and Answers
| Sblock Elements Quiz Question | Answer |
|---|---|
| Bleaching powder is obtained by treating chlorine with | Ca(OH)2 |
| Solvay process is used for the manufacture of | Na2 CO3 |
| On strong heating sodium bicarbonate changes into | sodium carbonate |
| The function of Sodium pump is a biological process operating in each and every cell of all animals. Which of the following biologically important ions is also a consituent of this pump : | K+ |
| Most powerful reducing agent is | Li |
| The activity of alkaline earth metals as reducing agent | increases from Be to Ba |
| In curing cement plasters water is sprinkled from time to time. This helps in | developing interlocking needle—like crystals of hydrated silicates |
| The wire of flash bulb is made of ... | Mg |
| Lithium shows diagonal relationship with | magnesium |
| Sodium carbonate is manufactured by | Solvay process |
Solutions MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14
Quiz 15
Quiz 16



Solutions Questions and Answers
| Solutions Quiz Question | Answer |
|---|---|
| The osmotic pressure is expressed in the units of | atm |
| The freezing point of 1% solution of lead nitrate in water will be | below 0° C |
| Which among the following is a non-colligative property | refractive index |
| The osmotic pressure of a solution is given by the relation | π/C = ST |
| The freezing point of 1 molal NaCl solution assuming NaCl to be 100% dissociated in water is | – 3.72oC |
| Now a days, divers uses the cylinder having gaseous mixture contains - | 11.7 % He, 56.2 % N2 and 32.1 % O2 |
| Due to which reason, O2 gas liberates from the blood of tissues of animal bodies | partial pressure of oxygen gas is less in tissues |
| Which of the following is not a substitutional solid solution ? | wc |
| Which of the following is a substitutional solid solution ? | wc |
 |
increases |
Some Basic Concepts Of Chemistry MCQ Questions



Some Basic Concepts Of Chemistry Questions and Answers
| Some Basic Concepts Of Chemistry Quiz Question | Answer |
|---|---|
| The number of oxygen atoms in 4.4 g of CO2 is | 1.2 × 1023 |
| Gram molecular volume of oxygen at STP is | 22400 cm3 |
| Which has maximum number of atoms? | 24 g of C |
| Which of the following has the smallest number of molecules ? | 0.1 mole of CO2 gas |
| The maximum number of molecules are present in | 15 L of H2 gas at STP |
| 4.6 x 1022 atoms of an element weigh 13.8 g. The atomic weight of that element is | 180 |
| Law of constant composition is same as the law of | definite proportion |
| A molar solution is one that contains one mole of a solute in | One litre of the solution |
| 1 mol of CH4 contains | 4 g atoms of hydrogen |
| The density of gas A is three times that of gas B. If the molecular weight of A is M, the molecular weight of B is | M/3 |
States Of Matter MCQ Questions



States Of Matter Questions and Answers
| States Of Matter Quiz Question | Answer |
|---|---|
| The compressibility factor for a real gas at high pressure is | 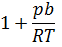 |
| The average velocity (in cm/s) of hydrogen molecule at 27°C will be | 17.8 × 104 |
| Which of the following will increase with the increase in temperature? | Vapour pressure |
| Dipole-induced dipole interactions are present in which of the following pairs : | HCl and He atoms |
| The compressibility factor of an ideal gas is | 1 |
| Equal moles of hydrogen and oxygen gases are placed in a container with a pin-hole through which both can escape. What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape ? | 1/8 |
| Which of the following is correct at freezing point? | Solid and liquid are in equilibrium |
| The term that corrects for the attractive forces present in a real gas in the van der Waal's equation is | n2 a/V2 |
| A gas deviates from ideal behaviour at a high pressure because its molecules | attract one another |
| Under which of the following conditions, van der Waals' gas approaches ideal behaviour? | Extremely low pressure |
Structure Of Atom MCQ Questions
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Quiz 5
Quiz 6
Quiz 7
Quiz 8
Quiz 9
Quiz 10
Quiz 11
Quiz 12
Quiz 13
Quiz 14
Quiz 15



Structure Of Atom Questions and Answers
| Structure Of Atom Quiz Question | Answer |
|---|---|
| [Ar] 3d 10, 4s1 electronic configuration belongs to | Cu |
| The most probable radius (in pm) for finding the electron in He+ is | 26·5 |
| The first emission line in the atomic spectrum of hydrogen in the Balmer series appears at | 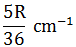 |
| The de-Broglie wavelength of a particle with mass 1g and velocity 100 m/s is | 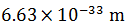 |
| Two electrons occupying the same orbital are distinguished by | Spin quantum number |
| For a f-orbital the values of m are | -3,- 2, -1, 0,+ 1,+ 2,+ 3 |
What is the maximum number of orbitals that can be identified with the following quantum
numbers?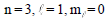 |
1 |
| Impossible orbital among the following is | 3f |
| What is the maximum numbers of electrons that can be associated with the following set of
quantum numbers?
n = 3, l = 1 and m = –1 |
2 |
| The orbital angular momentum of a p-electron is given as : | 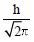 |
Surface Chemistry MCQ Questions



Surface Chemistry Questions and Answers
| Surface Chemistry Quiz Question | Answer |
|---|---|
| Which of the following statements is incorrect regarding physisorption? | Enthalpy of adsorption ( ∆Hadsorption ) is slow and positive  |
| Which of the following is a wrong statement for physisorption? | Reaction requires an energy of activation |
| In physical adsorption, the adsorbent and adsorbate are held together by the | van der Waals' forces |
| During the adsorption of krypton on activated charcoal at low temperature | ∆H < 0 and ∆S < 0 |
| Rate of physical adsorption increases with | decrease in temperature |
| Freundlich adsorption isotherm is | 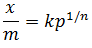 |
| Activated charcoal is used to remove colouring matter from pure substances. It works by | adsorption  |
| The extent of adsorption of a gas on a solid depends on | All of these |
| In the case of autocatalysis | product catalysis |
| Which of the following reaction requires catalyst? | 2SO2 + O2 → 2SO3 |
The D And F Block Elements MCQ Questions



The D And F Block Elements Questions and Answers
| The D And F Block Elements Quiz Question | Answer |
|---|---|
| In chromite ore, the oxidation number of iron and chromium are respectively | +2, +3 |
| The spin only magnetic moment of Fe2+ ion (in BM) is approximately | 5 |
| Anhydrous ferric chloride is prepared by | passing dry Cl2 gas over heated iron scrap |
| Copper exhibits only + 2 oxidation state in its stable compounds. Why ? | + 2 state compounds of copper are formed by exothermic reactions |
| German silver alloy contains | zinc, nickel and copper |
| Lanthanide contraction is caused due to | the imperfect shielding on outer electrons by 4f -electrons from the nuclear charge |
| The electronic configuration of Cu is | [ Ne ] 3s2, 3p6, 3d 10, 4s1 |
| Hair dyes contain | silver nitrate |
| Brass is an alloy of Cu with | Zn |
| Least paramagnetic property is shown by | Cu |
Thermodynamics MCQ Questions



Thermodynamics Questions and Answers
| Thermodynamics Quiz Question | Answer |
|---|---|
| A Beckmann thermometer is used to measure | Low temperature |
| The calorific value of fat is ………. | more than thant of carbohydrates and protein |
| A piece of ice kept at room temperature melts of its own. This reaction is governed by which law ? | Second law of thermodynamics |
| For a chemical reaction, ∆G will always be negative if | ∆H is negative and T∆S is positive |
| Which of the following processes is accompanied by an increase in entropy ? | Decomposition of N2O5 to N2O to O2 |
| Which of the following does not exhibit zero entropy at absolute zero | Glass |
| The favourable conditions for a spontaneous reaction are | T∆S > ∆H, ∆H = +ve, ∆S = +ve |
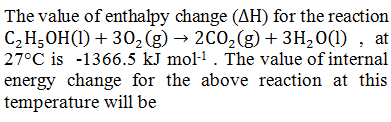 |
- 1364.0 kJ |
| The heat of combustion of carbon to CO2 is –393.5 kJ/mol. The heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas is | +315kJ |
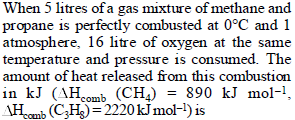 |
317 |
The Solid State MCQ Questions



The Solid State Questions and Answers
| The Solid State Quiz Question | Answer |
|---|---|
| The elements commonly used for making transistors are | Si and Ge |
| A metal crystallizes with a face-centered cubic lattice. The edge length of the unit cell is 408 pm. The diameter of the metal atom is : | 288 pm |
| Most crystals show good cleavage because their atoms, ions or molecules are | arranged in planes. |
| Glass is a | supercooled liquid |
| Iodine is a | molecular solid |
| The number of atoms contained in a fcc unit cell of a monatomic substance is | 4 |
| The ability of a substances to assume two or more crystalline structures is called | Polymorphism |
| For a crystal system a = b = c and α = β = γ ≠ 90o | rhombohedral |
| A solid compound XY has NaCl structure. If the radius of the cation is 100 pm, the radius of the anion (Y–) will be : | 241.5 pm |
| A body centric cubic lattice is made up of two different types of atoms A and B. Atom A occupies the body centre and B occupying the corner positions. One of the corners is left unoccupied per unit cell. Empirical formula of such a solid is | A8B7 |