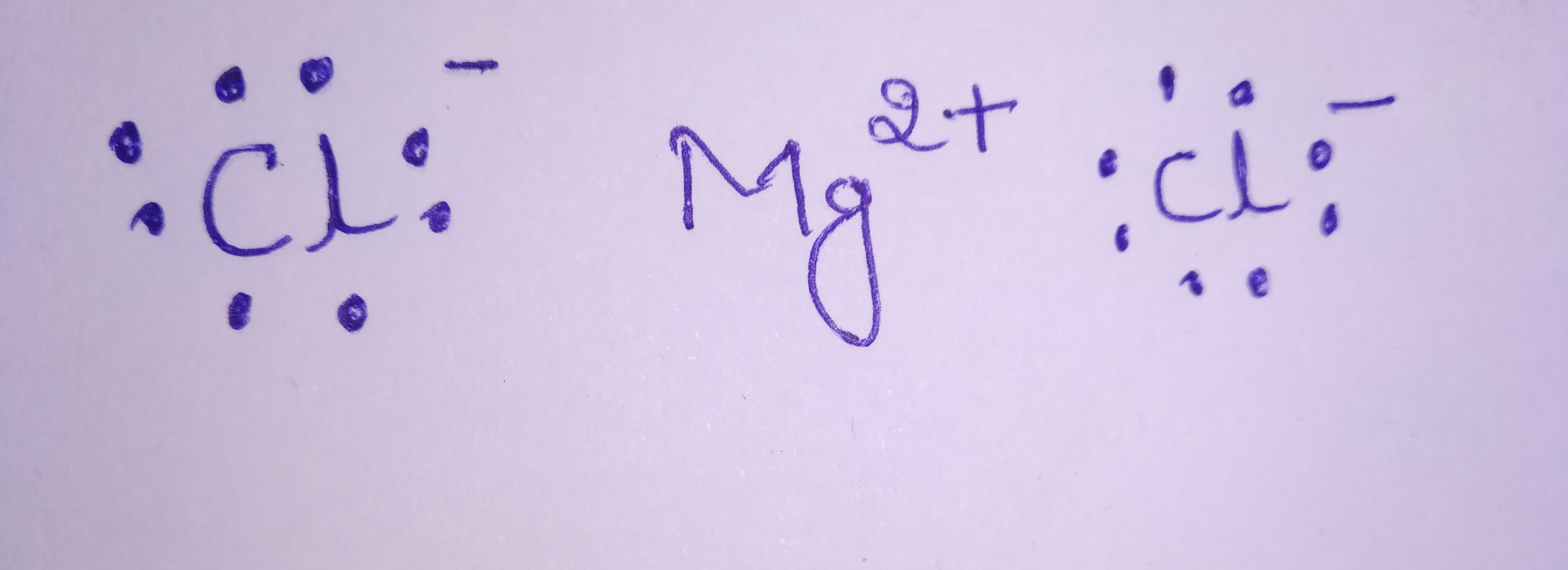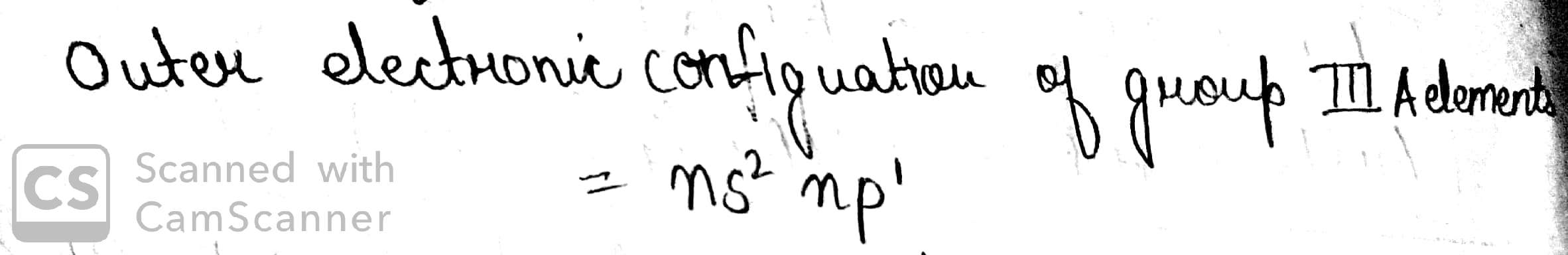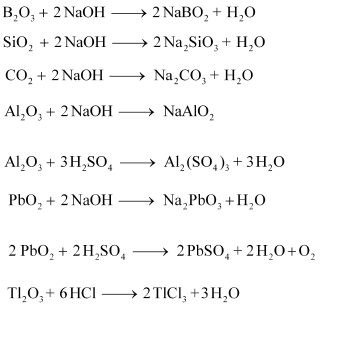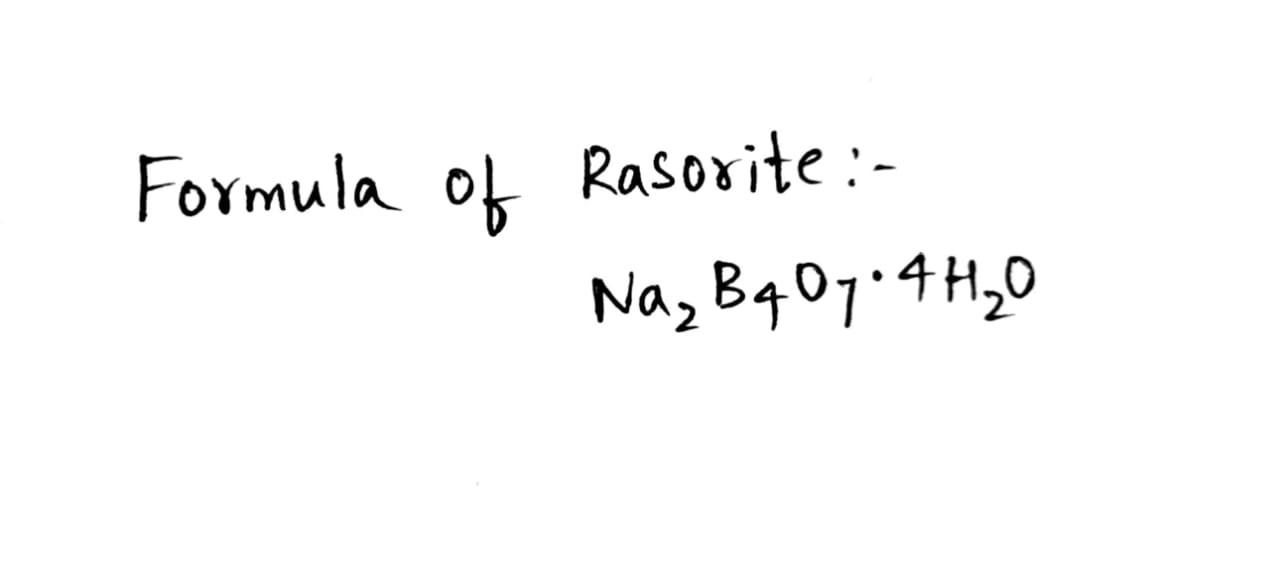The P-Block Elements - Class 11 Medical Chemistry - Extra Questions
Write two point of dissimilarities between boron and aluminium.
Match List I with List II.
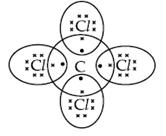
An element X is placed in groupState the formula and the nature of bonding in its chloride. Draw its electron dot structure of its chloride.
If true enter 1, else enter 0.
Although both carbon and silicon are in group IVA(14), very few Si - Si bonds are known.
Carbon is an essential element in the molecule on which life is based but silicon is only satisfactory.
If true enter 1, else enter 0.
The oxidation state of carbon in ionic carbide is $$x$$. The value of $$x\:+\:10$$ is :
Match the compounds given in list I with the oxidation state of their carbon given in list II.
State whether the following statement is true or false:
Tetra covalent nature of the element with following valence shell electronic configuration is : $$1s^2$$ $${2s}^2$$ $${2p}^2$$ explain on the basis of excitation of $$2s$$-electrons to $$2p$$-orbital.
Give the formula of a compound (given in list II) in which carbon exhibits an oxidation state of (given in list I):
Out of the elements $$C, S, Ge, Sn$$ and $$Pb$$, only $$+4$$ oxidation state is shown by how many element(s)?
How many elements in group $$14$$ are used as semiconductor?
Write the equation for the action of heat on a aluminium hydroxide.
Inert pair effect is shown by how many elements?
$$Ga, Al, Tl, Pb, Sn, Ge$$
The inert pair effect is the tendency to form ions two units lower in charge than expected from the group number of heavier $$p$$ - block elements.
If the statement is true enter 1 else enter 0.
If the statement is true enter 1 else enter 0.
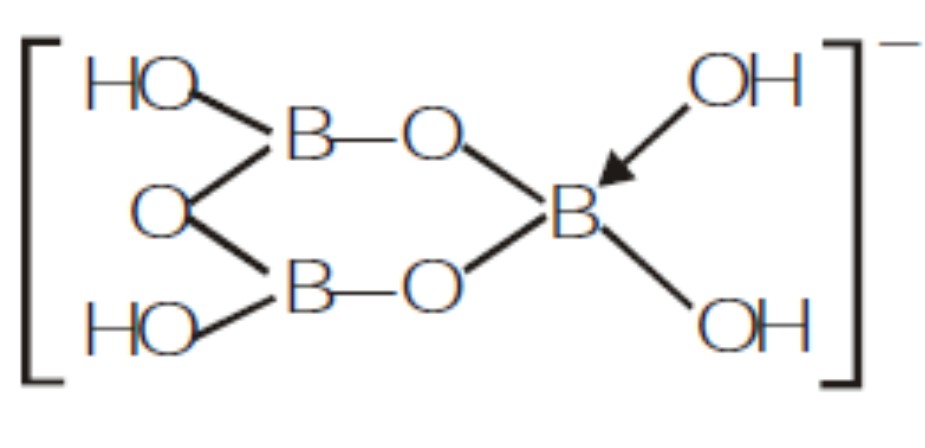
Draw the structure of following compound.$$[B_3O_3(OH)_4]^-$$
$$SiCl_4\xrightarrow {H_2O}X\xrightarrow {1000}Y$$
In the above reaction, how many oxygen atom(s) is/are present in X and Y ?
[If answer is 6 and 4, represent as 64]
In silica, $$SiO_2$$, each silicon atom is bonded to how many oxygen atom?
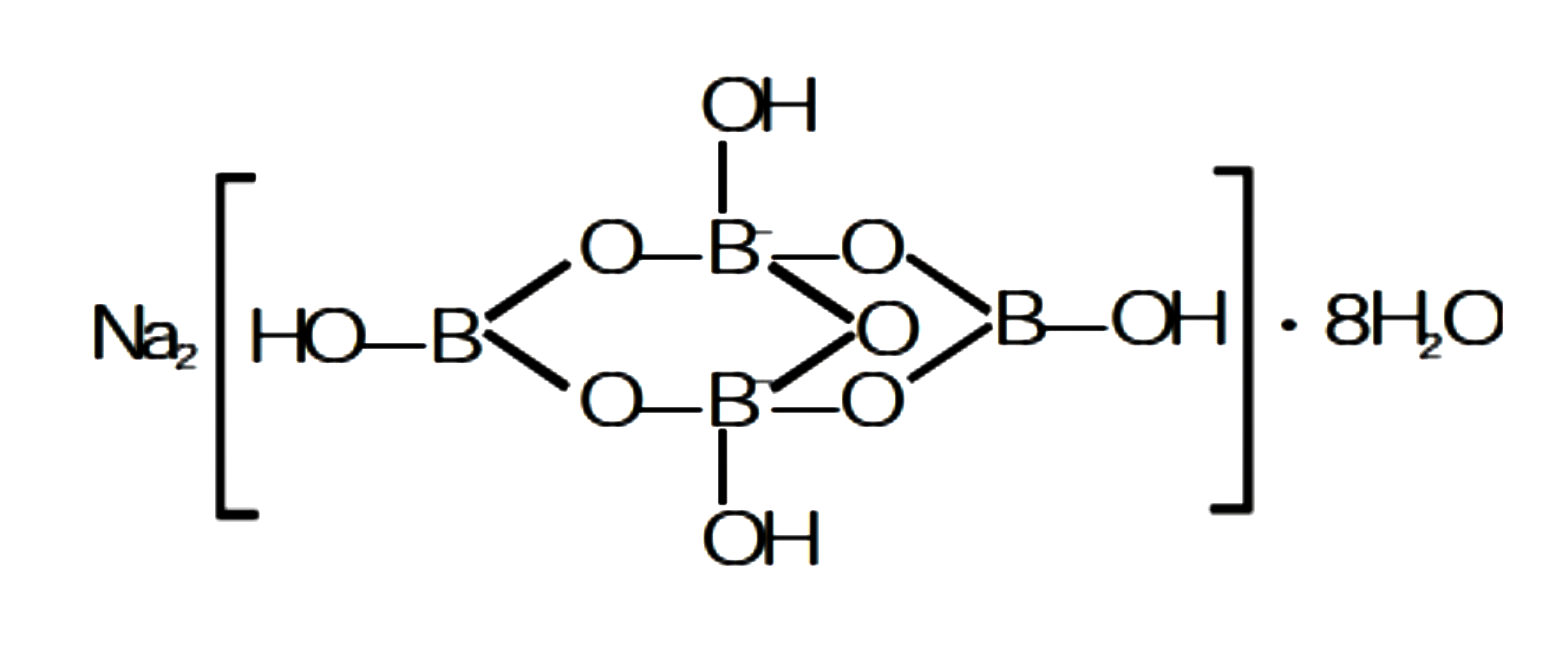
Draw the structure of following compound.
$$Na[B_4O_5(OH)_4].8H_2O$$
$$Na[B_4O_5(OH)_4].8H_2O$$
Match the following and select the correct option:
Sulphuric acid formula is [$$H_{2}SO_{3}$$]. If this is true enter 1 , if false enter 0.
Silicon is the most abundant element on earth
If this is true enter 1, if false enter 0.
Explain why is there a phenomenal decrease in ionization enthalpy from carbon to silicon?
How would you account the lower atomic radius of $$Ga$$ as compared to $$Al$$.
$$BF_3$$ dimerizes to form stable molecule.
Enter 1 if true else enter 0
Discuss the pattern of variation in the oxidation states of (i) $$B$$ to $$Ti$$ and (ii) $$C$$ to $$Pb$$.
Why is $$B_2$$ paramagnetic whereas $$C_2$$ diamagnetic?
The valencies of p block elements are equal to group number or 8 minus the group number. Therefore they show one lower valency and one higher valency. However, if we go to the bottom elements of any group, the compounds with lower valency are predominant whereas higher valency becomes rather uncommon. Explain.
Describe an activity which provides the evidence for
a) the motion of particles
b) attraction between particles
c) inter-particle space
What is inert pair effect?
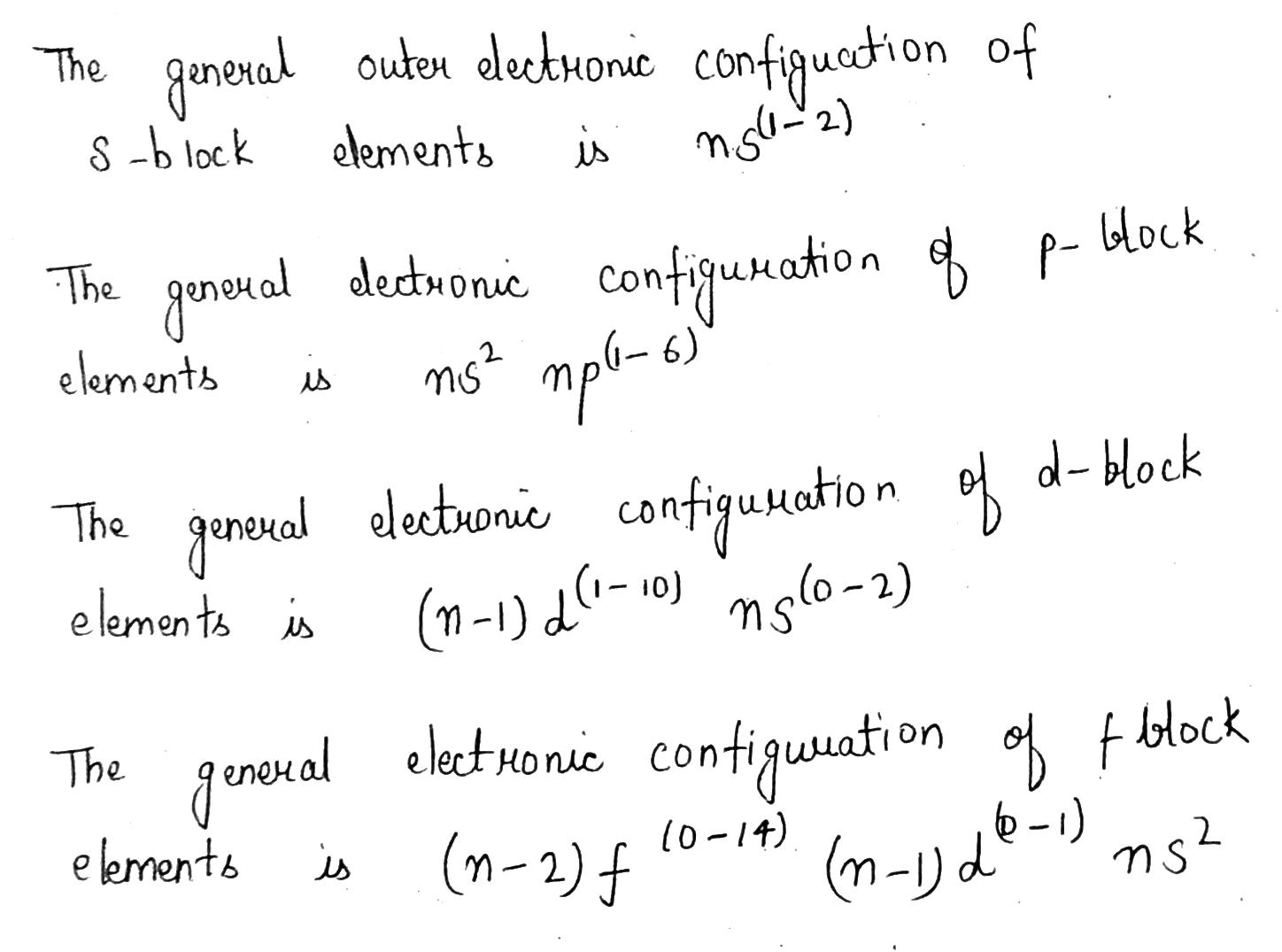
Write the general valence shell electronic configuration of p-block elements.
Based on M.O.T, explain the electronic configuration of the Hydrogen molecule and $$B{e_2}$$.
Draw electron dot representation for the formation of magnesium chloride.
Why does boron not form $${B}^{3+}$$ ion?
Name one metalloid of period 3.
Carban and silicon are mainly tetravalent but $$Ge,Sn$$ and $$Pb$$ show divalency.
$$CO_{2}$$ is gas, while $$SiO_{2}$$ is a solid at room temperatue. State a reason for it.
Why the compounds of $$Tl^+$$ are much more stable than those of $$Tl^{3 +}$$ ?
Mention the variation of atomic radii among the group 13 elements. Explain why ?
Which type of bond is formed in $$BF_{3}$$?
What is the reduction power of the hydrides of group 14 elements in increases order?
Write about the nature of carbon atom
The aluminium metal is obtained by the reduction of aluminium oxide with:
What are interhalogen compounds? Explain with two examples.
The element with atomic number 36 belongs to______ block in the periodic table.
Action of heat on aluminium hydroxide.
Which of the following is the most basic : $$Al_2O, Tl_2O_3, Tl_2O.$$
Account for the following
The tendency to form p$$\pi $$-p$$\pi $$ bonds of carbon is strong. Why $$Pb{ O }_{ 2 }$$ is a strong oxidizing agent than $$Sn{ O }_{ 2 }$$.
Fill in the blanks:
__________ is present in both living and non-living things.
How is boron absorbed by plants?
Give reasons for the following three statements.
a) Al is resistant to air and water
b) Boron halides are covalent in nature
c) $$PbCl_2$$ is more stable than $$PbCl_4$$
Write four characteristic properties of p - block element.
Write the general electronic configuration of s, p, d and f-block elements.
Fill in the blank:
Compounds that formally contain $$Pb^{4+}$$ are easily reduced to $$Pb^{2+}$$. The stability of the lower oxidation state is due to __________.
Does Ga has $$+2$$ oxidation state in $$GaCl_2$$?
Answer the following
Identify the most abundant metal in the earth's crust and enter the group number it belongs to.
Among group $$14$$ elements name:
The element having highest tendency to from $$p \pi -p \pi$$ bonds.
Lead prefers to form divalent compounds. Give reason.
Give reason for each of the following:
Why $$Ge, Sn$$ and $$Pb$$ show divalency?
Among group $$14$$ elements name:
The element which is commonly found in $$+2$$ oxidation state.
Answer the following
Name the element of group $$13$$ which forms the most stable compounds in $$+1$$ oxidation state.
Answer the following
What is the outer electronic configuration of group IIIA elements?
Explain the following with proper reasoning.
$$PbO$$ does not dissolve in $$H_2SO_4$$ while $$SnO$$ is soluble in $$H_2SO_4$$.
What is the oxidation state of iodine in $$ H_5IO_6? $$
Explain the following with relevant reason.
Although the ionisation potential of boron $$(8.30 eV)$$ is less than gold $$(9.22 eV)$$, yet former is a non-metal while the latter is a metal.
Three pairs of compounds are given below. Identify that compound in each of the pairs which has group 13 element in more stable oxidation state. Give reason for your choice. State the nature of bonding also
(a)$$TlCl_3$$, $$TlCl$$
(b)$$AlCl_3$$, $$AlCl$$
(c)$$InCl_3$$, $$InCl$$
Describe the general trends in the following properties of the element in group 13 and 14
(a) Atomic size
(b)Ionization enthalpy
(c)Metallic character
(d)Oxidation states
(e)Nature of halides
In group $$15$$ elements, the number of unpaired electrons in valence shell is:
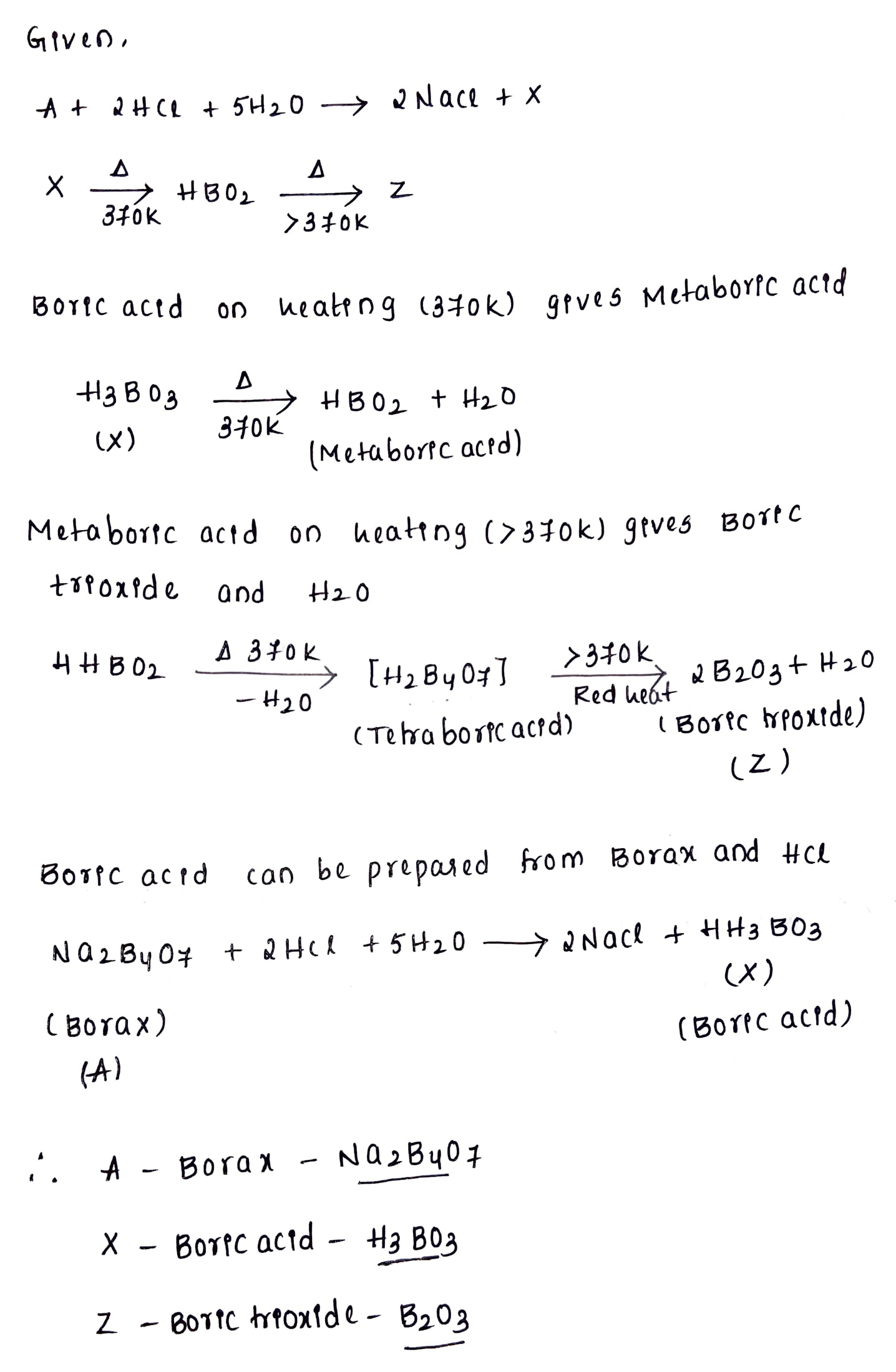
Identify the compounds A, X, and Z in the following reactions:
(a) $$A+2HCl +5H_2O \rightarrow 2NaCl +X$$
$$X\xrightarrow [ 370K ]
{ \Delta } HBO_2\xrightarrow [>370K] {\Delta}Z$$
Compare the properties of boron and aluminium under the following heads
Electronic configuration
What happens when water reacts with the following compound?
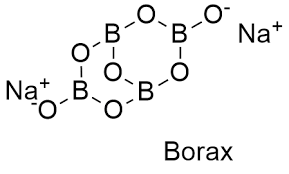
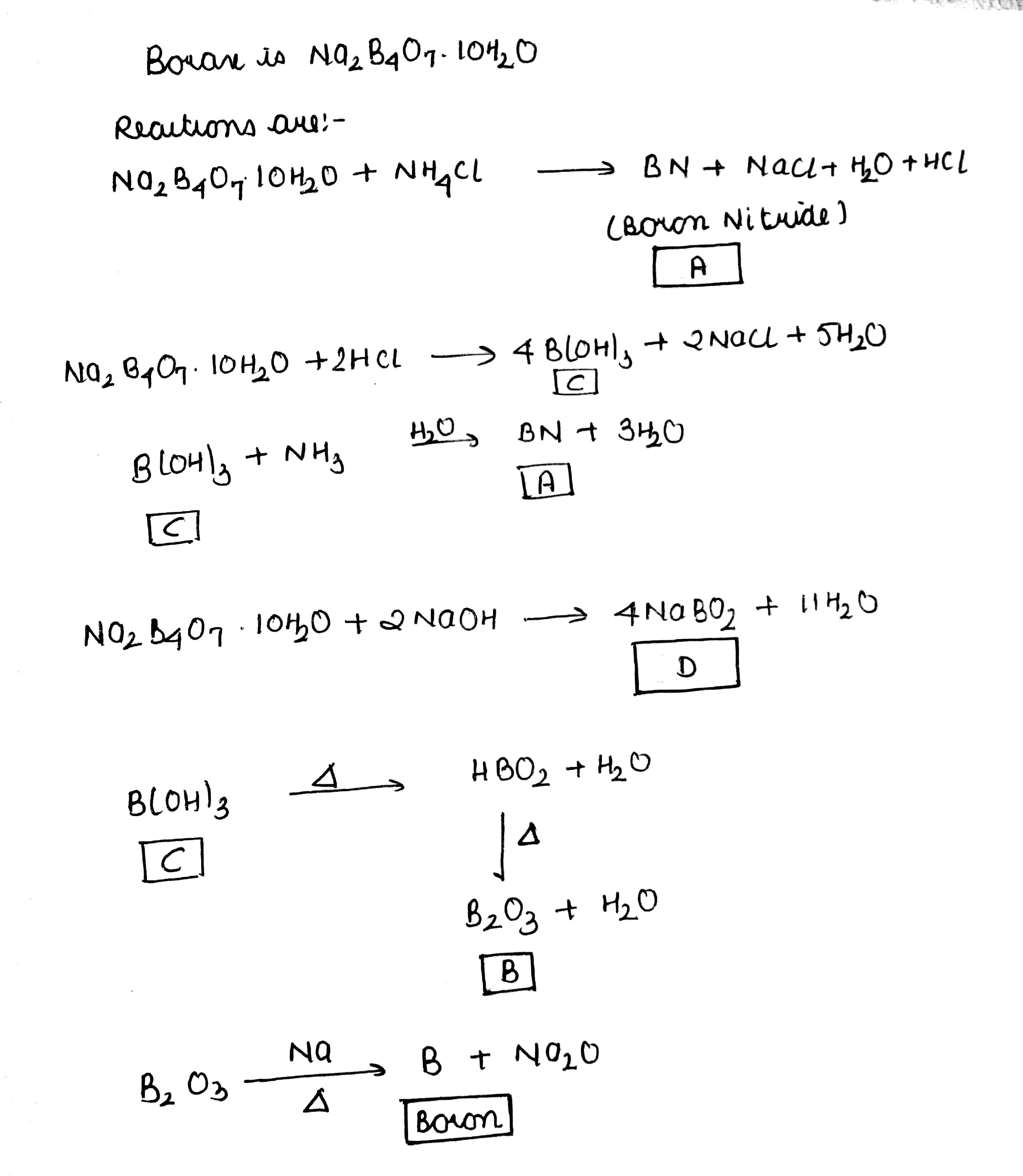
Borax
Borax
The number of unpaired electrons in the valence shell of the members of oxygen family is:
Compare the properties of boron and aluminium under the following heads
Nature of chlorides
Compare the properties of boron and aluminium under the following heads:
Nature of oxides
Name the various forms of carbon found on the Earth. How the carbon cycle may get disrupted.
Account for the following:
a. Ozone is thermodynamically unstable.
b. Solid $$PCl_{5}$$ is ionic in nature.
c. Fluorine forms only one oxoacid $$HOF$$.
How would you account for the following:
Both $$O_{2}$$ and $$F_{2}$$ stabilise higher oxidation states but the ability of oxygen to stabilise the higher oxidation state exceeds that of fluorine.
What happens when
a. Chlorine gas is passed through a hot concentrated solution of $$NaOH$$?
b. Sulphur dioxide gas is passed through an aqueous solution of $$Fe(III)$$ salt?
Account for the following:
a. $$SnCl_{4}$$ is more covalent than $$SnCl_{2}$$.
b. Tendency to form pentahalides decreases down the group in group $$15$$ of the periodic table.
Explain the following observations:
a. Oxygen is a gas but sulphur a solid.
b. Despite having greater polarity, hydrogen fluoride boils at a lower temperature than water.
c. The halogens are coloured.
Which of the following options is correct for the given statement:
Sand is a compound of
silicon and nitrogen
silicon and oxygen
oxygen and sulphur
none of the above
How does carbon attain a stable electronic configuration ?
Explain each of the following:
a. Nitrogen is much less reactive than phosphorus.
b. The stability of $$+5$$ oxidation state decreases down group $$15$$.
c. The bond angles $$(O-N-O)$$ are not of the same value in $$NO_{2}^{-}$$ and $$NO_{2}^{+}$$.
Explain the properties of carbon.
Give different forms in which carbon occurs in nature.
Write the characteristics of p-block elements.
Fill in the blanks.
The name 'carbon' is derived from the Latin word __________.
Write name.
The oxide that forms salt and water by reacting with both acid and base.
What is tincal? Give its chemical formula.
Why does boron form stable electron deficient compounds?
Why is boron metalloid?
In which pure form does carbon exists in nature?
Name two allotropes of boron.
Name two isotopes of boron
Complete the following
(i) $$ B + O_2 \rightarrow$$
(ii) $$ B + N_2 \rightarrow$$
(iii) $$ B + Cl_2\rightarrow$$
(iv) $$ BF_3 + NH_2\rightarrow$$
(v) $$ Na_2B_4O_7 + H_2O\rightarrow$$
(vi) $$Na_2B_4O_7\rightarrow$$
Which type of bonds are formed by boron and why?
Why is crystalline boron hard solid?
Name the oxidation states exhibited by $$Ge, Sn$$ and $$Pb$$.
Mention the state of hybridization of $$B$$ in $$BH_{4}^{-}$$
What is state of hybridisation of $$C$$ in $$CO_{3}^{2-}$$?
What is name give to elements which are neither metals nor non-metals?
Mention the chief reason for the anomalous behaviour of boron in group $$13$$ of the periodic table.
Write the state of hybridization in $$BF_3$$.
Carbon and Silicon are mainly tetravalent but $$Ge, Sn$$ and $$Pb$$ show divalency.
Give reason.
Which one of the following elements $$+1$$ oxidation state as well: $$Al, B, Ca, Tl, Be$$?
What is the oxidation state of $$Ni$$ in $$[Ni(CO)_4]$$?
Why does boron form electron deficient compounds?
What name is given to the compounds formed by more electropositive elements with carbon?
In terms of atomic size, ionization energy and charge, explain why are ionic compounds containing $$B^{3+}$$ not formed.
Give the chemical reaction as an evidence for each of the following observation?
$$\cdot$$ Tin (II) is a reducing agent whereas lead (II) is not
$$\cdot$$ Gallium (I) undergoes disproportionation reaction.
State the trends observed in case of following: Oxidation state of the elements of Group $$14$$.
As we move down in group $$13$$ elements increase in atomic size is comparatively very less. Explain.
Describe two similarities and two dissimilarities between $$B$$ and $$Al$$.
What is the oxidation state of $$C$$ in
(a) $$CO$$
(b) $$HCN$$
(c) $$H_2CO_3$$
(d) $$CaC_2$$.
What happens when a borax solution is acidified? Write a balanced equation for the reaction.
Arrange the following compounds in decreasing order of property indicated against each. Give reason for your answer : $$BCl_3, AlCl_3, GaCl_3, InCl_3, TiCl_3$$ (Stability of $$+3$$ oxidation state)
$$C$$ and $$Si$$ are always tetravalent but $$Ge, Sn, Pb$$ show divalency.
Mention the nature of an aqueous solution of borax.
How does electron deficient compound $$BF_3$$ achieve electronic saturation i. e., the fully occupied outer electron shells?
Explain the oxidation property of group $$14$$ elements.
Give the difference in structure of the following pair of compounds : $$CO_2$$ and $$SiO_2$$.
What is the electronic configuration of p-block elements ?
What are allotropy? Mention allotropy of $$Pb, Sn, Ge, Si$$.
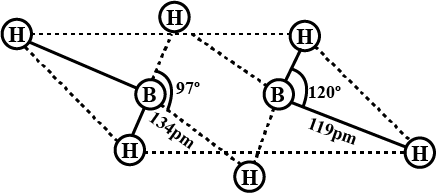
Compound $$X$$ on reduction with $$LiAH4$$ gives a hydride $$Y$$ containing $$21.72$$% hydrogen along with other products. The compound $$Y$$ reacts with air explosively resulting in boron trioxide. Identify $$X$$ and $$Y$$. Give balanced equations involved in the formation of $$Y$$ and its reaction with air. Draw the structure of $$Y$$.
How does metallic character vary among the group $$14$$ elements? How is it related to ionisation energy?
A compound $$'X'$$ or reduction with $$LiAH4$$ gives a hydride $$'Y'$$ that contains $$21.72$$% hydrogen along with other products. The compound $$'Y'$$ reacts with air explosively resulting in boron trioxide. Identify $$'X'$$ and $$'Y'$$. Give balanced reactions involved in the formation of $$'Y'$$ and its reactions with air. Draw the structure of $$Y$$.
Explain the oxidation state of carbon in $$Be_2 \ C$$ and $$Al_4 \ C_3$$.
Write the general electronic configuration of p-block elements.
Explain the following:
(i) Catenation
(ii) Inert Pair Effect
(iii) Allotropy.
Explain the various oxidation states found in group 14 elements.
Why Boron forms electron deficient compounds?
Analyze the chemical equation and find the reason for your observation.
$$C^0+2H_2{+1}S^{+6}O_4^{-2} \rightarrow C^{+4} O_2^{-2}+2H_2^{+1}O^{-2}+2S^{+4}O_2^{-2}$$
What is the oxidation state of element carbon?
Analyze the chemical equation and find the reason for your observation.
$$C^0+2H_2{+1}S^{+6}O_4^{-2} \rightarrow C^{+4} O_2^{-2}+2H_2^{+1}O^{-2}+2S^{+4}O_2^{-2}$$
What about the carbon in carbon dioxide?
Why the melting point of Boron is unexpectedly high?
Write the stability of +1 oxidation state of group 13 elements in increasing order.
How many electrons are there in the outermost shell of carbon?
Find the position of carbon in periodic table and the complete the table.
| Symbol | ................. |
| Atomic number | ................. |
| No. of electrons in the outermost shell | ................. |
| Valency | ................. |
| Metal/ non-metal? | ................. |
Total number of Boron atoms in anionic part of borax which participate in back bonding.
Fill in the blanks given in list I with items in list II.
Helium can be excited to the $${1s}^{1}{2p}^{1}$$ configuration by light of $$58.44nm$$. The lowest excited singlet state, with the configuration $${ 1s }^{ 1 },{ 2s }^{ 1 }$$ lies $$4857{c}^{-1}$$ below $${1s}^{1}{2p}^{1}$$ state. What would be the average $$He-H$$ bond energy ($$kJ{ mol }^{ -1 }$$) have to be in order that $$He{H}_{2}$$ could form non-endothermically from $$He$$ and $${H}_{2}$$? Assume that the compound would form from the lowest excited singlet state of helium. Neglect any differences between $$\Delta U$$ and $$\Delta H$$. Take $$\Delta {H}_{f}(H)=218.0kj/mol$$.[Give answer in nearest integer]
A certain salt (X) gives the following tests :
(i) Its aqueous solution is alkaline to litmus
(ii) On strongly heating it swells to give a glassy material.
(iii) When concentrated sulphuric acid is added to a hot concentrated solution of (X) , white crystals of a weak acid separate out
Write equations for all the above reactions and identify X, Y, Z.
In some of the reactions thallium resembles aluminium, whereas in others it resembles with group I metals. Support this statement by giving some evidences.
Explain the following reactions.
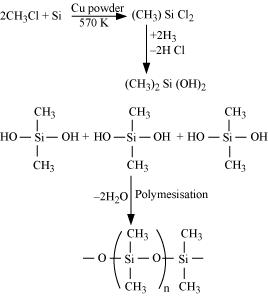
(a) Silicon is heated with methyl chloride at high temperature in the presence of copper.
(b) Silicon dioxide is treated with hydrogen fluoride.
(c) CO is heated with ZnO.
(d) Hydrated alumina is treated with aqueous NaOH solution.
A certain salt $$X$$, gives the following results.
(i) Its aqueous solution is alkaline to litmus.
(ii) It swells up to a glassy material $$Y$$ on strong heating.
(iii) When conc. $$H_2SO_4$$ is added to a hot solution of $$X$$, white crystal of an acid $$Z$$ separates out.
Write equations for all the above reactions and identify $$X, Y$$ and $$Z$$.
(a) Classify following oxides as neutral, acidic, basic or amphoteric:
$$CO,\, B_2O_3,\, SiO_2,\, CO_2,\, Al_2O_3,\, PbO_2,\, Tl_2O_3$$
(b) Write suitable chemical equations to show their nature.
Number of $$B-O-B$$ bond in borax is ________.
Which is most abundant element in $$17th$$ group?
Explain the synthesis of ammonia occurs by Haber's process.
Explain: (i) $${[Si{F}_{6}]}^{2-}$$ is known whereas $${[Si{Cl}_{6}]}^{2-}$$ is not known.
(ii) Boron is unable to form $${[{BF}_{6}]}^{3-}$$ ion
(iii) conc. $$H{NO}_{3}$$ can be stored in Aluminium container.
What happens when
i) White phosphorous is heated with concentrated $$NaOH$$ solution in an inert atmosphere of $$CO_2$$.
ii) $$XeF_6$$ undergoes partial hydrolysis.
Which of the following reacts with water and aqueous solution become acidic $$SiCl_4$$ or $$CCl_{4}$$?
Write down the balanced equations for the reaction when calcium phosphate is heated with a mixture of sand and carbon.
Name the metals with which the following ores/ minerals are associated :
Borax
Match the list I and List II
Explain why the tendency to show-$$2$$ oxidation state diminishes from sulphur to polonium?
An inoganic halide $$(A)$$ reacts with water to form two acids $$(B)$$ and $$(C). (A)$$ also reacts with $$NaOH$$ to form two salts $$(D)$$ and $$(E)$$ which remain in solution. The solution gives white precipitates with both $$AgNO_{3}$$ and $$BaCl_{2}$$ solution respectively. $$(A)$$ is a useful organic reagent.
Answer the following
Which element among group $$13$$ elements has the highest ionisation enthalpy?
Why $$BiH_3$$ is the strongest reducing agent?
Give reason for each of the following:
$$SnCl_2$$ is a solid while $$SnCl_4$$ is a liquid at room temperature.
Starting from borax, how would you obtain?
Boron trioxide
Answer the following
Which has higher ionisation enthalpy?
$$Al \ or \ Ga$$
Answer the following
What is Tincal?
Explain the following with relevant reason.
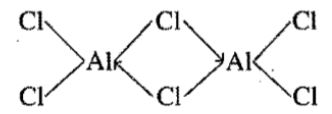
$${AlCl}_{3}$$ forms a dimer but $${BCl}_{3}$$ does not form dimer.
Identify the unknown species and complete the following equation:
$$Na_2B_4O_10H_2O+ conc. H_2SO_4 \xrightarrow [ ]{ Heat } (A)$$
$$Na_2B_4O_10H_2O+ conc. H_2SO_4 \xrightarrow [ ]{ Heat } (A)$$
Describe the preparation, properties, and uses of the following compound
Borax.
A colourless solid $$(A)$$ on hydrolysis produces a heavy white precipitate $$(B)$$. Solid $$(A)$$ gives a clear solution in conc. $$HCl$$; however when added to large amount of water, it again gives precipitate of $$(B)$$. When $$H_2S$$ passed through a suspension of $$(A)$$ or $$(B)$$, a brown black precipitate of $$(C)$$ is obtained. Compound $$(A)$$ liberates a gas $$(D)$$ on treating with $$H_2SO_4$$. The gas $$(D)$$ is water soluble and gives white precipitate $$(E)$$ with solution of mercurous salt but not with mercuric salt. Identify $$(A)$$ to $$(E)$$.
When aqueous solution of borax is acidifies with hydrochloric acid, a white crystalline solid is formed which is soapy to touch. Is this solid acidic or basic in nature? Explain
The +1 oxidation state in group 13 and +2 oxidation state in group 14 becomes more and more stable with increasing atomic number. Explain.
Explain the following with relevant reason.
Why boron does not form $${B}^{3+}$$ ion?
Give formula of the following:
Rasorite
Rasorite
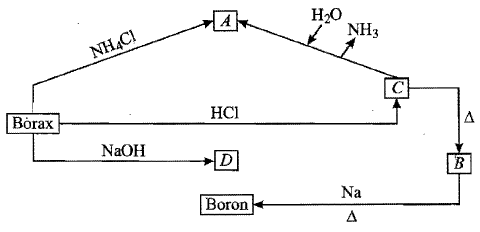
Properties of borax are represented by the following road map. Identify the compounds $$'A'$$ to $$'D'$$ and explain the reaction involved.
What happens when:
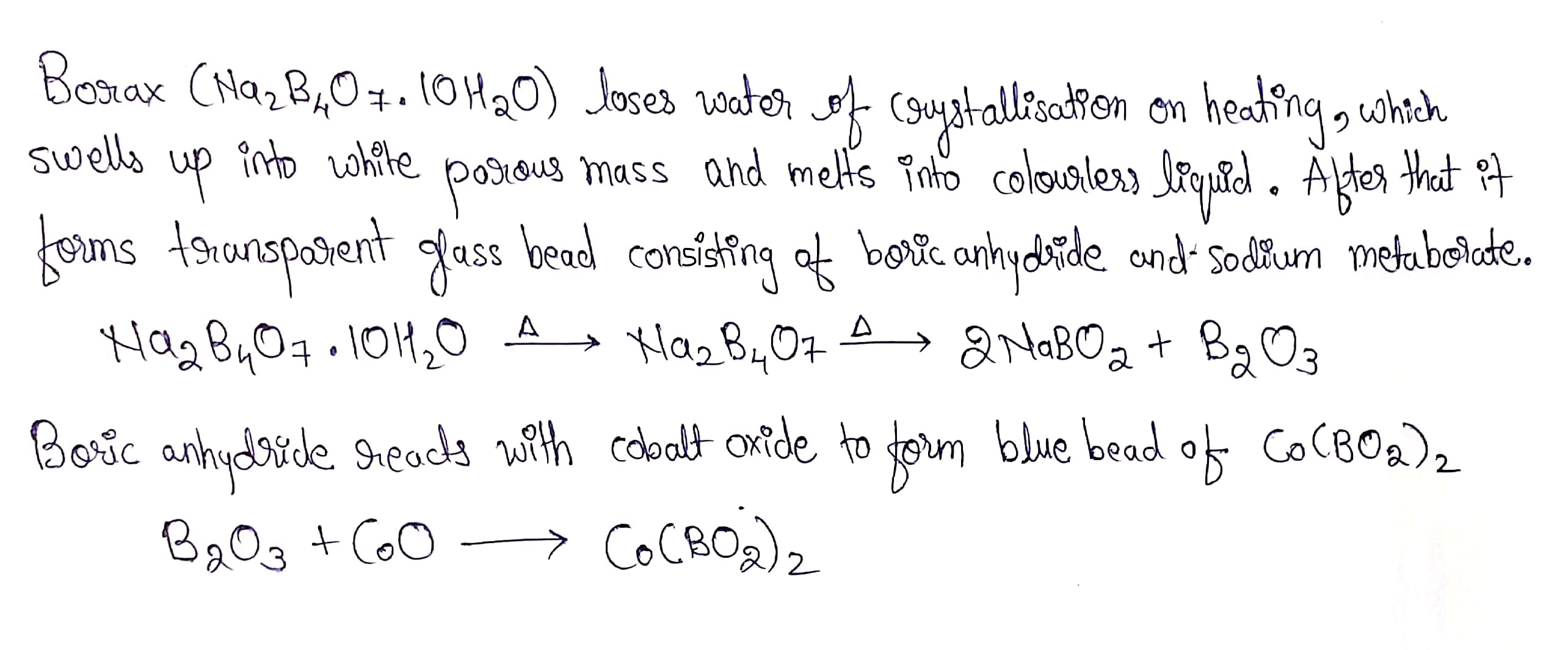
A mixture of borax and cobalt oxide is heated in a flame.
Show the effect of heat on the following commercially important compounds:
Borax, $$Na_2B_4O_10H_2O$$.
Borax, $$Na_2B_4O_10H_2O$$.
A white crystalline solid $$'A'$$ dissolves in water to give an alkaline solution. On heating $$'A'$$ first loses water molecules and swells up. On further heating it turns into a transparent liquid which solidifies into a glassy bead.
Explain the chemistry of borax bead test.
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with —ic suffix.
B. If product is oxy acid with —ous suffix.
C. If product are two oxy acids one with —ic suffix and another one with —ous suffix.
D. If product is not oxy acid, neither with —ic suffix nor with —ous suffix.
$$H_4\underline B_2O_5+H_2O \longrightarrow H_3BO_3$$
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions