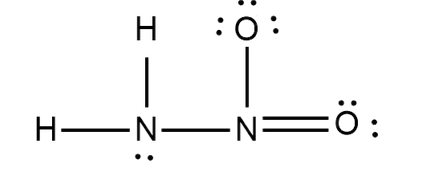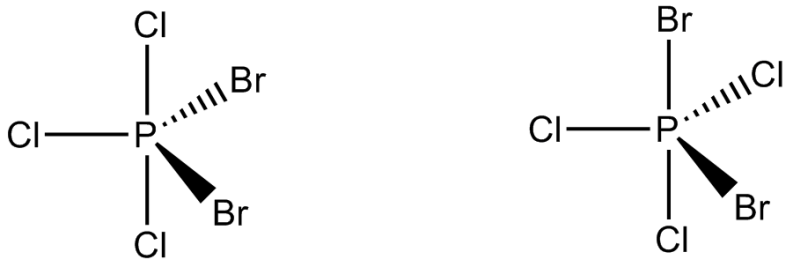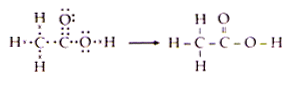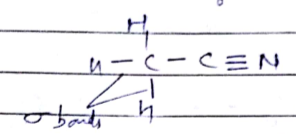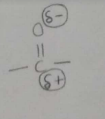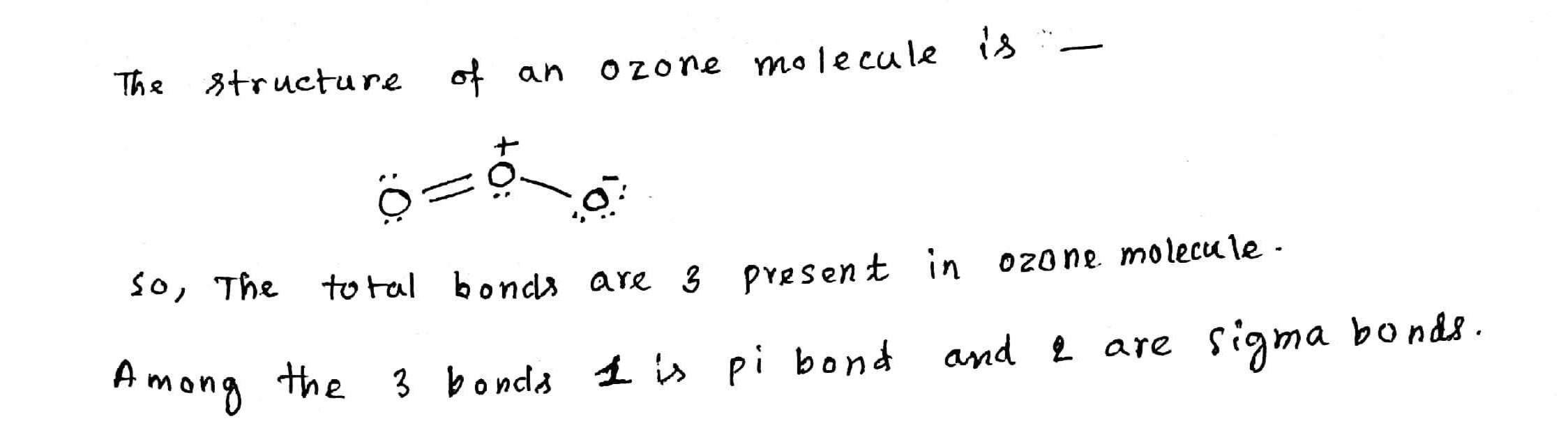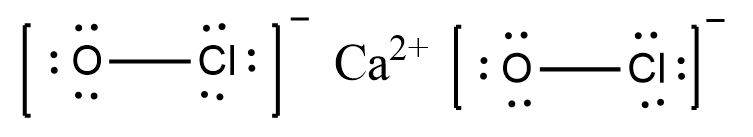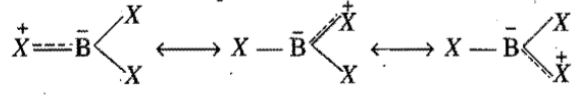Chemical Bonding And Molecular Structure - Class 11 Medical Chemistry - Extra Questions
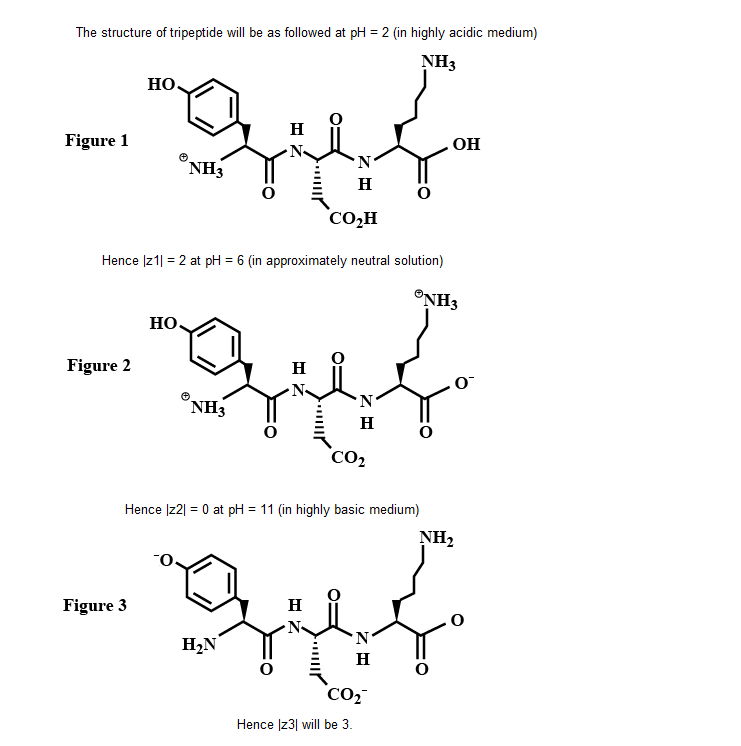
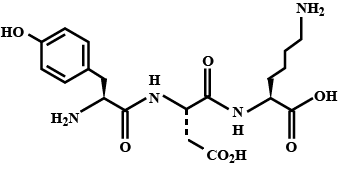
The structure of a peptide is given below.
If the absolute values of the net charge of the peptide at $$pH = 2, pH = 6$$ and $$pH = 11$$ are $$|z_1|, |z_2|,$$ and $$|z_3|$$, respectively, then what is $$|z_1|+|z_2| + |z_3| ?$$
What is meant by the term chemical bond and chemical bonding?
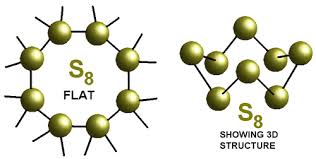
The covalency of sulphur in the sulphur molecule ($$S_8$$) is :
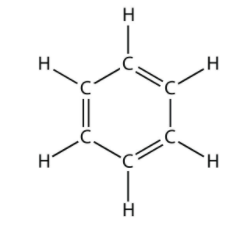
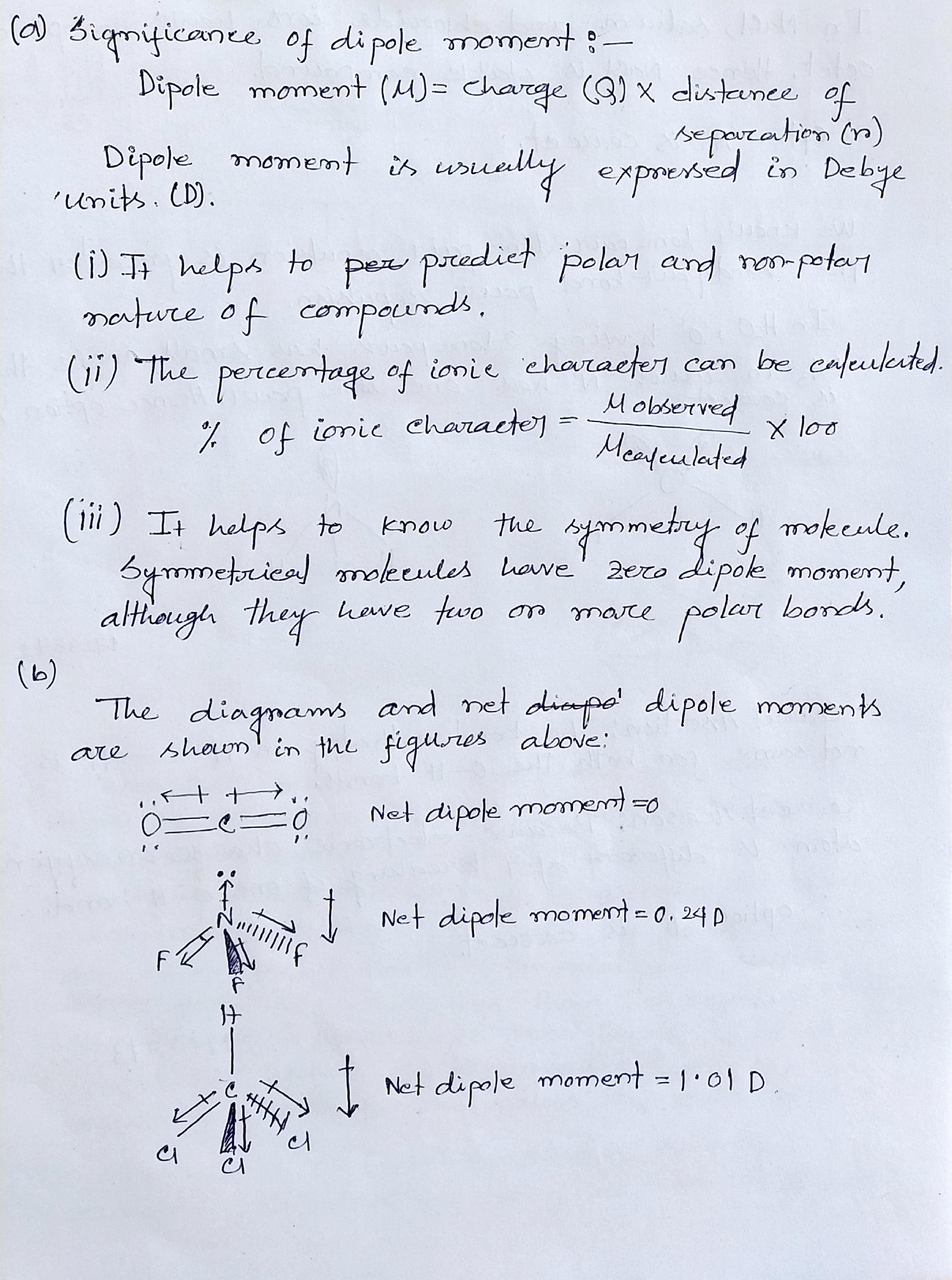
Total number of the sigma bonds present in benzene ring are:
__________ overlapping between orbitals leads to $$\pi$$ bond formation.
$$1$$ mole of $$Na_2
S_2 O_3 $$ looses $$10$$ moles of $$e^{-}$$ . Determine final Oxidation number of S.
Isotopes are the atoms of same element, with same atomic number. But with different __________.
Define isotopes.
What are co-ordinate bonds?
What is a lone pair of electrons?
A certain mass of a gas occupies a volume of 2 L at STP. To what temperature the gas must be heated to double its volume, keeping the pressure constant?
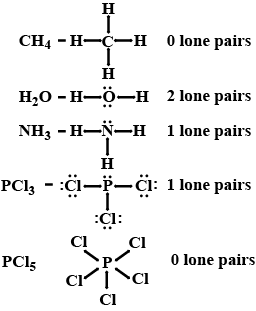
How many lone pair of electrons are present on the central atom of $$CH_4, H_2O, NH_3, PCl_3$$ and $$PCl_5$$ molecules?
What types of overlapping take place in a sigma and a pi bond?
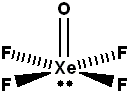
Total number of lone pair of electrons on $$Xe$$ in $$XeOF_{4}$$ is ________.
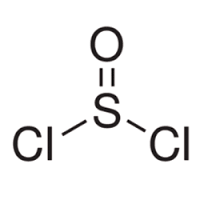
By using an expanded octet for the sulphur atom in thionyl chloride (SOCl$$_{2}$$), you can write a Lewis structure with no formal charge.
If true enter 1 else 0
If true enter 1, else enter 0.
When ethene reacts with $$H_2$$, ethane is formed. The $$ \pi$$ bond of C=C is affected. Thus, $$ \pi$$ bond is more reactive than $$ \sigma $$-bond.
Consider the following Lewis Dot structure:
$$\left[: \ddot{N} = N = \ddot{N}:\right]^-$$
The formal charge on the central $$N$$ atom is:
The formal charge on the central $$N$$ atom is:
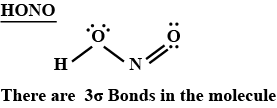
How many $$\sigma$$ bonds are present in the Lewis structure of $$HONO$$?
Consider the following Lewis Dot structure:
$$\left[: \ddot{O} = N = \ddot{O}:\right]^+$$
The sum of formal charges on $$N$$ and $$O$$ is:
The sum of formal charges on $$N$$ and $$O$$ is:
How many $$\sigma$$ bonds are present in the Lewis structure of $$H_2N-OH$$ ?
How many $$\sigma$$ bonds are present in $$H_2N-NO_2$$?
Formal charge on sulphur in $$SO_2$$ is:
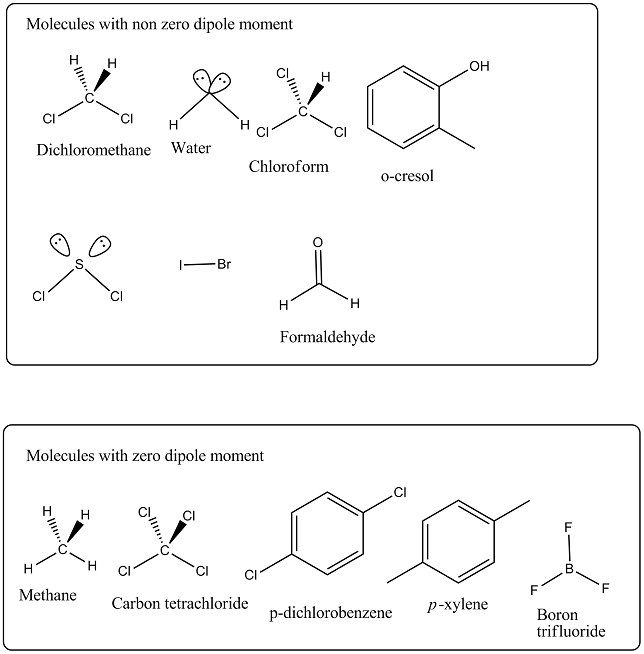
Out of $$CH_2Cl_2, CH_4, CCl_4, H_2O, CHCl_3, d-$$dichlorobenzene, $$o$$-cresol, $$p$$-xylene, $$SCl_2, BF_3, IBr$$ and $$CH_2O$$, non zero value of dipole moment are:
Numbers of geometric isomers of $$PBr_2Cl_3$$ molecule which have non-zero dipole moment are:
Ratio of bond pair-lone pair electrons $$XeO{F}_{2}$$ is:
Maximum number of atoms which can be attached on $$N$$-atom are :
If true enter 1, else enter 0.
Hydrogen bond is weaker than a covalent bond.
If the statement is true enter 1, else enter 0.
Repulsion between bond pair-bond pair electron is lesser than in between lone pair-lone pair.
Number of Sugden's singlet bonds in $$P{Cl}_{5}$$ is :
If true enter 1, else enter 0.
The $$H$$-bond energy is around $$8.4kJ$$ $${mol}^{-1}$$.
Number of unpaired electrons in $${O}_{2}[As{F}_{4}]$$ is :
In acetylene, there are ______ (two/four) $$\pi$$ - bonding electrons between carbon atoms.
The dipole moment of $${CH}_{3}OH$$ ____ (more/less) than that of $${CH}_{3}SH$$
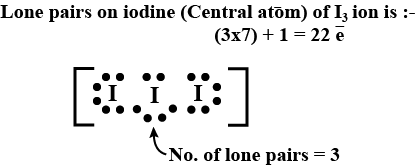
The number of lone pairs of an electron on central iodine atom of $${I}_{3}^{-}$$ ion is:
Find the number of lone pairs of electrons on $$Xe$$ in $$XeOF_4.$$
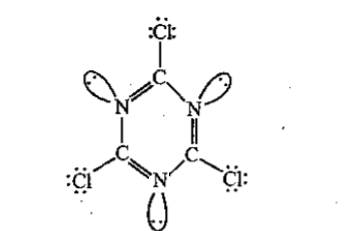
Find the ratio of the total number of bonds to the total number of lone pairs in $$C_3N_3Cl_3$$.
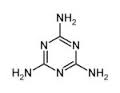
Find the ratio of lone pairs to $$\pi $$ bond (present in ring).in $$C_3N_3\, (NH_2)_3$$.
Draw the electron dot structure of Ethanoic acid
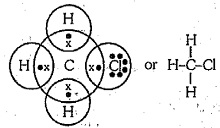
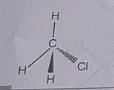
Explain the nature of the covalent bond using the bond formation in $$ C{ H }_{ 3 }Cl$$
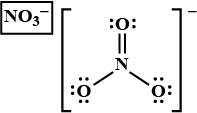
In $${NO_3}^-$$ ion, find the sum of bond pairs and lone pairs of electrons on nitrogen atom.
1.
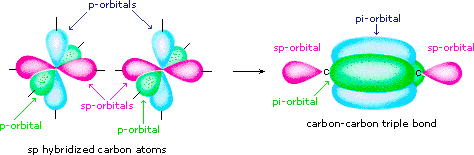
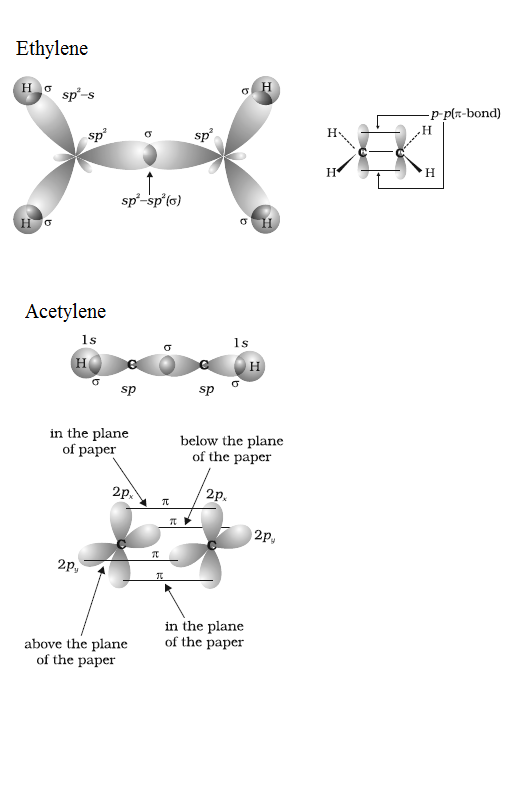
Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in $$ \displaystyle C_{2}H_{4} $$ and $$ \displaystyle C_{2}H_{2} $$ molecules.
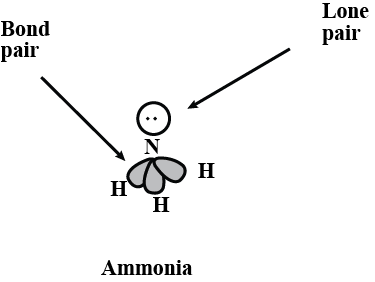
What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
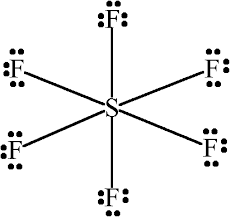
Find the number of lone pairs and bond pairs in $${SF}_{6}$$.
Linear molecules possess _______ dipole moment.
Lone pairs and bond pairs possess electrons. However, the repulsion experienced by lone pairs is much greater than that experienced by bond pairs. What reason can be attributed to this?
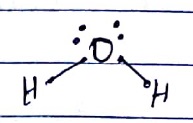
In the formation of water molecule, _________ number of lone pairs and ________ number of bonds pairs are present.
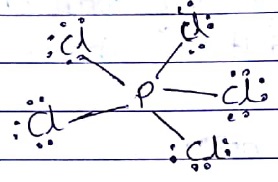
The number of bond pairs in the molecule $$PCl_5$$ is __________.
Why do only valence electrons involve in bond formation? Why not electron of inner shells? Explain.
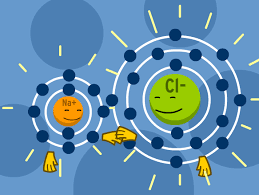
Explain the formation of sodium chloride and calcium oxide on the basis of the concept of electron transfer from one atom to another atom.
What is a Chemical bond?
Why water drops (dew) form on flowers and grass during morning hours of winter season ?
A, B, and C are three elements with atomic number 6, 11, and 17 respectively. Which of these can form an ionic bond as well as covalent bonds?
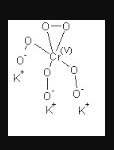
Oxidation number of Cr in $${ K }_{ 3 }{ CrO }_{ 8 }$$ is
(1) $$+6$$ (2) $$+5$$
(3) $$+3$$ (4) $$+2$$
Calculate the fractional charge value of HCl the dipole moment is $$1.02$$D.
Which of the following molecules posses more dipole moment and why?$${ NH }_{ 3 }$$ or $${ NF }_{ 3 }$$
What is the total number of sigma bonds and pi bonds in the following molecules?
$$C_2H_3Cl$$, $$CH_2Cl_2$$, $$H_3C-\overset{H}{\overset{|}{C}}=\overset{H}{\overset{|}{C}}-C\equiv C-H$$
Number of $$\sigma $$ bonds and $$\pi$$ bonds in methyl cyanide are $$x$$ and $$y$$ respectively. Value of $$x+y$$
Explain the formation of a chemical bond.
Give reasons:
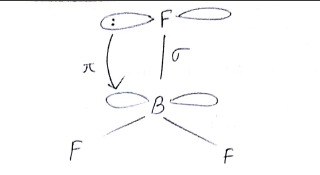
(i) $${BF}_{3}$$ has zero dipole moment although the $$B-F$$ bonds are polar.
(ii) $$NaCl$$ is harder than $$Na$$ metal.
How many bond pair and lone pair are there in $$CO_3^{2-}$$?
What is the dipole moment of $$BeF_2$$ molecule? Account for your answer.
What is the differnece between $$\sigma$$ and $$\pi$$ bonds?
Compare and justify the dipole moment of $${ NH }_{ 3 }$$ and $${ NF }_{ 3 }.$$
A diatomic molecule has a dipole moment equal to 1.2 D. If the bond length is 1 $${ A }^{ 0 }$$, what fraction of electronic charge $$'e'$$ exists on each atom.
Number of molecules having non-zero dipole moment.
$$BeCl_{2},XeF_{2}, NH_{3},PCl_{3}F_{2},PCl_{2}F_{3},BCl_{3},SF_{6}, XeF_{4}$$

The H-OH bond angle in water isThe dipole distance being $$0.94{ A }^{ 0 }$$. The dipole moment for molecule is 1.85 debye. Calculate the charge on oxygen atom.
[$$\cos { { 105 }^{ 0 } } $$= 0.259(approx)].
Explain the terms
(i) bonding molecular orbitals,
(ii) antibonding molecular orbitals,
(iii) non-bonding molecular orbitals.
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electronic shells but different number of electrons in their outermost shell. It was found that elements A and G combine to form an ionic compound which can also be extracted from sea water. This compound is added in a small amount to almost all vegetable dishes during cooking. Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral. Based on the above information answer the following question.
What would be the nature of compound formed by a combination of elements B and F?
What would be the nature of compound formed by a combination of elements B and F?
What type of interaction hold the molecules together in a polar molecular solid?
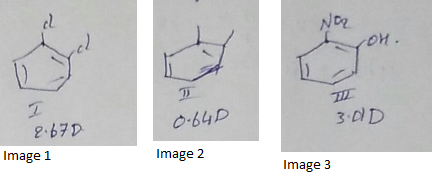
Compare the dipole moment of $$o-dichlorobenzene (I) ; o - xylene (II) and o - nitrophenol(III)$$
The strength of sigma bonds formed by axial overlap of s- orp-orbitals of 2nd shell of participating atoms decreases as:
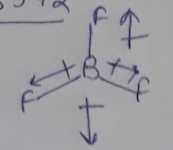
Why dipole moment of $$PF_2Cl_3$$ is zero but that of $$PF_3Cl_2$$ is non-zero?
Explain the difference between the valence electrons and the covalency of an element.
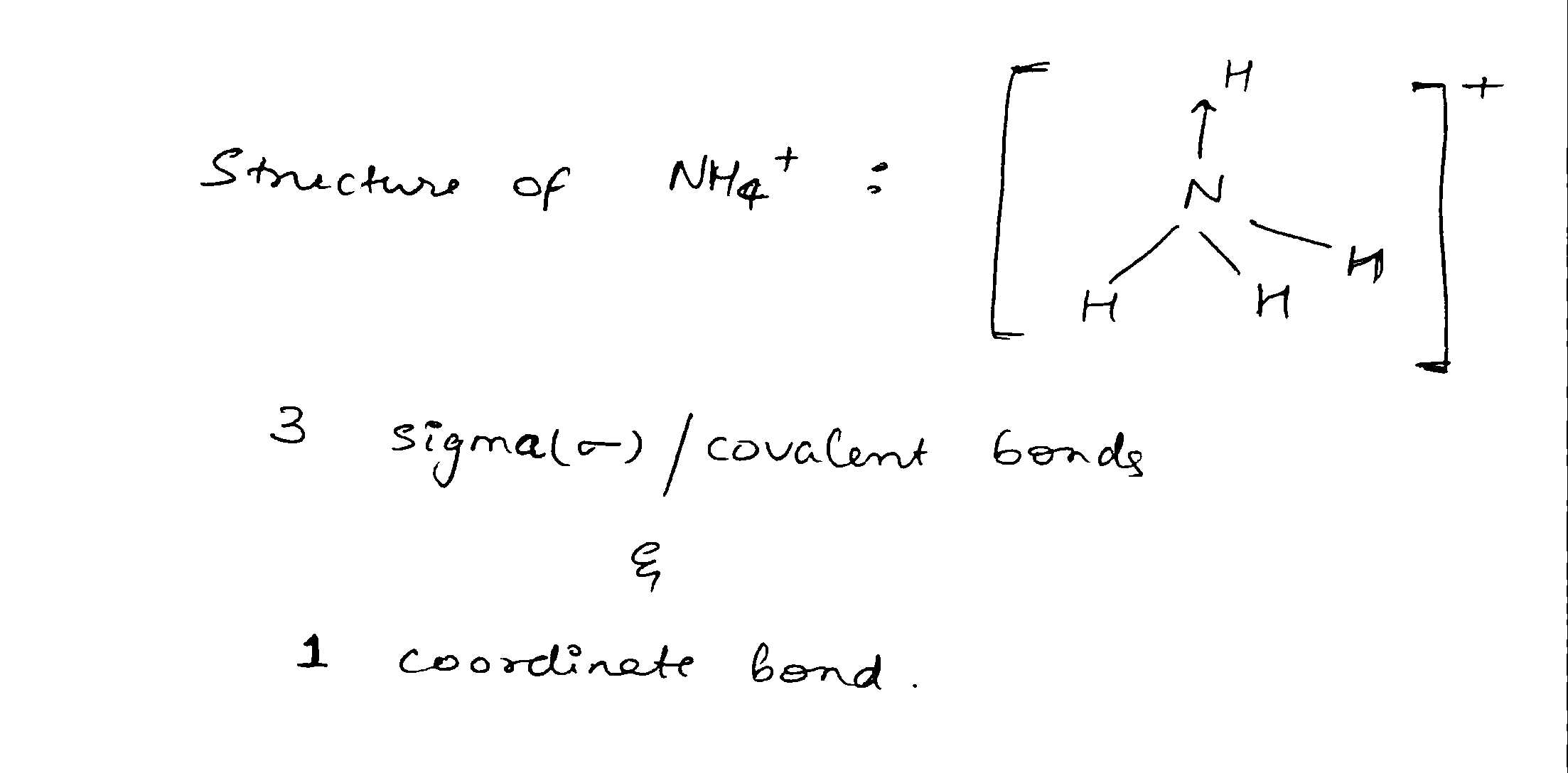
Calculate the formal charge on the all atoms present in the molecules $$NH_{4}^{+}$$.
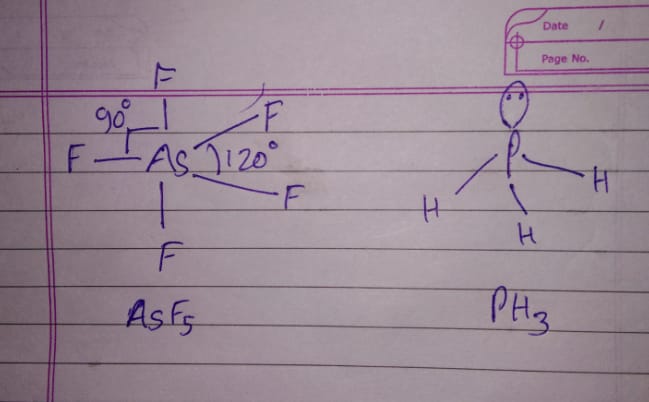
Discuss the shape of the following molecule using VSEPR model:
$$As{ F }_{ 5 }$$ and $${ PH }_{ 3 }$$
$$LiF$$ is ionic but $$BeF_2$$ is covalent. Comment on the statement.
What is the nature of bond in $$CaH_{2}$$?
Comment on the nature of bonding formed in $$BF_{3}$$.
Write short notes on synergic bonding.
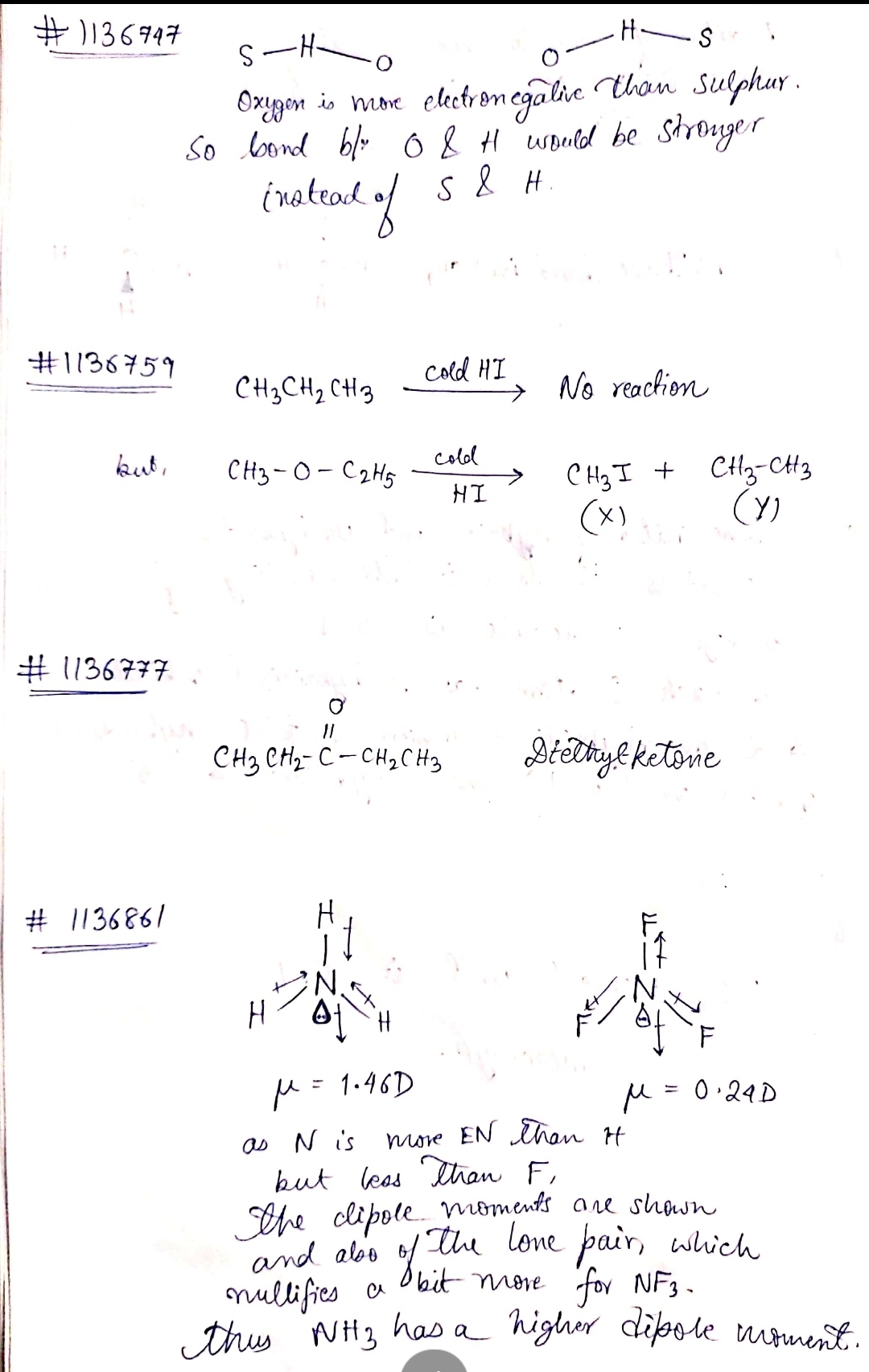
Which hydrogen bond is stronger?
$$S-H-O$$ or $$O-H-S$$
Account for the following:
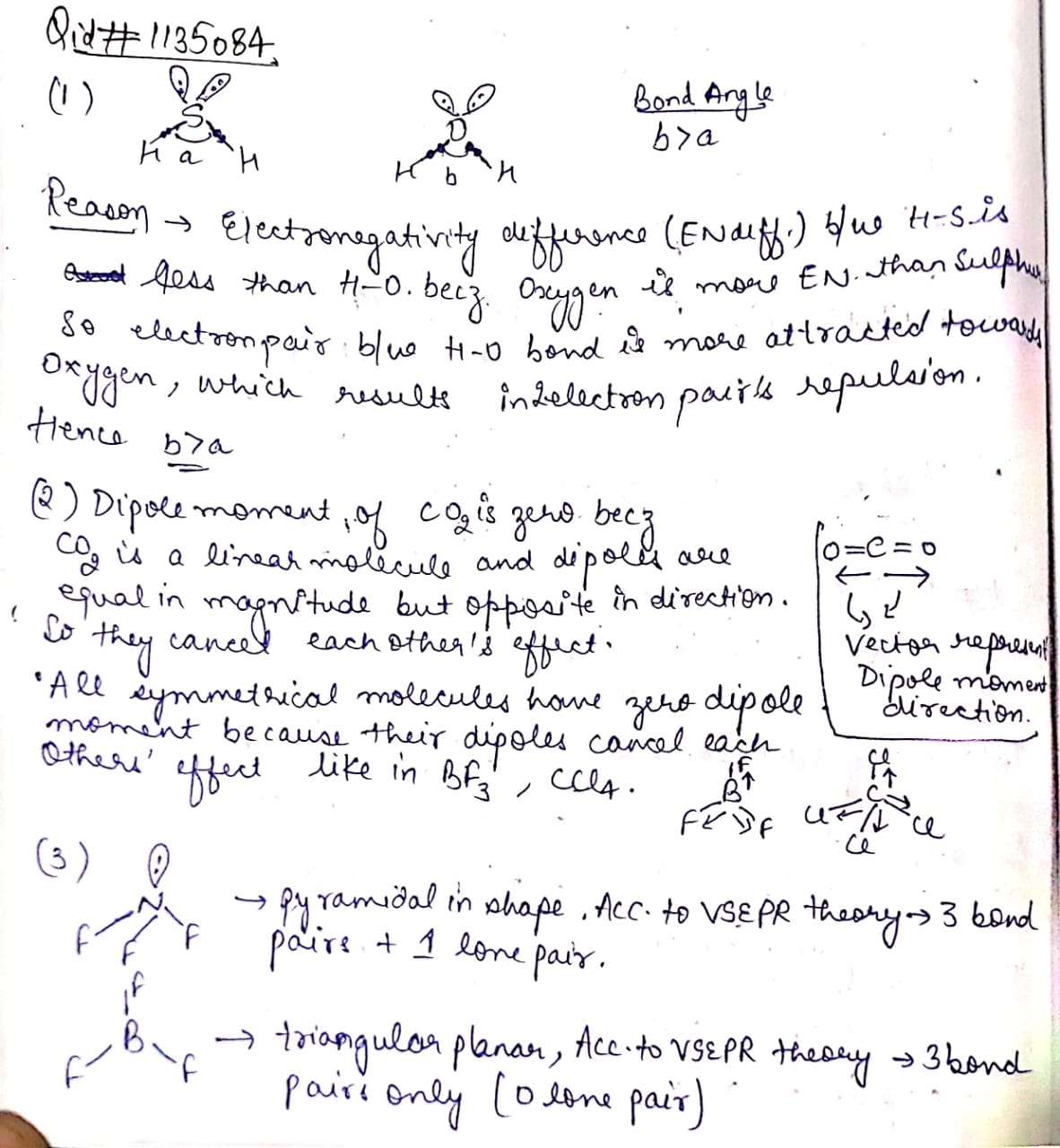
1) The $$H-S-H$$bond angle in $${ H }_{ 2 }S$$is less than the $$H-O-H$$bond angle in $${ H }_{ 2 }O$$
2) Dipole moment of$$C{ O },\quad B{ F }_{ 3 } , CC{ I }_{ 4 } $$ are zero
3) $$N{ F }_{ 3 }$$is pyramidal but $$B{ F }_{ 3 }$$is triangular planar
In $$O_{2},O_{2} and O_{2}^{^{-2}}$$ molecular species, the total number of antibonding electrons respectively are ?
Bond line Formula
C-atom $$\rightarrow $$ denoted by corner
H-atom $$\rightarrow $$ Not shown
Other atom $$\rightarrow $$ usual rotation.
What is covalency?
Write a short note on hydrocarbons?
What is partial -ve & +ve
Molecular compounds are usally formed by the combination between _________.
Boron trifluoride $$(BF_3)$$ has no dipole moment $$(\mu = 0D)$$. Explain how this observation confirms the geometry of $$BF_3$$ predicted by VSEPR theory.
The dipole moment of water is $$6.17 \times 10^{-3} cm$$. The H-O-H bond angle is $$104^0$$ and 0-H bond length is 96 pm. Calculate the % ironic character.
Find the formal charge of each $$'O'$$ in ozone.
A diatomic molucule has a dipole moment of 2.4 D. If the bond length is 2 $$\mathring { A } $$. What fraction of electronic charge exist on one of the poles?
Compare the $$C-N$$ bond-length in the following species.
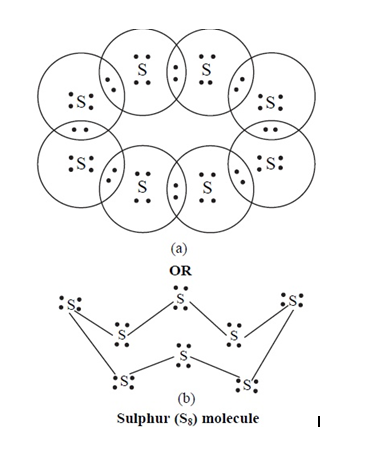
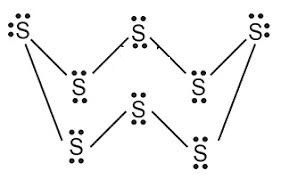
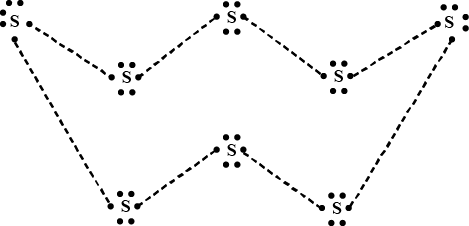
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur ?
If dipole moment of is $$ 1.5 D$$, dipole moment of is:
Can we get hydrogen and oxygen by setting up an arrangement for passing current through distilled water? Why?
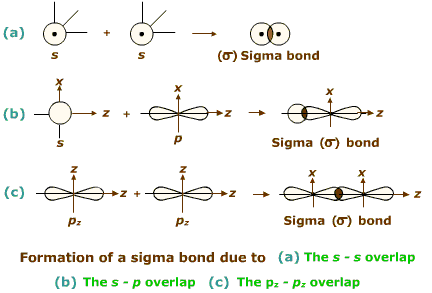
Explain why a Sigma $$(\sigma)$$- bond is stronger than a Pi$$(\pi)$$- bond?
Explain the electronic configuration :- $${\text{n}}{{\text{s}}^{{\text{2}}\,}}\,\,{\text{n}}{{\text{p}}^{\text{3}}}$$. are more stable as compared $$np^3$$
Write the significance/applications of dipole moment.
Atomicity of oxygen in ozone molecule is____________.
What do you mean by noble metals?
Dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.Give reason :
(i) Discuss the significance/applications of dipole moment.
(ii) Represent diagrammatically the bond moments and the resultant dipole moment in C02, NF3
and CHCl3.
How many $$\sigma$$ - and $$\pi$$ -bonds are present in each of the following molecules?
(a) $$H C \equiv C- C H = C H- C H _ { 3 }$$
(b ) $$C H _ { 2 } = C = C H- C H _ { 3 }$$
What's the difference between electrovalency, covalency and valency?
Write three - dimensional ( wedge - darked wedge line ) representation for the methyl chloride $$CH_{3}Cl$$ compound.
Find the number of $$ \sigma $$ bond $$ \pi $$ bond in $$1, 3$$-butadiene.
(a) Discuss the significance / applications of dipole moment.
(b) Represent diagrammatically the bond moments and the resultant dipole moment in $$ CO_2 , NF_3$$ and $$CHCl_3 $$
How many $$ \sigma $$ molecules and how many $$ \pi $$ bonds are present in a benzene molecule?
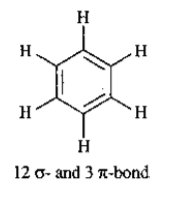
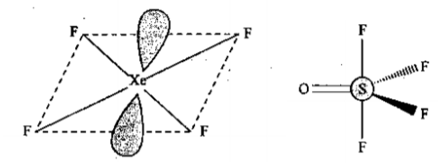
Draw the shape $$ XeF_4 $$ and $$ OSF_4 $$ according to VSEPR theory. Show the lone pair of electrons on the central atoms.
State four major physical properties that can be used to distinguish between covalent and ionic compounds. Mention the distinguishing features in each case.
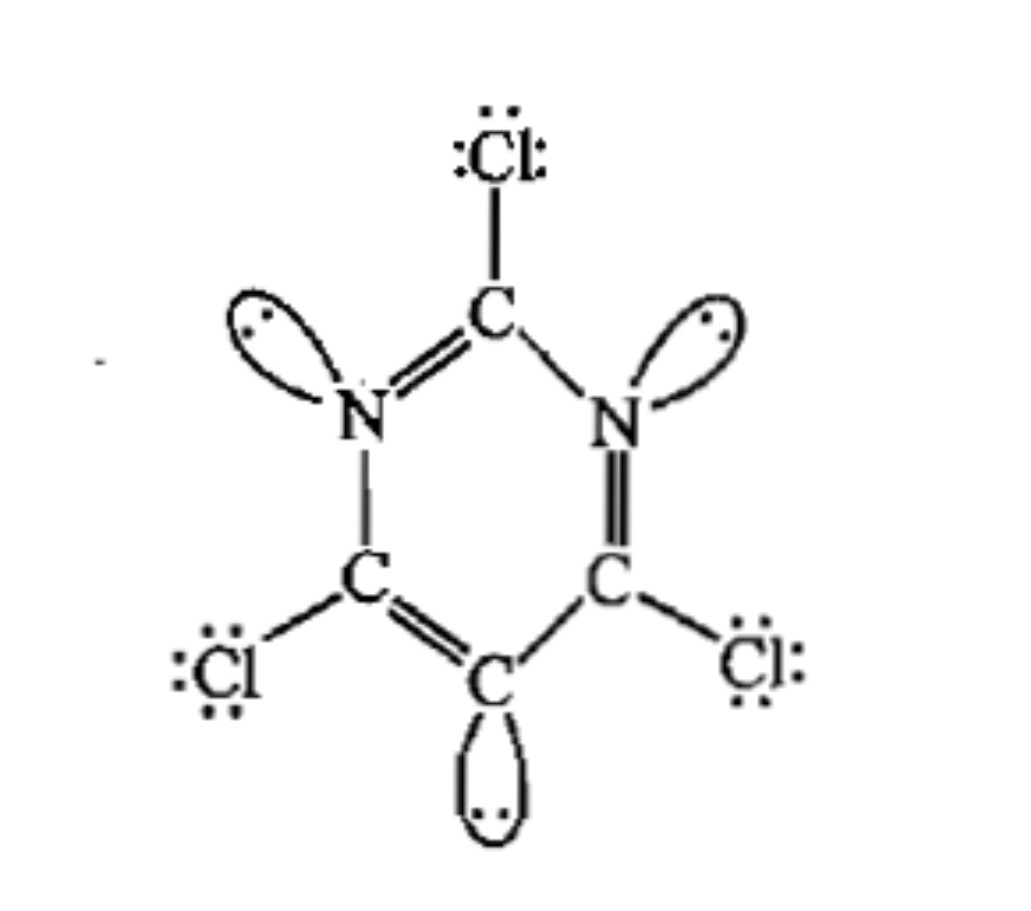
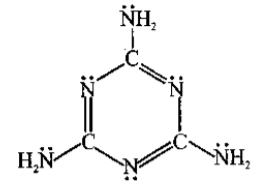
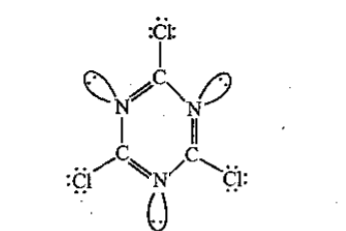
The total number of lone-pairs of electrons in melamine is .............
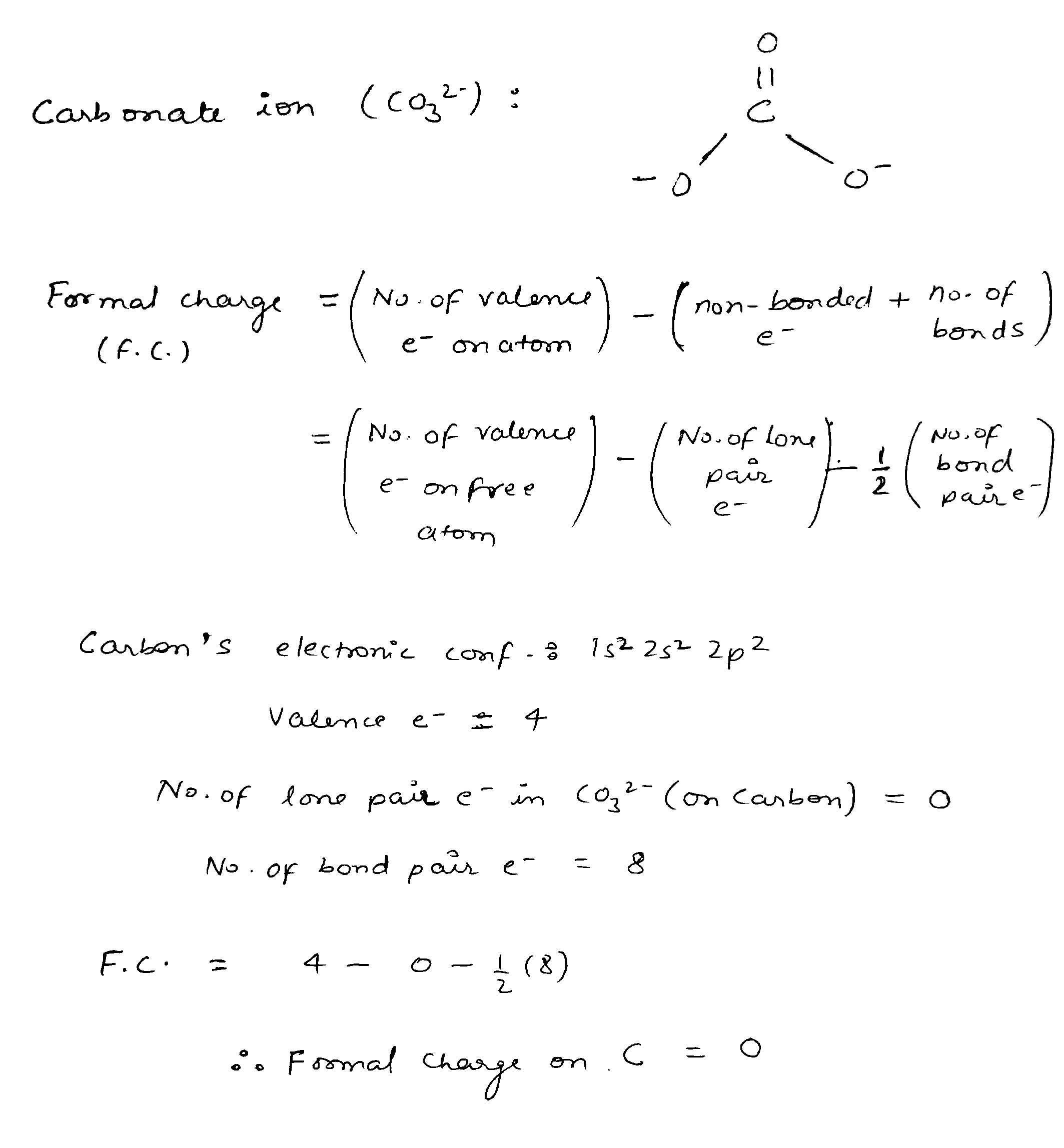
The formal charge on the carbon atom in the carbonate ion is:
What types of bonds and how many of each are present in $$ NH_4^+ ? $$
Which one of the following has strongest bond?
HF, HCl , HBr, HI
How many total bonds (sigma and pi bonds) are present in ozone molecule?
The number of lone pairs on Xe in $$ XeF_6 $$ is:
What type of bonds are present in the following molecules?
(i) $$ MgF_2 $$ (ii) BrCI (iii) $$ CBr_4 $$ (iv) $$ C_2N_2 $$ (v) CuS (vi) $$ H_2O $$ (vii) $$ H_2SO_4 $$ (viii) $$ SO_2 $$ (ix) $$ HNO_3 $$ (x) $$ K_4 Fe (CN)_6 $$
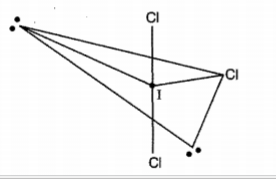
In the molecule $$ ICl_3 $$ how many lone pairs of electrons are associated with iodine ?
How many lone pairs are present in nitrogen molecule?
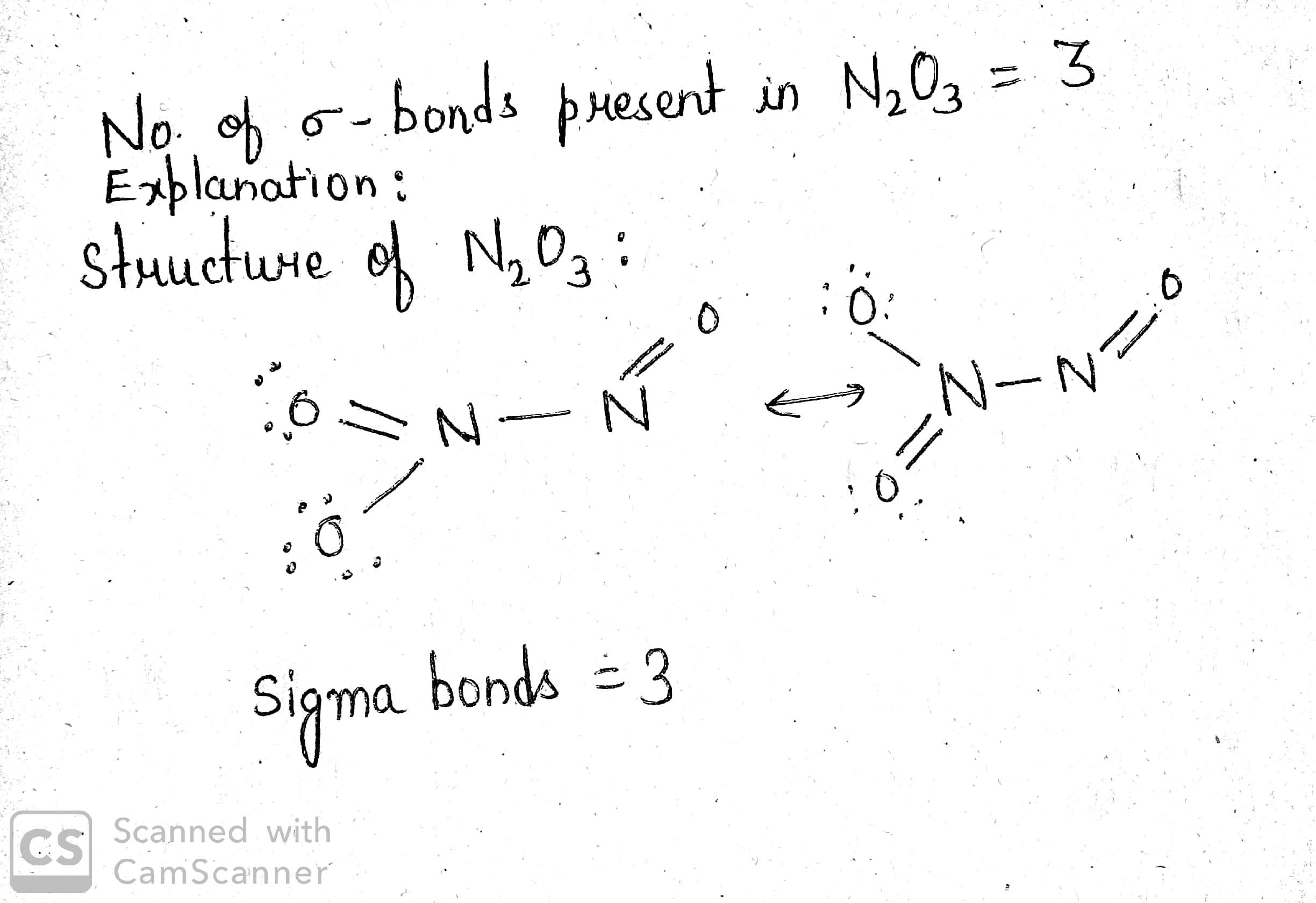
How many $$\sigma$$- bonds are present in $$N_2O_3$$?
How many lone pairs of electrons are present on Xenon atom in $$XeF_4$$?
The ratio of lone pairs and the number of $$S-S$$ bonds in $$S_8$$ molecules is:
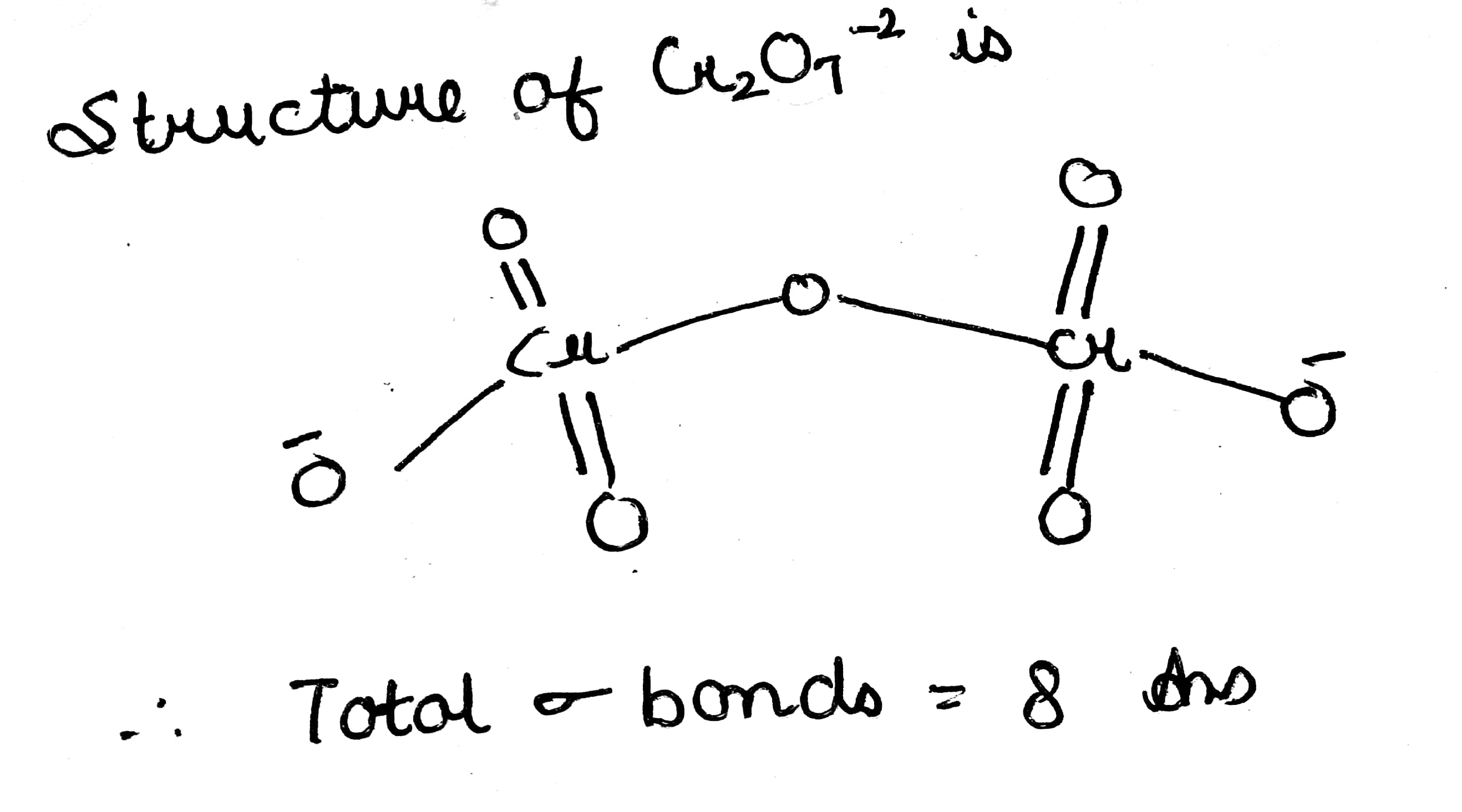
How many sigma bonds are present in $$Cr_2O_7^{2-}$$ ion?
The difference in the number of $$\sigma $$ and $$ \pi $$ bonds in trimer of $$ SO_{3}\ i.e \ S_{3}O _{9}$$ (consider no coordinate bond to be present )
How many lone pairs are present in $$OF_{2}$$ molecule?
Number of $${ sp }^{ 2 }-{ sp }^{ 2 }$$ sigma bonds in given compound $$A$$ is:
Explain the following statement:
Some alkali metals can be cut with a knife.
Arrange the followint compounds in increasing order of diple moment:
$${ CH }_{ 3 }{CH}_{2}{ CH }_{ 3 },{ CH }_{ 3 }{ CH }_{ 2 }{NH}_{2},{ CH }_{ 3 }{ CH }_{ 2 }OH$$
Define Chemical bond.
What do you understand by lone pair and shared pair?
Define :
a chemical bond
Match the pairs.
Define a Chemical bond.
What do you understand by a chemical bond?
In $$XeF_2\ ,XeF_4,\ and\ XeF_6$$, the number of lone pairs on $$Xe$$, is ________.
Calculate the value of X-Y, for $$XeOF_{4}$$. (X = Number of $$\sigma$$ bond pair and Y = Number of lone pair on central atom)
Calculate x + y + z for $$H_{3}PO_{3}$$ acid, where x is no. of lone pairs , y is no. of bonds and z is no.of $$\pi$$ bonds
What is dipole moment ?
What is covalency ?
How many sigma bonds are present in ethane ?
What is chemical bonding ?
Draw the electron-dot structure of $$S_8$$ molecule.
What is a sigma bond ?
What do you mean by lone pair of electron ?
Give the scientific reason for the following:
In the atmosphere, oxygen is available as $$O_2$$ but not as O.
In the atmosphere, oxygen is available as $$O_2$$ but not as O.
Calculate the value of "x + y - z" here x,y and z are total number of non-bonded electron pair(s), pie bond(s) and sigma bonds in hydrogen phosphite ion respectively .
How are atoms in these compounds held together?
Which one of the following has the highest dipole moment?
$$CCl_4$$
Define dipole moment.
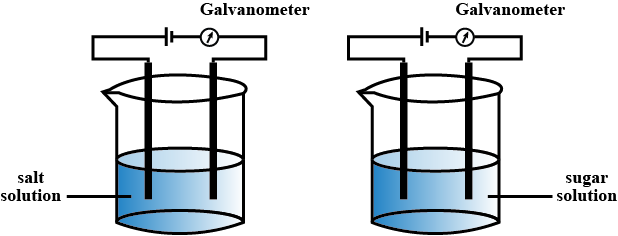
Perform the experiments arranging the apparatus as shown in the figure.
Record your observations and identify what type of compounds sodium chloride and sugar are.
What is meant by Chemical Bonding?
The electronic configuration of magnesium and chlorine are $$ 2 , 8 , 2 $$ and $$2 , 8 , 7 $$ respectively. How many electrons does a magnesium atom donate ? What charge will it get ?
In the formation of sodium chloride which atoms are combined?
Which of the following molecule / molecules will have zero dipole moment ?
$$CO_{2}, H_{2}O, CCl_{4}, CHCl_{3}, BF_{3}, BeF_{2}, NH_{3}.$$
Complete the equation of this process
$$ Cl+ 1e^- \rightarrow $$
Mention the chemical bond in following molecules or compounds:
Sodium fluoride
Taking x-axis as the internuclear axis, which out of the following will form $$\sigma$$-bond ?
(i) 1s and 1s (ii) 1s and 2$$p_x$$ (iii) 2$$p_y$$ and 2$$p_y$$ (iv) 1s and 2s.
What is chemical bond? Explain its types giving one example of each.
Let us complete the equation for this process,
$$ Mg \rightarrow Mg^{2+} + $$ .................
Mention the chemical bond in following molecules or compounds:
Potassium bromide
What is the valency of a molecule?
Fill in the blanks:
The atom which loses electrons acquires a ............... charge.
What are the SI units of dipole moment ?
Compound $$A [C_{4}H_{8}N_{2}]$$ on hydrolysis in acidic medium gave $$B$$ ($$[C_{4}H_{9}O_{2}N]$$). A on reduction with $$LiAlH_{4}$$ gave $$C$$ $$([C_{4} H_{12} N_{2}])$$.'B' on treatment with $$NaHCO_{3}$$ solution liberates $$CO_{2}$$. 'B' on heating forms $$D$$ ($$[C_{4}H_{7}ON]$$). 'D' on reduction with $$LiAlH_{4}$$ forms secondary amine $$E$$ ($$[C_{4}H_{9}N]$$). Number of single bonds in final compound E are:
The number of $$P-O-P$$ bonds in tricyclic metaphosphoric acid is:
Match the species in List-I with the number of lone pairs given in List-II.
Number of covalent bonds present in $$Ca (OCl)_2$$.
Match the list of type of bonds (List I) with list of compounds (List II).
Match type of bond (given in List I) with method of formation (given in List II).
By the following method you can predict the geometrical shape of species with only one central atom, without first drawing the Lewis structure :
Total number of electron pairs = (number of valence electrons $$\pm$$ electrons (for ionic) charge)/2
Number of bond electron pairs = number of atoms - 1
Number of electron pairs around central atom = total number of electron pairs - 3 [number terminal atoms (except H)]
Number lone pair = (number of central electron pairs - number of bond pairs)
How many of the following have trigonal bipyramidal shape based on this method?
$$(a) PCl_5\, \, \, (b) NH_3\, \, \, (c) H_2O$$ $$(d) {ClO_3}^- \, \, \, (e) {ICl_4}^-$$
How many of the following have trigonal bipyramidal shape based on this method?
$$(a) PCl_5\, \, \, (b) NH_3\, \, \, (c) H_2O$$ $$(d) {ClO_3}^- \, \, \, (e) {ICl_4}^-$$
Considering $$x-$$axis as the internuclear axis, the overlap of which of the following orbitals will not form a sigma bond and why? (a) $$1s$$ and $$2s$$
(b) $$1s$$ and $$ \displaystyle 2 p_{x}$$
(c) $$ \displaystyle 2p_{y}$$ and $$ \displaystyle 2p_{y} $$ (d) $$1s$$ and $$2s$$
(c) $$ \displaystyle 2p_{y}$$ and $$ \displaystyle 2p_{y} $$
The following table shows the electronic configuration of the elements $$W, X, Y, Z$$ :
| Element | W | X | Y | Z |
| Electronic configuration | 2, 8, 1 | 2, 8, 7 | 2, 5 | 1 |
(i) What type of Bond is formed between :
$$W$$ and $$X$$ $$Y$$ and $$Z$$
(ii) What is the formula of the compound formed between :
$$X$$ and $$Z$$ $$W$$ and $$X$$
How many $$\sigma$$ and $$\pi$$ bonds are presents in each of the following molecule?
(a) $$HC \equiv C -CH =CH - CH_3$$ (b) $$CH_2 = C = CH - CH_3$$
Calculate formal charge of atoms $$HClO_{4}, C{{O}_{3}}^{2}$$.
In the formation of compound between two atoms $$A$$ and $$B$$, $$A$$ looses two electrons and $$B$$ gain one electron.
(a) What is the nature of bond between $$A$$ and $$B$$?
(b) Explain the formation of $$MgCl_2$$ molecule.
(c) Common salt conducts electricity only in the molten state. Why?
(d) Why is melting point of $$NaCl$$ high?
Dipole moment of $$H_2S$$ is $$0.95$$D. Calculate the bond moment if the bond angle is $$97^0$$. $$(cos \ 48.5^0 = 0.662)$$
Based on $$MO$$ theory compare the relative stabilities of $$O_{2}$$ & $$O_{2}^{2-}$$ and indicate their magnetic properties.
Arrange-$$NH_3;BF_3$$ and $$NF_3$$ in the increasing order of their Dipole moment, giving reasons.
Write down correct order of covalent character of the following.
a . LiCl , NaCl , RbCl , KCl
b. NaCl , Mg$$Cl_2$$ , Si$$Cl_4$$
c . Sn$$Cl_2$$ , Sn$$Cl4$$
Write chemical equation for the following reaction.
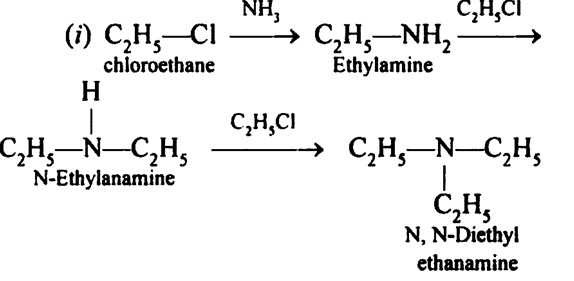
Reaction of ethanolic $$NH_3$$ with $$C_2H_5Cl$$.
How many of the following substance have higher lattice energy than $$NaBr$$?
$$CaCI_{2}, NaI, CsBr, LiF, MgO, AI_{2}O_{3}, TiO_{2}, KI_{3}$$
How many sigma (T) bond present in $$CH_2=C=CH-CH_3$$
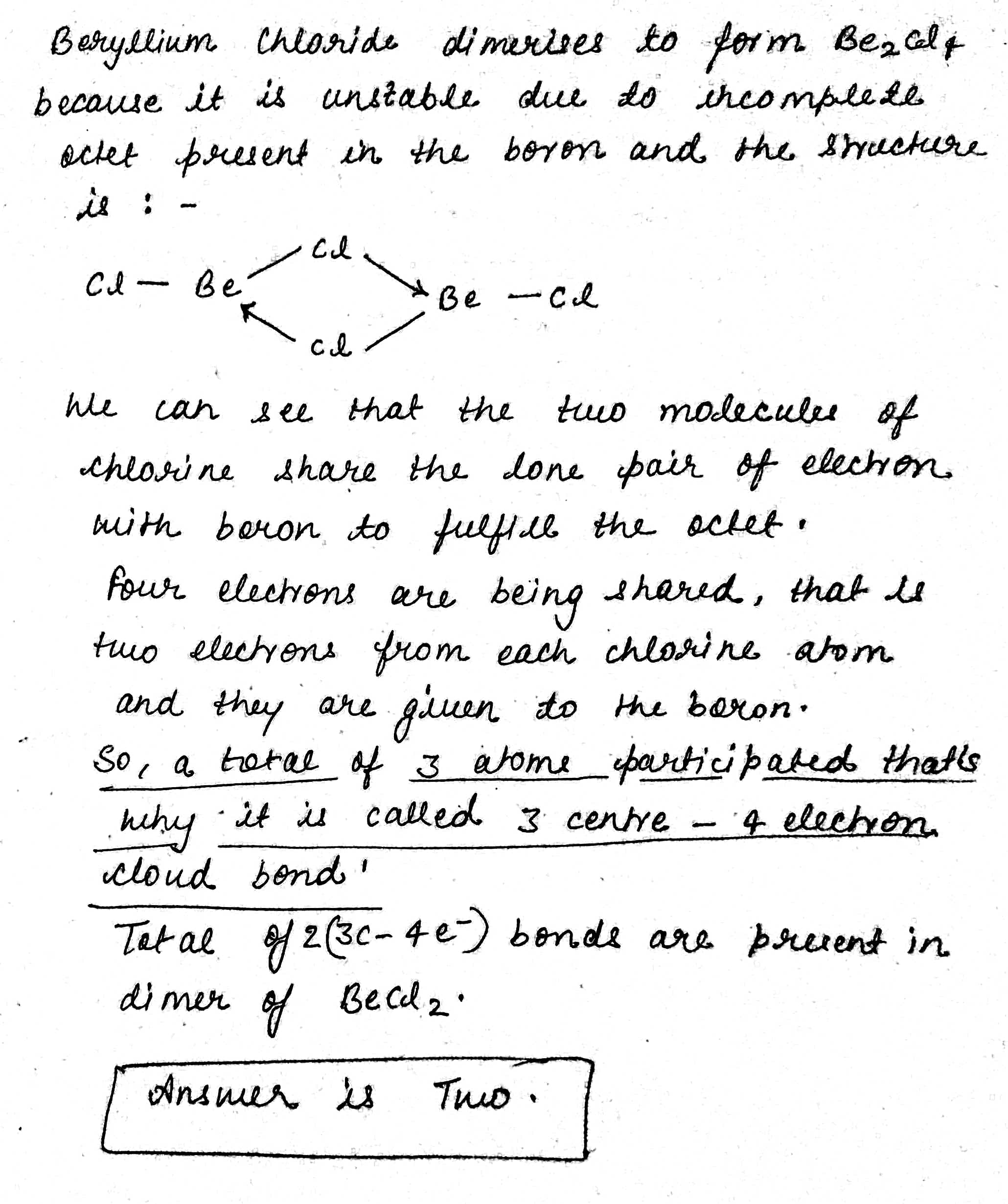
How many $$3C - 4e^- $$ bonds are present in dimer of $$BeCl_2$$ ?
Find the ratio of the total number of bonds to the total number of lone pairs in $$ C_3N_3Cl_3 .$$
Find the ratio between $$ \sigma $$ - bond and $$ \pi $$ - bond in $$ C_3N_3Cl_3 $$.
Find the ratio of $$ \sigma $$ -bonds to $$ \pi $$ -bonds in $$ P_5O_{16} ^{7-} $$.
Explain the following with relevant reason.
The $$B-X$$ distance is shorter than what is expected theoretically in $${BX}_{3}$$ molecule $$(X=Cl, F, Br, I)$$?
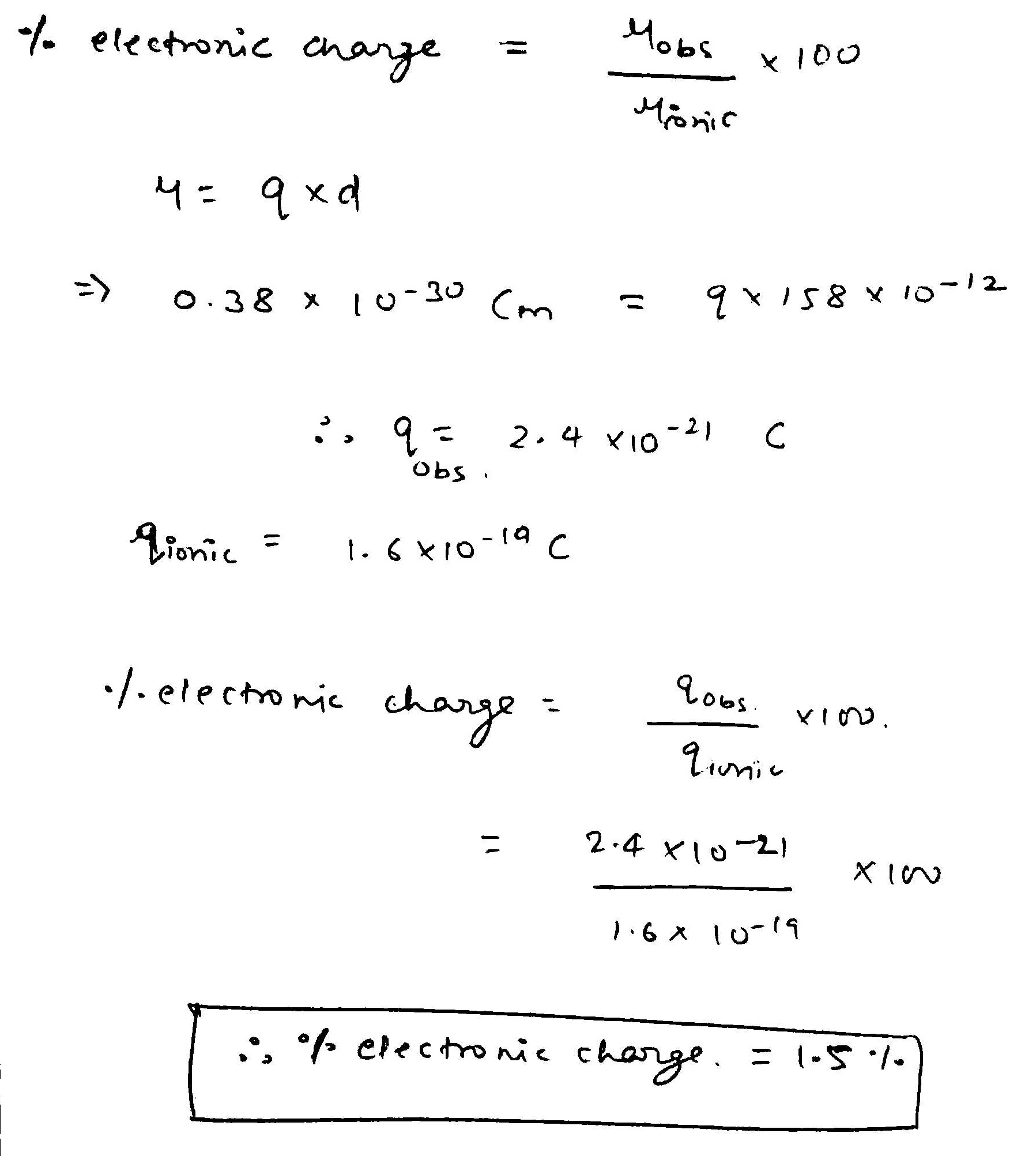
Dipole moment of certain diatomic molecule X-Y is 0.38 D. If the X-Y distance is 158 pm, the percentage of electronic charge developed on X-atom is:
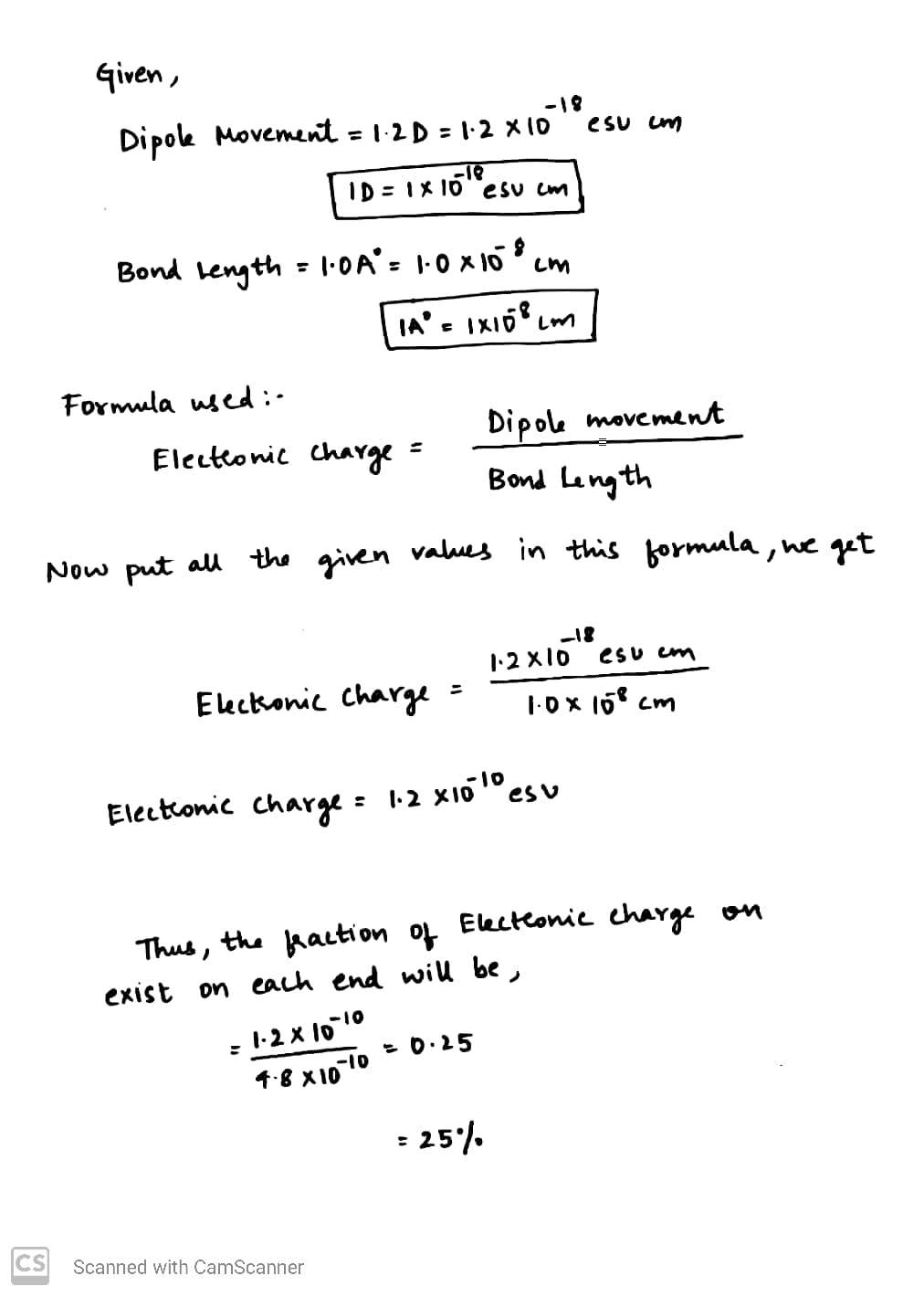
A diatomic molecule has a dipole moment of $$1.2$$D. If its bond distance is $$1.0\overset{o}{A}$$, what fraction of an electronic charge, e, exists on each atom? ($$1 D=10^{-18}$$ esu cm and $$e=4.8\times 10^{-20}$$ esu).
Account for the following:
The bond angle in $${NH_4}^+$$ is higher than $$NH_3$$.
An oxyacid of phosphorus is triprotic in nature. Identify the compound and draw its electron dot formula.
Taking the $$HOH$$ bond angle in $$H_{2}O$$ molecule as $$105^\circ,$$ calculate the charge on oxygen atom. Given that the dipole moment of $$H_{2}O$$ molecule is $$1.84\,D$$ and $$O-H$$ bond distance is $$0.94 \,\mathring {A}.$$
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions