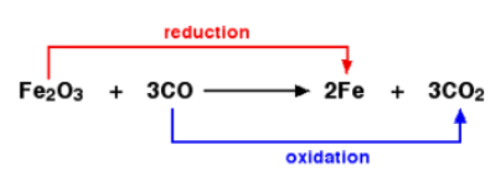
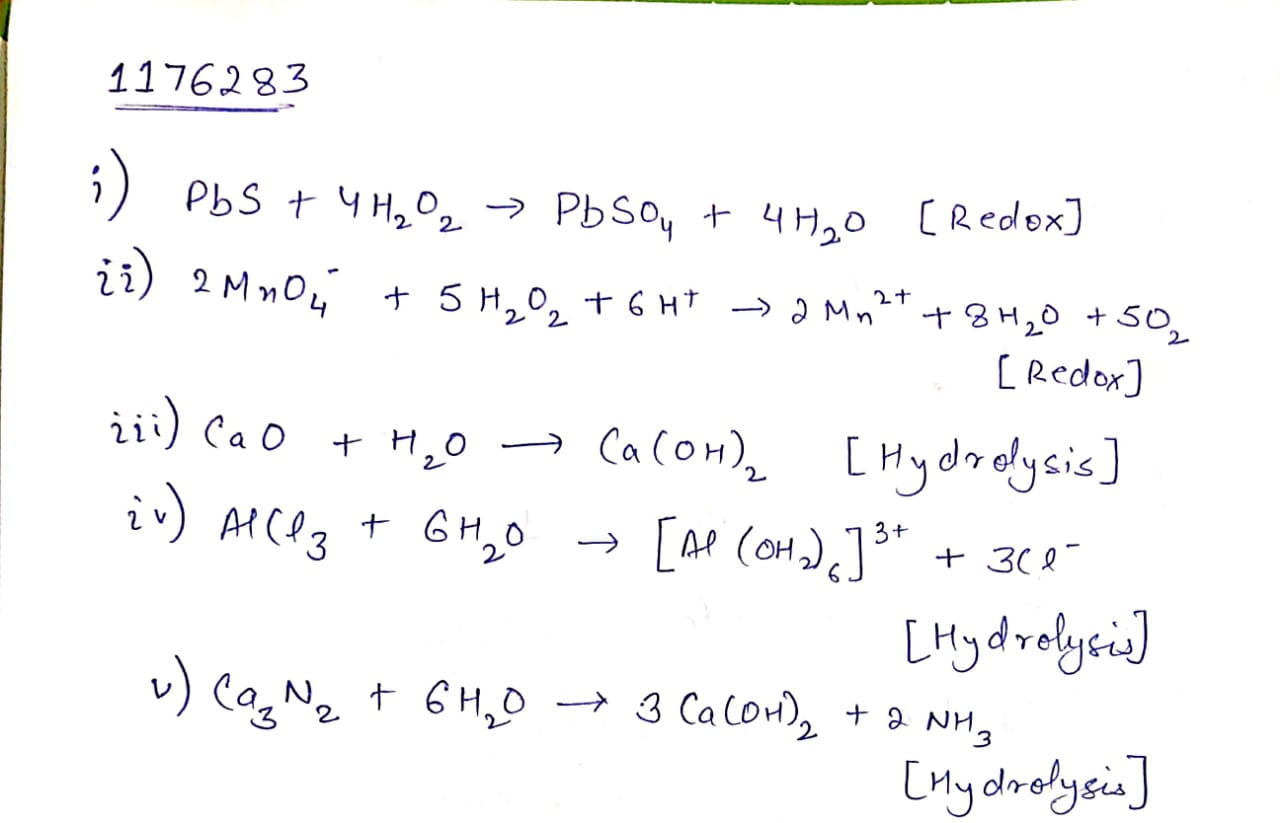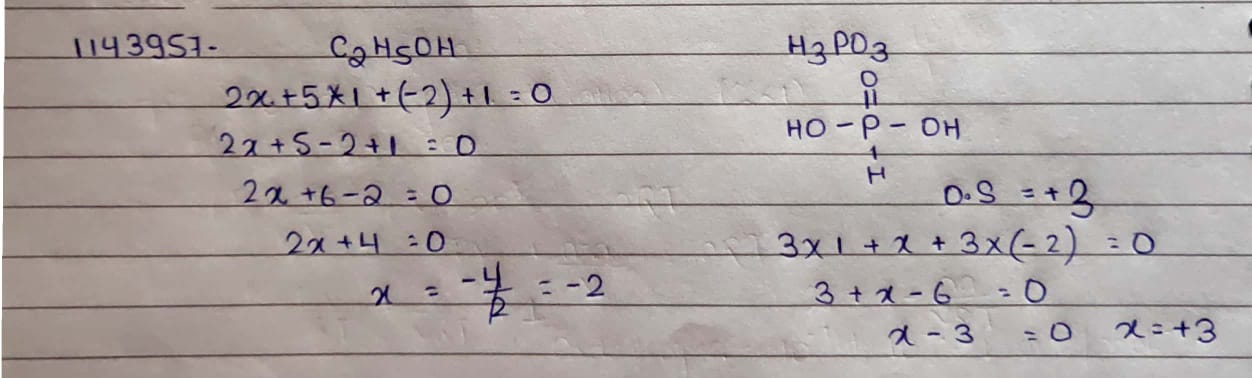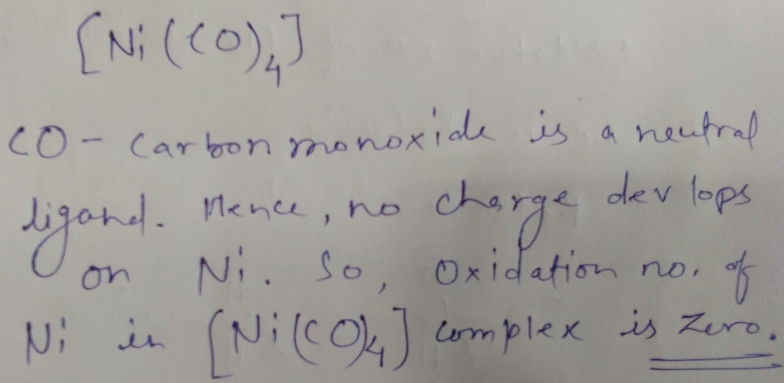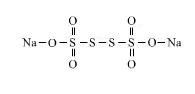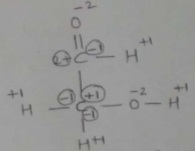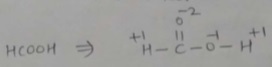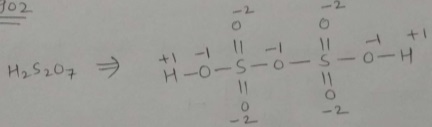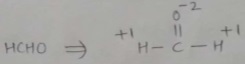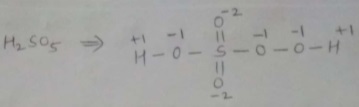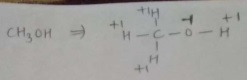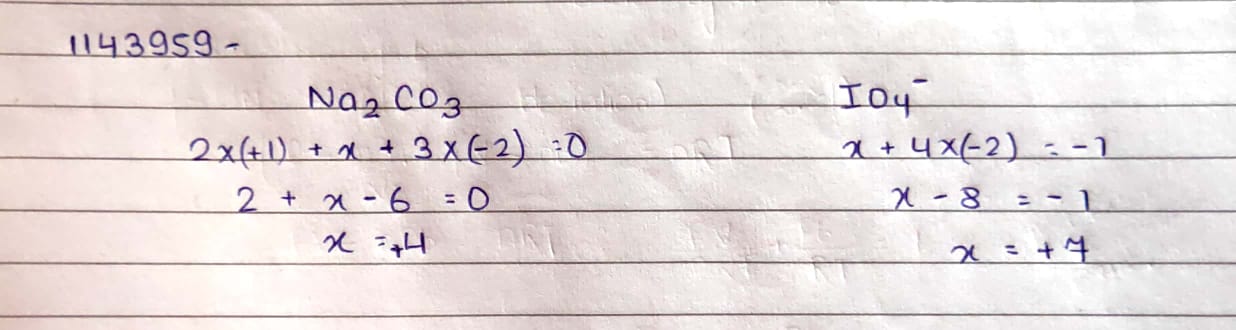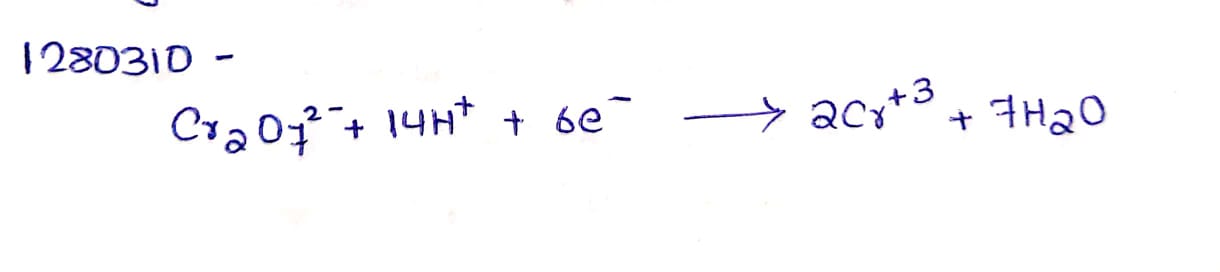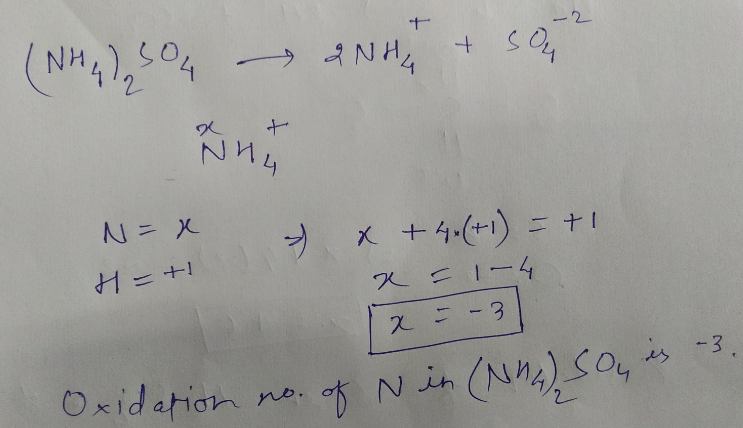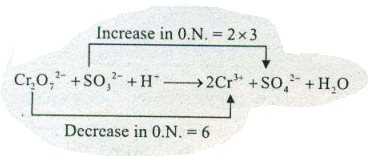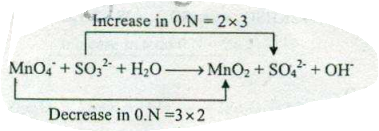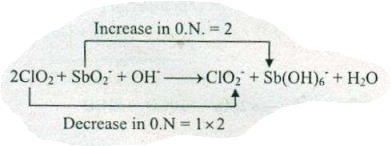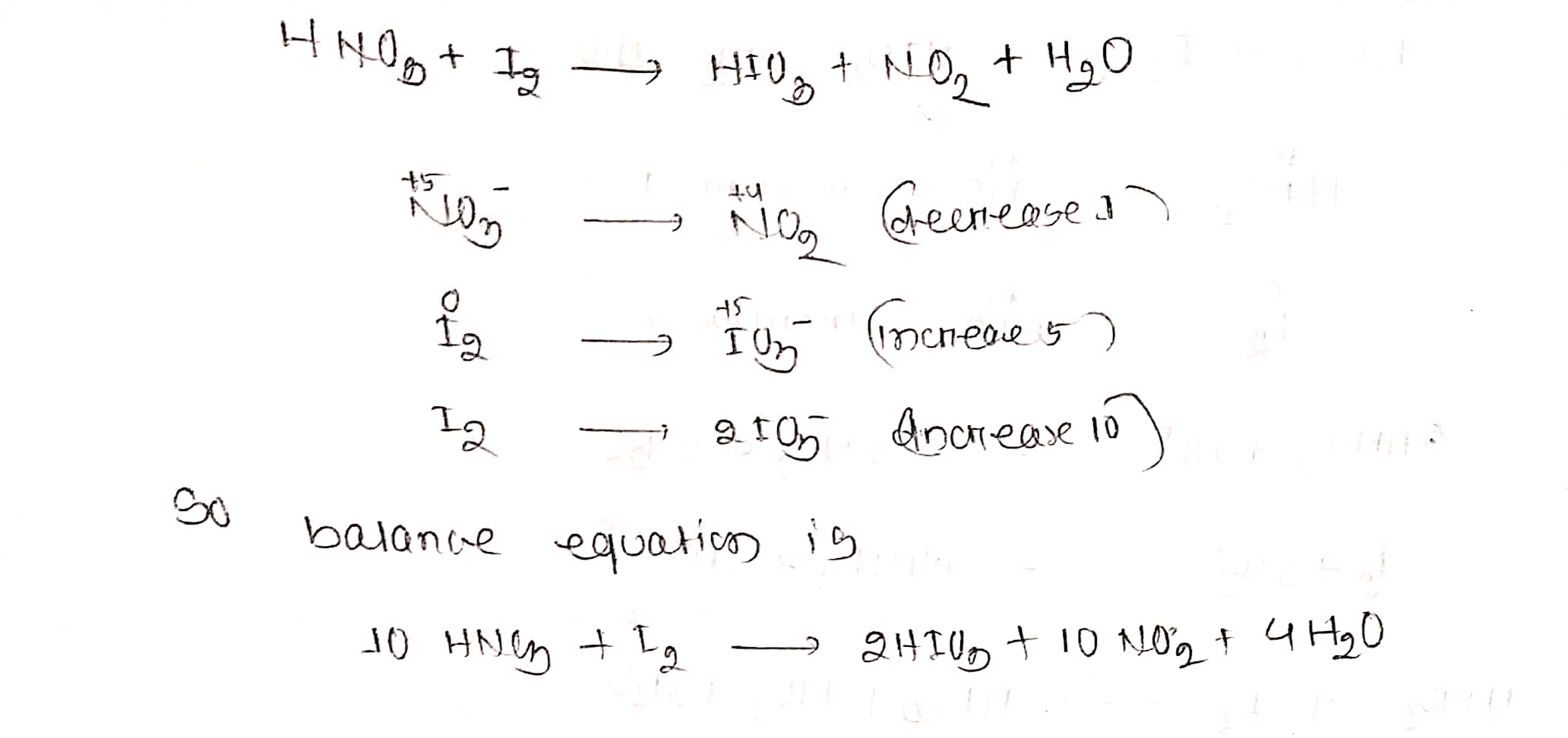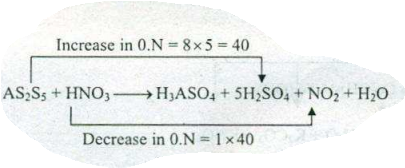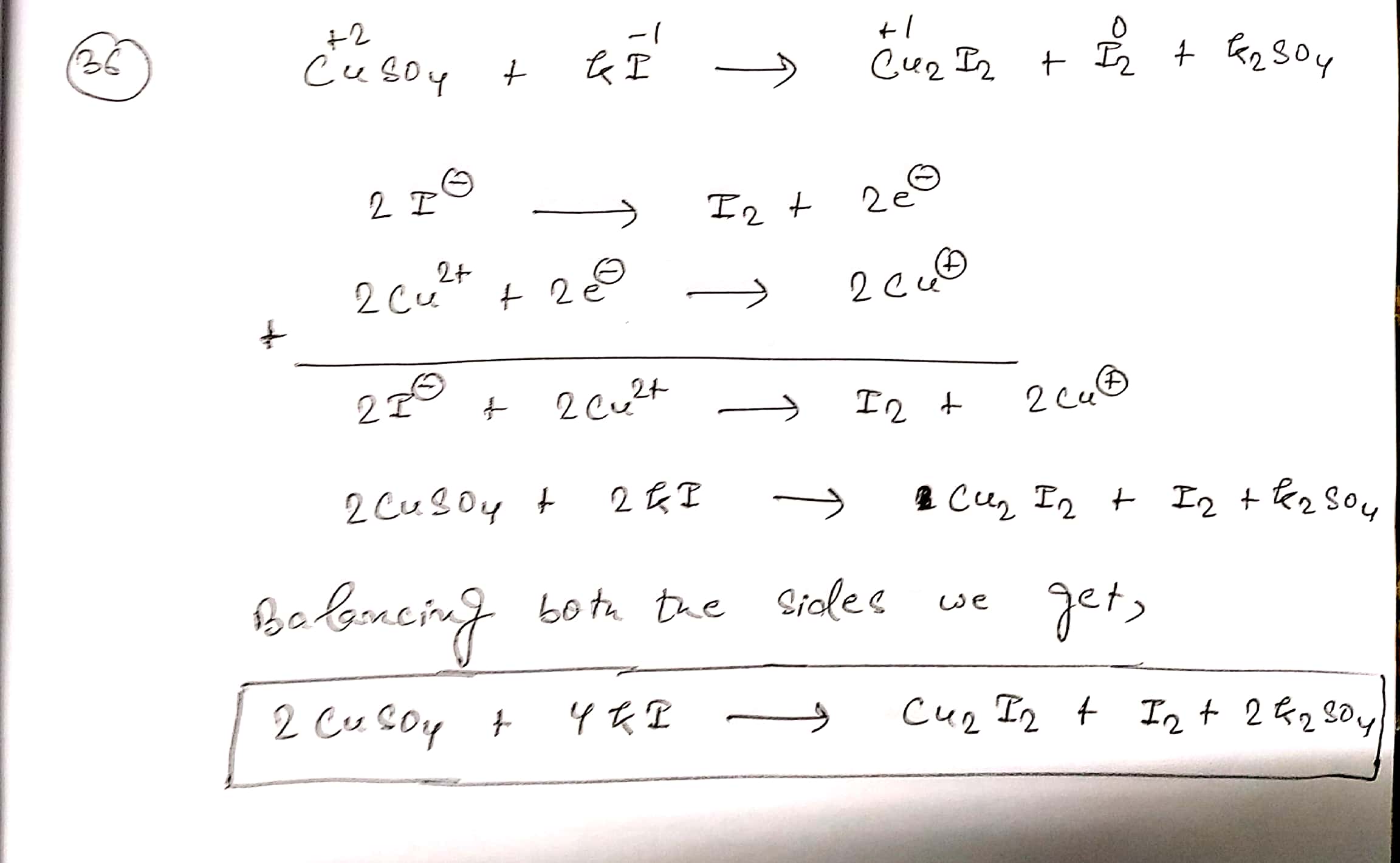Redox Reactions - Class 11 Medical Chemistry - Extra Questions
Arrange the following in the order of increasing oxidation number of chlorine: $$Cl_2O_7, Cl_2O, HCl, ClF_3, Cl_2$$.
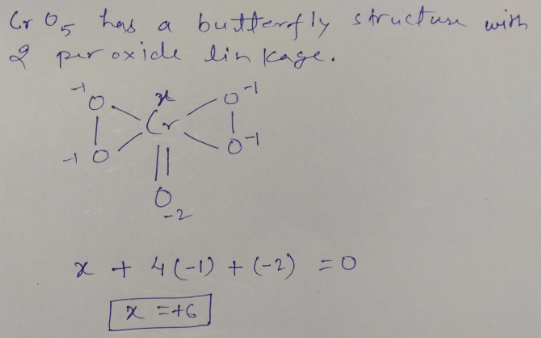
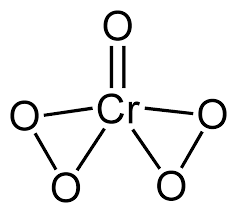
Write oxidation. no of chromium in $$CrO_{5}$$.
Complete the following chemical reactions.
(i) $${ PbS(s) } + { H }_{ 2 }{ O }_{ 2 }(aq)\quad \rightarrow $$
(ii) $${ MnO }_{ 4 }^{ - }(aq) + { H }_{ 2 }{ O }_{ 2 }(aq) \rightarrow$$
(iii) $${ CaO }(s) + { H }_{ 2 }O(g) \rightarrow $$
(Iv) $${ AlCl }_{ 3 }(g) + { H }_{ 2 }{ O }(l) \rightarrow $$
(v) $${ Ca }_{ 3 }{ N }_{ 2 }(s) + { H }_{ 2 }O(l) \rightarrow $$
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reaction.
The sum of the oxidation numbers of both the nitrogen atoms in $${ NH }_{ 4 }{ NO }_{ 3 }$$ is :
Mention the oxidation number of Oxygen in Superoxides.
Find oxidation number of boron in $$NaBH_{4}$$.
Is the following reaction is a redox reaction?$$BaO_{2}+H_{2}SO_{4}\rightarrow BaSO_{4}+H_{2}O_{2}$$
The oxidation number of $$Mn$$ in the product of alkaline oxidative fusion of $$Mn{ O }_{ 2 }$$ is
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$C_2H_5OH$$
$$H_3PO_3$$
Calculate the oxidation number of
$$S$$ in $$Na_{2}S_{4}O_{6}$$
Calculate the oxidation number of
$$Fe$$ in $$Fe_{3}O_{4}$$
Oxidation number of O in $$H_2O_2$$ is:
What is oxidation number of nickel in $$[Ni(CO)_{4}]$$?
Calculate oxidation number of $$S$$ in $${H}_{2}{SO}_{4}$$ and $$C$$ in $${C}_{3}{O}_{2}$$
Calculate the oxidation number of
$$Pb$$ in $$Pb_{3} O_{4}$$
What is an oxidation number or oxidation state ? how is it useful to identify redox reactions, oxidant and reductant ?
Solve;-
Oxidation state of $$Cr.$$ in $$C{r_2}{(S{O_4})_3}$$
Oxidation states of nitrogen in the explosive formed by reaction of ammonia iodine is .
How has this black coloured substance formed ?
Match the List I with List II.
In the following reactions, find the sum of x+y+z:
(i) $$xNa+O_2\rightarrow yNa_2O$$
(ii) $$H_2SO_4+2NaOH\rightarrow Na_2SO_4+yH_2O$$
(iii) $$yH_2O_2\rightarrow 2H_2O+O_2$$
(iv) $$2Al+2H_3PO_4\rightarrow 2AlPO_4+zH_2$$
(v) $$Ca(OH)_2+2HCl\rightarrow CaCl_2+yH_2O$$
(i) $$xNa+O_2\rightarrow yNa_2O$$
(ii) $$H_2SO_4+2NaOH\rightarrow Na_2SO_4+yH_2O$$
(iii) $$yH_2O_2\rightarrow 2H_2O+O_2$$
(iv) $$2Al+2H_3PO_4\rightarrow 2AlPO_4+zH_2$$
(v) $$Ca(OH)_2+2HCl\rightarrow CaCl_2+yH_2O$$
$$SO_2$$ reduces $${Cr_2O_7}^{2-}$$ to $$Cr^{2+}$$. The change in oxidation number of $$Cr$$ is:
When $${SO}_{2}$$ gas is passed into aqueous $${ K }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }$$ solution (orange), it changes to green coloured solution. What is the oxidation number of the species that has this green color?
The oxidation state of "C" in diamond is :
What is the oxidation number of Mg in magnesium nitride?
The brown ring complex $$[Fe(H_2O)_5NO^+]SO_4$$ has oxidation number of Fe as :
On combustion of $$CH_4$$ to $$CO_2$$ and $$H_2$$, the oxidation number of carbon changes by :
Number of peroxy linkage in $${ K }_{ 3 }Cr{ \left( { O }_{ 2 } \right) }_{ x }$$ having oxidation number of $$Cr$$ as $$+5$$ is :
Oxidation number of $$Cr$$ in $${ \left( { NH }_{ 3 } \right)}_{ 2 }Cr{ \left( { O }_{ 2 } \right)_2}$$ is:
The oxidation state of $$I$$ in $$H_4IO_6^-$$ is :
The oxidation number of chlorine is $$NaClO_3$$ is :
The oxidation state of $$N$$ in $$NaNO_3$$ is $$+x$$. Here, $$x$$ is :
Find the sum of oxidation state of phosphorus in $$H_3PO_4$$ and $$H_3PO_3$$.
What is the oxidation number of underlined element?
$$ \underline S^{2-}$$
Among the compounds given, what is the total number of compounds having +5 oxidation state of the underlined elements?
a. $${ K }_{ 4 }\underline { { P }_{ 2 } } { O }_{ 7 }$$
b. $$Na\underline { Au } { Cl }_{ 4 }$$
c. $${ Rb }_{ 4 }Na\left[ H\underline { { V }_{ 10 } } { O }_{ 28 } \right]$$
d. $$\underline { I } Cl$$
e. $${ Ba }_{ 2 }\underline { Xe } { O }_{ 6 }$$
f. $$\underline { O } { F }_{ 2 }$$
g. $$Ca{ \left( \underline { Cl } { O }_{ 2 } \right) }_{ 2 }$$
h. $$\underline { N } { O }_{ 2 }^{ \ominus }$$
Among the following, what is the total number of compounds having +3 oxidation state of the underlined elements?
a. $${ K }_{ 4 }\underline { { P }_{ 2 } } { O }_{ 7 }$$
b. $$Na\underline { Au } { Cl }_{ 4 }$$
c. $${ Rb }_{ 4 }Na\left[ H\underline { { V }_{ 10 } } { O }_{ 28 } \right]$$
d. $$\underline { I } Cl$$
e. $${ Ba }_{ 2 }\underline { Xe } { O }_{ 6 }$$
f. $$\underline { O } { F }_{ 2 }$$
g. $$Ca{ \left( \underline { Cl } { O }_{ 2 } \right) }_{ 2 }$$
h. $$\underline { N } { O }_{ 2 }^{ \ominus }$$
Among the following, what is the total number of compounds having zero oxidation state of the underlined elements?
a. $$\underline { S } { O }_{ 3 }^{ 2- }$$
b. $${ H }_{ 2 }\underline { C } O$$
c. $$\underline { C } { H }_{ 2 }{ Cl }_{ 2 }$$
d. $${ Na }_{ 2 }\underline { { Cr }_{ 2 } } { O }_{ 7 }$$
e. $$\underline { { O }_{ 3 } } $$
The oxidation number of $$Ni$$ in $$K_4[Ni(CN)_4]$$ is :
The oxidation states of sulphur in $$ SO_{3}^{2-}$$ is :
Oxidation number of iodine in $$IF_3$$ is:
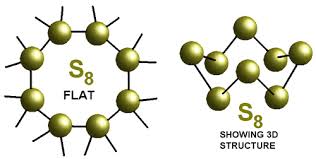
The oxidation number of sulphur in the sulphur molecule ($$S_8$$) is :
The oxidation states of sulphur in $$S_2 O_{4}^{2-}$$ is :
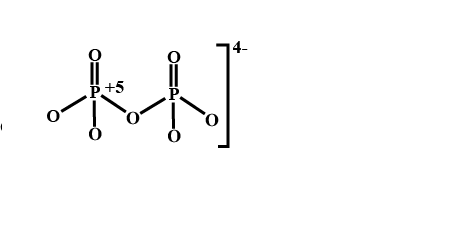
The oxidation number of phosphorus in $$P_2O_7^{4-}$$ is
The oxidation state of sulphur in $$S_2 O_{6}^{2-}$$ is :
Justify that the following reactions are redox reactions:
$$CuO(s)\, +\, H_2(g)\, \rightarrow Cu(s)\, +\, H_2O(g)$$
$$CuO(s)\, +\, H_2(g)\, \rightarrow Cu(s)\, +\, H_2O(g)$$
For a titration of $$100\:{cm}^{3}$$ of $$0.1$$ $$M$$ $${ Sn }^{ 2+ }$$ to $${ Sn }^{ 4+ }$$, $$50$$ $${cm}^{3}$$ of $$0.40$$ $$M$$ $${ Ce }^{ 4+ }$$ solution was required. The oxidation state of cerium in the reduction product is :
Consider the reactions:
Why it is more appropriate to write these reactions as: $$\displaystyle 6\, CO_2(g)\, +\, 12H_2O(l)\, \rightarrow \, C_6H_{12}O_6(aq)\, +\, 6O_2(g)+6H_2O(l)$$
Also suggest a technique to investigate the path of the above (a) and (b) redox reactions.
$$6\, CO_2(g)\, +\, 6H_2O(l)\, \rightarrow \, C_6H_{12}O_6(aq)\, +\, 6O_2(g)$$
Why it is more appropriate to write these reactions as: $$\displaystyle 6\, CO_2(g)\, +\, 12H_2O(l)\, \rightarrow \, C_6H_{12}O_6(aq)\, +\, 6O_2(g)+6H_2O(l)$$
Also suggest a technique to investigate the path of the above (a) and (b) redox reactions.
What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?$$K\underline{I}_3$$
Assign oxidation numbers to each atom of the following reaction equation:
$$2Fe\left( s \right) +{ O }_{ 2 }\left( g \right) +2{ H }_{ 2 }O\left( l \right) \rightarrow 2Fe{ \left( OH \right) }_{ 2 }\left( s \right) $$
$$NaOH + H_{2}SO_{4} \rightarrow Na_{2}SO_{4} + H_{2}O$$. Balance the given chemical equation
What is the oxidation state of sulphur in the following.
a) $$SF_6$$ b) $$Na_2S_2O_3$$
A greenish yellow gas burns in a rotten egg smelling gas giving an acidic gas and a yellow coloured non metal. Write the balanced chemical equation of the reaction.
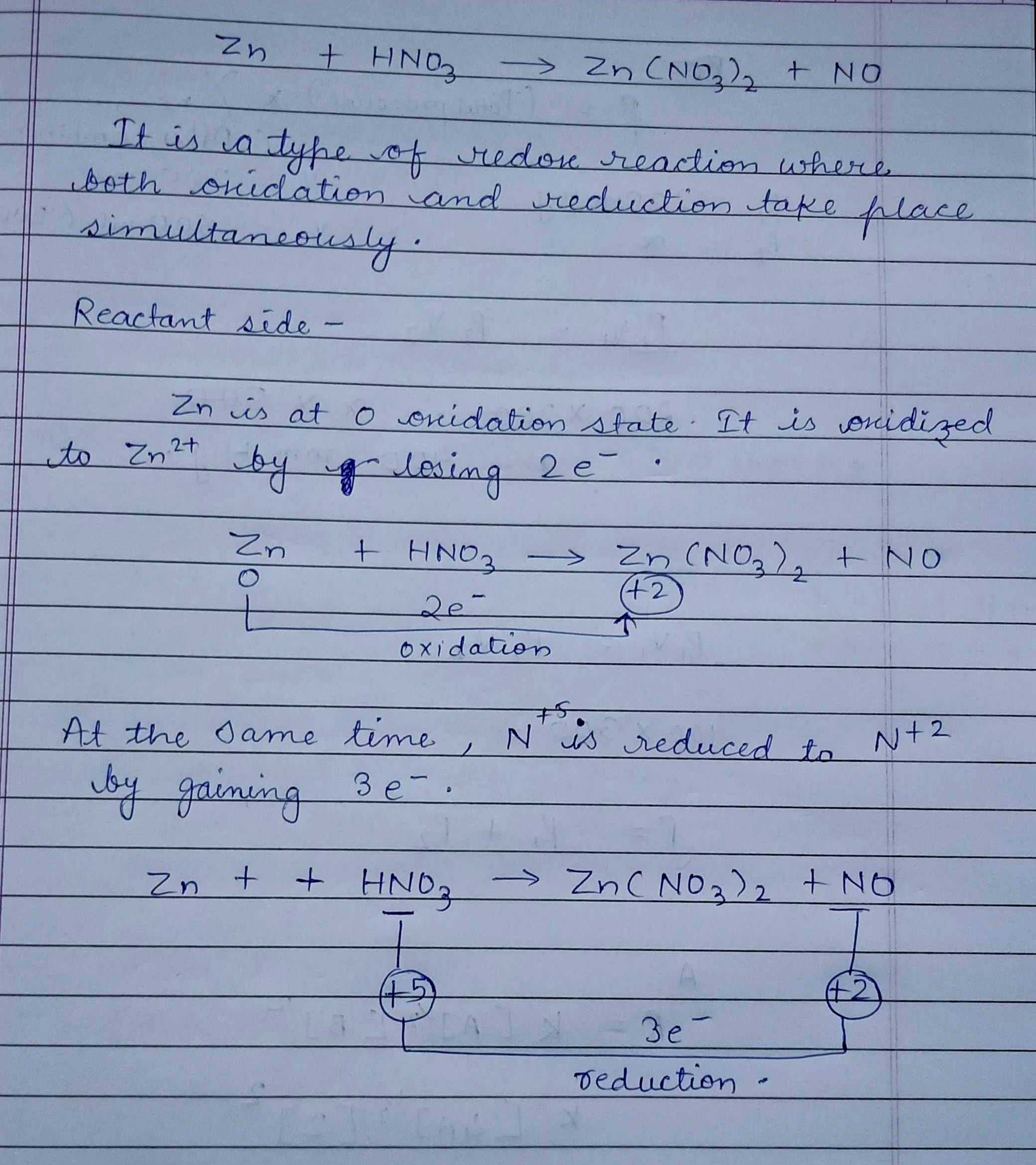
Explain how $$Zn+2AgNO_3\rightarrow 2Ag+Zn(NO_3)_2$$ is a redox reaction.
Give two examples for oxidation-reduction reaction.
Read the redox reaction given below and answer the questions:
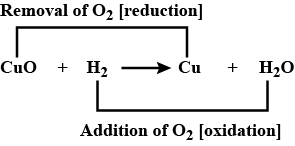
$$CuO+{ H }_{ 2 }\longrightarrow Cu+{ H }_{ 2 }O$$
(a) Conversion of $$CuO$$ into $$Cu$$ is called _______.
(b) Conversion of $${H}_{2}$$ into $${H}_{2}O$$ is called _______.
What is the oxidation number of $$Mn$$ in $$KMnO_4$$ and of $$S$$ in $$Na_2S_2O_3$$?
Find the oxidation number of $$S$$ in $${HSO_3}^-$$ ion.
Find the oxidation number of $$Mn$$ in $$[MnO_4]^-$$ ion.
Which compound amongst the following has the highest oxidation number for $$Mn$$? $$KMnO_4, K_2MnO_4, MnO_2$$ and $$Mn_2O_3$$.
Find the oxidation number of $$Pt $$ in $$[PtCl_6]^{2-}$$ ion.
Balance the following equation by oxidation number method.
$$PbS+H_2O_2\rightarrow PbSO_4+H_2O$$.
Find the oxidation number of P in $$NaH_2PO_4$$.
Find the oxidation number of Fe in $$[Fe(CN)_6]^{4-}$$.
Find the oxidation number of Ni in $$[Ni(CN)_6]^{4-}$$.
Find the oxidation number of P in $$PH^+_4$$.
Find the oxidation number of Pt in $$[PtCl_6]^{2-}$$.
Find the oxidation number of C in $$C_{12}H_{22}O_{11}$$.
Find the oxidation number of S in $$H_2S_2O_8$$.
Find the oxidation number of P in $$Mg_2P_2O_7$$.
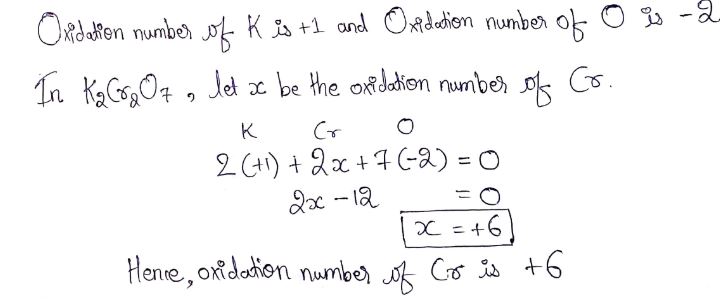
Find the oxidation number of Cr in $$K_2Cr_2O_7$$.
Find the oxidation number of Mn in $$MnO^-_4$$.
Find the oxidation number of N in $$NO^-_3$$.
Find the oxidation number of S in $$S_2Cl_2$$.
Find the oxidation number of Fe in $$Na_2[Fe(CN)_5NO]$$.
Which compound among the following has the lowest oxidation number of Mn?
$$KMnO_4, K_2MnO_4, MnO_2$$ and $$Mn_2O_3$$.
Which compound among the following has the highest oxidation number of P?
$$PH_3, H_3PO_2, PCl_3$$ and $$H_3PO_4$$.
Which compound among the following has the lowest oxidation number of chlorine?
$$HClO_4, HOCl, ClF_3, HClO_3$$ and HCl.
Find the oxidation number of Cr in $$(NH_4)_2Cr_2O_7$$.
Find the oxidation number of Ni in $$Ni(CO)_4$$.
Find the oxidation number of Cl in $$Ca(ClO_2)_2$$.
Give the oxidation state of underlined.
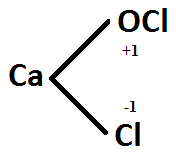
$$CaO\underline{Cl_2}$$.
Give the oxidation state of underlined.
$$\underline{Fe_3}O_4$$.
Give the oxidation state of underlined.
$$\underline{N}H4\underline{N}O_3$$.
Give the oxidation state of underlined.
$$\underline{N}H_4\underline{N}O_2$$.
Give the oxidation state of underlined.
$$Na_2\underline{S_4}O_6$$.
Give the oxidation state of underlined.
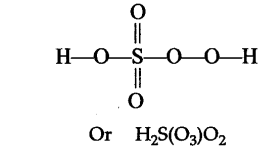
$$H_2\underline{S}O_5$$.
Give the oxidation state of underlined.
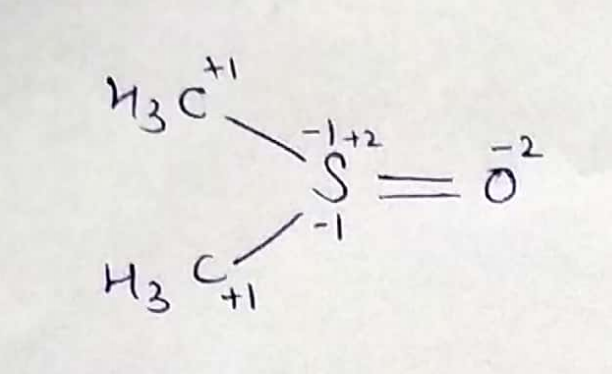
$$(CH_3)_2\underline{S}O$$.
Give the oxidation state of underlined.
$$K\underline{O_2}$$.
Give the oxidation state of underlined.
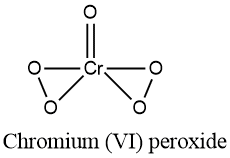
$$\underline{Cr}O_5$$.
Give the oxidation state of underlined.
$$\underline{Co}SeO_4$$.
What is the value of $$n$$ in the molecular formula $$Be_nAl_2Si_6O_{18}$$?
Write balanced chemical equation for the following.
Action of ozone on hydrogen peroxide.
What is redox reaction? Explain with one example.
What is an oxidizing agent in $$Cl_2 + 2I^{-1} \rightarrow 2Cl^{-} + I_2$$?
Name a reducing agent that can be used to obtain manganese from manganese dioxide. Write the balanced chemical equation for the reaction.
State the characteristic of chemical reaction which takes place when:
1) Lead reacts with KI
2) Zinc reacts with HCI
3) Reaction of candle wax
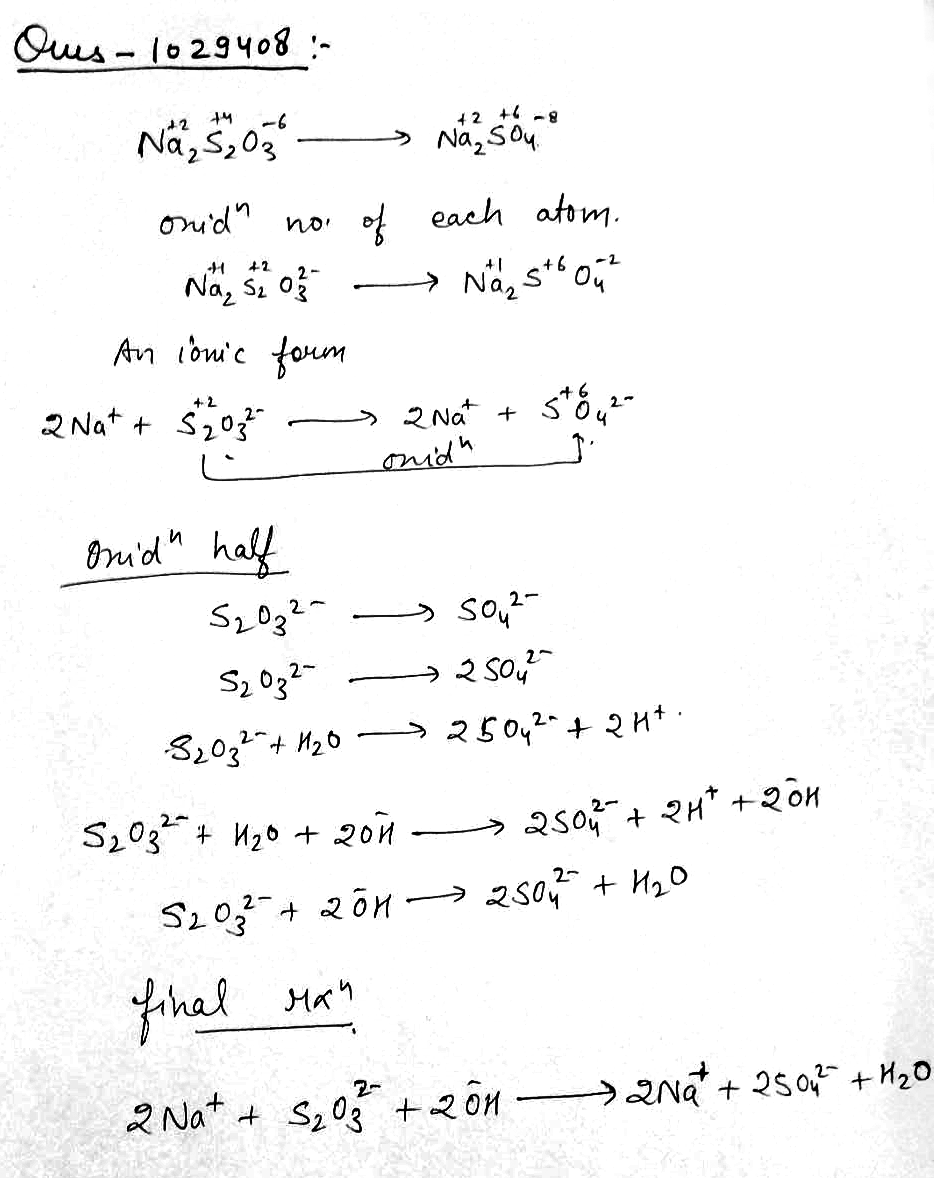
In basic medium $$\overset{+2}{Na_2}\overset{+4}{S_2}\overset{-6}{O_3}\rightarrow \overset{+2}{Na_2}\overset{+6}{S}\overset{-3}{O_4}$$.Write the balanced chemical equation.
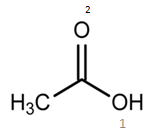
Calculate the oxidation number of oxygen in the following : $$OF_2, O_2, Na_2O_2$$ and $$CH_3COOH$$
Calculate the oxidation number of $$C$$ in the following: $$C_2H_6, C_4H_{10}, CO, CO_2$$ and $$HC{ O }_3^-$$
Give one example each of displacement, decomposition and combination reaction with explaination.
Consider the reactions :
$$2S_2O^{2-}_{3} (aq) + I_2(s) \rightarrow S_4O^{2-}_{6} (aq) + 2I^-(aq)$$
$$S_2O^{2-}_{3}(aq) + 2Br_2(l) + 5H_2O(l) \rightarrow 2SO^{2-}_{4}(aq) + 4Br^-(aq) + 10H^+(aq)$$
Why does the same reductant, thiosulphate react differently with iodine and bromine ?
Oxidation number of iron in $$Na_2[Fe(CN)_5NO^+]$$ is?
After balancing the equation,
$${K_2}C{r_2}{O_7} + x{H_2}S{O_4} + yS{O_2} \to {K_2}S{O_4} + C{r_2}{\left( {S{O_4}} \right)_3} + z{H_2}O$$
The values $$X,Y$$ and $$Z$$ obtained will be respectively
Give one example each of the componds of chlorine which show +1, +3, +4 and +6 oxidation states.
$$\overset { \underset { || }{ O } }{ \underset { \overset { | }{ \underset { \underset { H }{ | } }{ H-C-O-H } } }{ \quad \quad C-H } }$$
Write oxidation No. of each C atom.
$$LiAlH_{4}$$
Find oxidation no.of $$Al$$.
Explain the term reduction reaction with an example involving the reaction of hydrogen sulphide with chlorine .
Find oxidation number of $$Fe$$ in $$K_{3}[Fe(CN)_{6}]$$.
What is the reaction called when oxidation and reduction take place simultaneously? Explain with one example.
Balance the following equations and write sown the stoichiometric proportions.
a) $$CaCO_3 + HCl \to CaCl_2+H_2O + CO_2$$
b) $$MnO_2+HCl \to MnCl_2 + H_2O + Cl_2$$
c) $$Mg(OH)_2+HCl \to MgCl_2 + H_2O$$
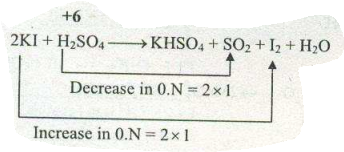
d) $$KI + H_2SO_4 \to KHSO_4 + I_2+SO_2$$
Oxidation no. of $$Fe$$ in $$ { K }_{ 3 }[Fe({ CN })_{ 6 }]$$
What is the oxidation number of Cl in $$HClO$$ and $$HClO_2$$?
Calculate the oxidation number of the metal in following
(i) $${ K }_{ 4 }\left[ Fe{ \left( CN \right) }_{ 6 } \right] $$
(ii) $$KAg{ \left( CN \right) }_{ 2 }$$
Find the oxidation number of carbon in HCOOH.
Find oxidation number of S in $$PbSO_4$$.
Find oxidation number of Sulphur in $$H_{2}S_{2}O_{7}$$.
Find oxidation number of oxygen in $$CaO_{2}$$.
Find oxidation number of Sulphur in $$KAl{ \left( SO_{ 4 } \right) }_{ 2 } . 12H_{2}O$$.
Find oxidation number of Magnese in $$K_{2}MnO_{4}$$.
Find the oxidation number of carbon in HCHO.
Find oxidation number:-
$$Br_{3}O_{8}$$ [Br]
Find oxidation number of Sulphur in $$H_{2}SO_{5}$$.
Find the oxidation number of carbon in $$CH_3OH$$.
Consider the reactions:
$$2{ S }_{ 2 }{ O }_{ 3 }^{ 2- }(aq)+{ I }_{ 2 }(s)\longrightarrow { S }_{ 4 }{ O }_{ 6 }^{ 2- }(aq)+2{ I }^{ - }(aq)$$
$${ S }_{ 2 }{ O }_{ 3 }^{ 2- }(aq)+2{ Br }_{ 2 }(l)+5{ H }_{ 2 }O(l)\longrightarrow 2{ SO }_{ 4 }^{ 2- }(aq)+4{ Br }^{ - }(aq)+10{ H }^{ + }(aq)$$
Why does the same reductant thiosulphate react differently with iodine and bromine?
$$ Cr_2O_7^{_2} +H_2O+3SO_2 \rightarrow 2Cr^{+3} +2 OH^- +3SO_4^{-2} $$write down to oxidation and reduction reaction
It requires $$ 40 mL $$ of $$ 0.5M Ce^{4+} $$ to titrate $$ 10 mL $$ of $$ 1M \ Sn^{4+} $$. What is the oxidation state of the cerium in the product?
Balance the following redox reaction.
$$(Cr_{2}O_{7})_{(aq)}^{2-} + Fe_{(aq)}^{2+} \rightarrow Cr_{(aq)}^{3+} + Fe_{(aq)}^{3+}$$ (acidic medium)
Write the oxidation number of K and B in the given species:
$${ K }_{ 2 }Mn{ O }_{ 4 }$$ and $$Na{ BH }_{ 4 }$$
Calculate the oxidation number of $$Xe$$ in $$XeF_{4}$$ and $$XeO_{2}$$.
Calculate oxidation number of central atom in $$[K_4[Fe(CN)_6]$$.
Write balanced net ionic equation for the following reaction in acidic solution.
$$HSO_4(aq)+As_4(s)+Pb_3O_4(s)\rightarrow PbSO_4(s)+H_2AsO_4^-(aq)$$
Calculate oxidation number of central atom in $$[Fe(CO)_5]$$.
Write balanced net ionic equation for the following reaction in acidic solution.
$$HNO_2(aq) \rightarrow NO_3^-+NO(g)$$
What is the oxidation number of $$Cu$$ in $$[Cu(NH_{3})_{4}]^{2+}$$?
What is the oxidation number of $$V$$ in $${ K }_{ 4 }Na[{ HV }_{ 10 }{ O }_{ 28 }]$$?
$$(CH_3)_2 \underline { S} O \Longrightarrow oxd^n$$ number of S _____
The oxidation state (integer) of chlorine in perchlorie acid $$(HClO_{4})$$ is:
Find the Oxidation number of $$Fe$$ in $${K}_{2}Fe{O}_{4}$$.
Define Redox reactions. Identify oxidising and reducing agents in following reactions:
$${I}_{2(aq)}+2{S}_{2}{O}_{3}^{2-}(aq)\rightarrow {S}_{4}{O}_{6}^{2-}(aq)+2{I}^{-}(aq)$$
Write the oxidation reaction of diphenyl amine indicator :
What is the atomic number of the following elements Potassium iodide, Iodine is
Arrange the following in increasing order of oxidation states of $$Mn$$,
$$K_2MnO_4, MnO_2, KMnO_4$$
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$V_2O_7^{4-}$$
$$H_5P_3O_{10}$$
Which of the following is the strongest oxidising agent : $$CrO_{4}^{2-}MoO_{4}^{2-}, WO_{4}^{2-}.$$
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$VO_4^{3-}$$
$$Ni_2O_3$$
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$Mn(OH)_3$$
$$I_3^-$$
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$CuSO_4$$
$$BiO_3^-$$
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$K_3[Fe(CN)_6]$$
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$Na_2CO_3$$
$$IO_4^-$$
Find the oxidation numbers to the underlined species in the following compounds or ions.
$$PF_6^-$$
$$NaIO_3$$
Hydrogen is a good reducing agent.What do you understand by the above statement? Explain with the help of copper oxide as an example .
Balance the equation in basic medium $$Al+NO_3^-\rightarrow Al(OH)^-_4+NH_3$$
Write the following equation redox reation in the oxidation and reduction half reaction
$$2K_{(s)}+Cl_{2(g)}----- \to 2KCL_{(s)}$$.
Answer the following question : Complete the equation $$Cr2O7^{2-}+14\,H^++6e : \rightarrow$$
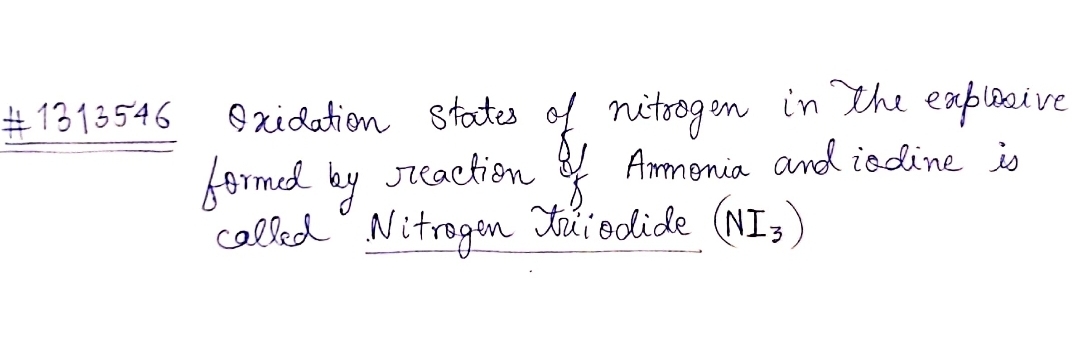
Oxidation states of nitrogen in the explosive formed by reaction of ammonia iodine is
Complete the reaction :
$$I^- + IO_3^- \rightarrow I_2^ +$$
The reaction $$Cl_{2}(g) + 2OH^{-} (aq) \rightarrow ClO^{-}(aq) + Cl^{-1} (aq) + H_{2}O(l)$$
represents the process of bleaching. Identify and name the species that bleaches the
substances due to its oxidising action.
Calculate the oxidation number of
$$N$$ in $$(NH_{4})_{2}SO_{4}$$
explain the reaction
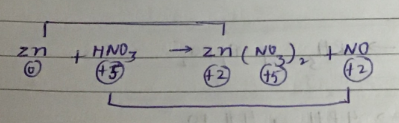
Three oxides of nitrogen, $$NO, NO_{2}$$ and $$N_{2}O$$, can be formed under different conditions.
Complete the table to give the oxidation numbers of nitrogen in $$NO$$ and $$NO_{2}$$.
| Compound | $$NO$$ | $$NO_{2}$$ |
| Oxidation number of $$N$$ |
Calculate the oxidation number of Mn in $$KMnO_{4}$$ and $$Cr$$ in $$K_{2}Cr_{2}O_{7}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reaction and then balance the$$ Kl+H_{2}SO_{4} \to KHSO_{4}+SO_{2}+l_{2}+H_{2}O $$.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
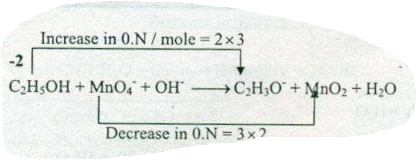
$$ C_{2}H_{5}OH +MnO_{4}^{-}+ OH^{-} \to C_{2}H_{3}O^{-} + MnO_{2}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ H_{2}S+Cr_{2}O_{7}^{2-}+ H^{+}\to Cr^{3+}+S_{8}+H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ KNO_{3}+FeSO_{4}+H_{2}SO_{4} \to K_2SO_{4}+Fe_{2}(SO_{4})_{3}+ NO+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ H_{2}S+K_{2}Cr_{2}O_{7}+H_{2}SO_{4}\to KHSO_{4}+ Cr_{2}(SO_{4})_{3}+S+H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ ZnS+O_{2}\to ZnO+SO_{2}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ Fe_{3}O_{4}+ MnO_{4}^{-} + H_{2}O \to Fe_{2}O_{3}+MnO_{2}+OH^{-}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ Cr_{2}O_{7}^{2-}+SO_{3}^{2-}+ H^{+} \to Cr^{3+}+ SO_{4}^{2-}+H_{2}O $$.
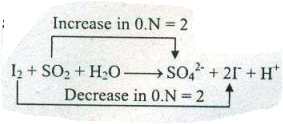
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ I_{2}+SO_{2}+H_{2}O \to SO_{4}^{2-}+ I^{-}+ H^{+}$$.
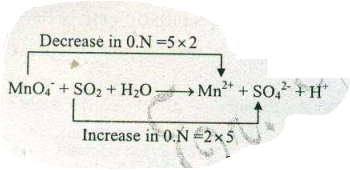
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ MnO_{4}^{-} + SO_{2} + H_{2}O \to Mn^{2+}+SO_{4}^{2-} +H^{+}$$.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ MnO_{4}^{-}+SO_{3}^{2-}+H_{2}O \to MnO_{2} +SO_{4}^{2-}+OH^{-}$$.
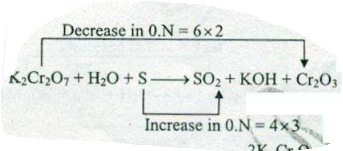
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the$$ K_{2}Cr_{2}O_{7} +H_{2}O + S \to SO_{2}+KOH+ Cr_{2}O_{3}$$.
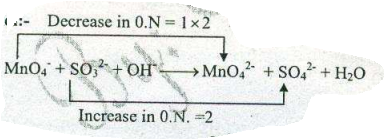
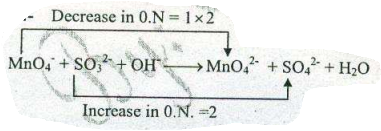
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ MnO_{4}^{-}+SO_{3}^{2-} +OH^{-} \to MnO_{4}^{2-}+H_{2}O $$.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the$$ MnO_{4}^{-} + SO_{2} + H_{2}O \to Mn^{2+}+SO_{4}^{2-} +H^{+}$$.
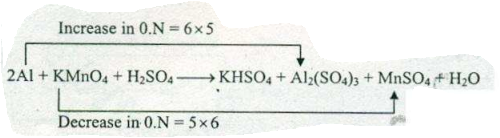
Assign ON to atoms of only those elements which undergo ON change in the following redox reaction and then balance the reaction:
$$ Al +KMnO_{4}+H_{2}SO_{4} \to KHSO_{4}+Al_{2}(SO_{4})_{3}+MnSO_{4}+H_{2}O $$
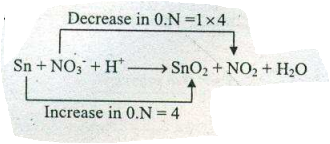
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the$$ Sn +NO_{3}^{-}+H^{+} \to SnO_{2}+NO_{2}+ H_{2}O $$.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the$$ ClO^{-} +Br^{-} \to BrO_{3}^{-}+Cl^{-}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ KMnO_{4}+H_{2}O_{2}+H_{2}SO_{4}\to MnSO_{4}+K_{2}SO_{4}+O_{2}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ Cl_{2}+IO_{3}^{-}+OH^{-}\to Cl^{-}+IO_{4}^{-}+H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ KMnO_{4}+HCl\to Cl_{2}+KCl+MnCl_{2}+H_{2}O$$
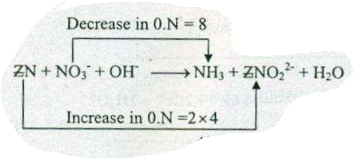
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the$$ Zn+NO_{3}^{-}+OH^{-} = NH_{3}+ZnO_{2}^{2-}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ Zn+ NO_3^{-}+H^{+}\to Zn^{2+}+NH_{4}^{+}+H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ ClO+SbO_{2}^{-}+OH^{-}\to ClO_{2}^{-}+Sb (OH)_{6}^{-}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ H_{2}O_{2}+PbS\to PbSO_{4}+H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the$$ H_{2}SO_{3}+Cr_{2}O_{7}^{2-}+H^{+}=HSO_{4}^{-}+Cr^{3+}+H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ BaCrO_{4}+KI+HCl\to BaCl_{2}+I_{2}+KCl+CrCl_{3}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
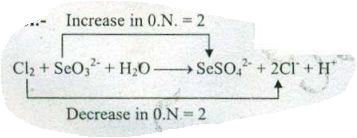
$$ Cl_{2}+SeO_{3}^{2-}+ H_{2}O \to SeO_{4}^{2-}+Cl^{-}+H^{+}$$
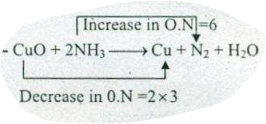
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ CuO +NH_{3} \to Cu+N_{2}+H_{2}O $$.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ Cu_{3}P+H^{+}+Cr_{2}O_{7}^{2-}\to Cu^{2+}+H_{3}PO_{4}+Cr^{3+}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ H_{2}O+SbCl_{3}\to SbOCl+2HCl $$
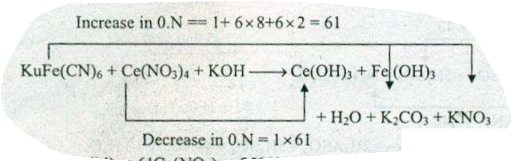
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$K_{4}Fe(CN)_{6}+Ce(NO_{3})_{4}+KOH \to Ce(OH)_{3}+Fe(OH)_{3}+H_{2}O+K_{2}CO_{3}+KNO_{3}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ HNO_{3}+I_{2}\to HIO_{3}+NO_{2}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ As_{2}S_{5}+HNO_{3}=H_{3}AsO_{4}+H_{2}SO_{4}+NO_{2}+H_{2}O $$.
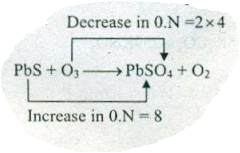
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the $$ PbS+O_{3} \to PbSO_{4}+O_{2}$$.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ AsO_{3}^{3-}+I_{2}+H_{2}O \to AsO_{4}^{3-}+ H^{+}+I^{-}$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ K_{2}Cr_{2}O_{7}+H_{2}SO_{4}+KI\to I_{2}+K_{2}SO_{4}+Cr_{2}(SO_{4})_{3}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
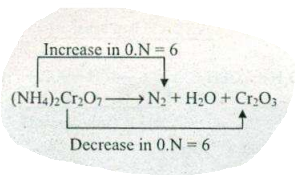
$$ (NH_{4})_{2}Cr_{2}O_{7}\to N_{2}+H_{2}O +Cr_{2}O_{3} $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ CuSO_{4}+KI\to Cu_{2}I_{2}+I_{2}+K_{2}SO_{4}$$
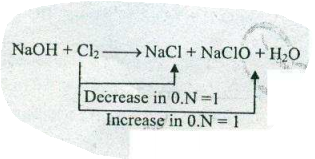
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ NaOH+Cl_{2}\to NaCl+NaClO+H_{2}O$$
Point out the oxidation number of $$C$$ in the following:
$${ CH }_{ 4 },\ { C }_{ 3 }{ H }_{ 8 },\ { C }_{ 2 }{ H }_{ 6 },\ { C }_{ 4 }{ H }_{ 10 },\ CO,\ { CO }_{ 2 },\ H{ CO }_{ 3 }^{ - }$$ and $${ CO }_{ 3 }^{ 2- }$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
$$ As_{2}S_{5}+HNO_{3}\to H_{2}SO_{4}+NO_{2}+H_{3}AsO_{4}+H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ Zn+HNO_{3} \to Zn(NO_{3})_{2}+N_{2}O+ H_{2}O $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
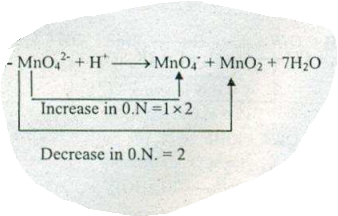
$$MnO_{4}^{2-}+H^{+}\to MnO_{4}^{-} + MnO_{2} +H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
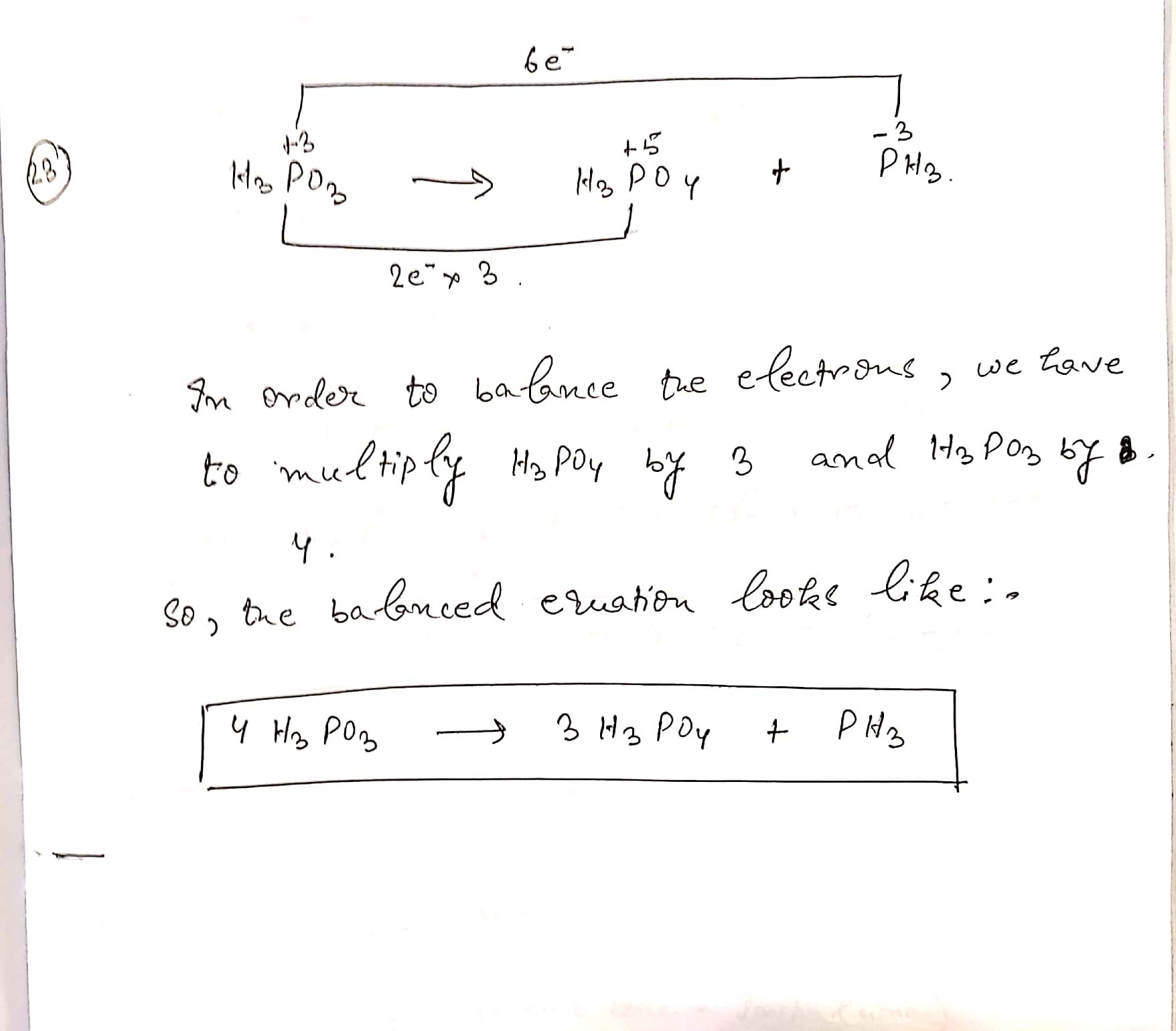
$$H_{3}PO_{3}\to H_{3}PO_{4} + PH_{3} $$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
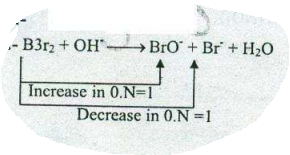
$$ Br_{2}+OH^{-}\to BrO^{-}+Br^{-}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
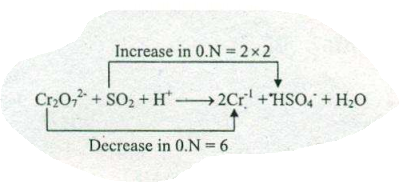
$$ Cr_{2}O_{7}^{2-}+SO_{2}+H^{+}\to Cr^{3+}+HSO_{4}^{-}+H_{2}O$$
Fill in the blanks:
(a) Hydrogen is ______ than air.
(b) Hydrogen is ______soluble in water.
(c) Hydrogen burns with a ___________ flame and _____ sound is heard.
(d) A metal _______ hydrogen in the reactivity series gives hydrogen with ______.
(e) Hydrogen reacts with metal oxides to form ______ and ______.
(f) Oxidation is the removal of ________ and addition of _______. (g) In redox reaction oxidation and reduction occur ___________.
Write a balanced chemical equation for each of the following reactions and also classify them.
(c) Iron (III) oxide on heating with carbon monoxide gas reacts to form solid iron and liberates carbon dioxide gas.
Name the following:
(a) Two metals which give hydrogen with cold water.
(b) A metal which liberates hydrogen only when steam is passed over red hot metal.
(c) The process in which oxygen is added or hydrogen is removed.
(d) A metallic oxide which can be reduced into metal by hydrogen.
Give reasons:
Carbon cannot reduce the oxides of Na or Mg.
Write a balanced chemical equation for the following reaction and also classify them.
Hydrogen sulphide gas reacts with oxygen gas to form solid Sulphur and liquid water.
What do you mean by redox reaction? Explain with the help of an example.
Balance the following chemical equation and identify the type of chemical reaction.
$$H g O(s) \stackrel{H e a t}{\longrightarrow} H g(l)+O_{2}(g)$$
$$H g O(s) \stackrel{H e a t}{\longrightarrow} H g(l)+O_{2}(g)$$
Give two examples each of:
Redox reactions
Define:
(a) catalytic hydrogenation
(b) oxidation
(c) reduction
(d) redox reaction
Calculate the oxidation number of the element underline in each of the following cases
$$ Al $$ in $$ Al_2O_3 $$
Calculate the oxidation number of the element underline in each of the following cases
$$ Na_2PdCl_4 $$
Balance $$ Fe^{2+} + Cr_2O^{2-}_7 \rightarrow Fe^{3+} Cr^{3+} $$ is acid media.
Calculate the oxidation number of the element underline in each of the following cases
$$ S $$ in $$ Na_2S_2O_3 $$
Calculate the oxidation number of the element underline in each of the following cases
$$ HA_4Cl_4 $$
Calculate the oxidation number of the element underline in each of the following cases
$$ Mn $$ in $$ KMnO_4 $$
Calculate the oxidation number of the element underline in each of the following cases
$$ P $$ in $$ P_2O_5 $$
What do you understand by redox reactions?
What do you understand redox reactions? Explain oxidation and reduction in terms of loss or gain of electrons.
What does $$(II)$$ in lead $$(II)$$ nitrate indicate?
Calculate the oxidation number of the element underline in each of the following cases
$$ H\underline{S}O^-_3 $$
Find the oxidation number of the underlined atoms.
$$ H\underline{P}O^-_2 $$
Calculate the oxidation number of the element underline in each of the following cases
$$ \underline {Cl}O^-$$
Find the oxidation number of the underlined atoms.
$$ Na_2\underline{B}_4O_7$$
Find the oxidation number of the underlined atoms.
$$ \underline {C}H_4 $$
Find the oxidation number of the underlined atoms.
$$ \underline{C}Cl_4 $$
Find the oxidation number of the underlined atoms.
$$\underline {C}IO^-_3$$
What is oxidation number of sulphur in the following molecules / ions $$ (n) (i) H_2S (ii) SO^{2-}_4 (iii) H_2SO_4 $$
Calculate the oxidation number of the element underline in each of the following cases
$$ \underline{Cr_2}O_7^{-2} $$
Calculate the oxidation number of the element underline in each of the following cases
$$\underline{Si}0_3^{- 2}$$
What is the oxidation number of $$Li$$ in $$LiAlH_{4}$$?
In the given structure, the electronegativity of $$Y$$ is more then $$Z$$. Find the oxidation number of $$Z$$.
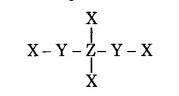
Calculate the oxidation number of $$Ni$$ in $$[Ni(CN)_{4}]^{2-}$$.
Calculate the oxidation number of $$Mo$$ in $$(NH_{4})_{2} Mo O_{4}$$.
What is the oxidation number of oxygen in $$KO_{2}$$?
Find the oxidation number of the underlined atoms.
$$ Fe_3O_4 $$
Calculate the oxidation state of $$Na $$ in $$Na_2O_2 $$.
Observe the following chemical equation
$$Cu +2H_2SO_4\to CuSO_4 +SO_2 +2H_2O$$
Which one has oxidation state as $$0$$?
Find the oxidation number of $$Mn$$ in $$KMnO_4$$ (oxidation number of $$K$$ is $$+1$$, oxidation number of $$O$$ is $$-2$$)
Observe the following chemical equation
$$Cu +2H_2SO_4\to CuSO_4 +SO_2 +2H_2O$$
What is its oxidation state of Cu after the chemical reaction?
$$\displaystyle \overset{0}{H_2} + \overset{0}{Cl_2} \longrightarrow \overset{+1}{2H} \overset{-1}{Cl} $$
In the formation of hydrogen chloride does the oxidation number of hydrogen increase or decrease ?
Whether the oxidation number of chlorine decrease or increase ?
Based on oxidation number which is the oxidizing agent and reducing agent ?
How do you determine the oxidation number of sulfur in $$H_2SO_4$$ ?
Find the oxidation number of $$Mn$$ in $$MnO_2 , Mn_2O_3 $$ and $$ Mn_2O_7$$
Analyze the following equation, fill-up the blanks.
$$ \overset{0}{Zn} + 2 \overset{+1}{H} \overset{-1}{Cl} \rightarrow \overset{+2}{Zn} \overset{-1}{Cl_2} + \overset{0}{H_2} $$
The oxidation number of hydrogen increases/decreases from ............. to ........
Consider the equation $$ H_2 + Cl_2 \rightarrow 2 HCl $$ What is the oxidation number of hydrogen in $$H_2$$ ?
What is the oxidation number of chlorine in $$Cl_2$$ ?
In this reaction , does the oxidation number of hydrogen increase or decrease ?
What change takes place in the oxidation number of chlorine ?
Analyze the following equation and fill-up the blanks.
$$ \overset{0}{Zn} + 2 \overset{+1}{H} \overset{-1}{Cl} \rightarrow \overset{+2}{Zn} \overset{-1}{Cl_2} + \overset{0}{H_2} $$
The oxidation number of zinc increases/decreases from ............to ........
Find the oxidation number of the ions in ionic compound ?
In the following chemical reactions which is the oxidizing agent which is the reducing agent ? Analyze the oxidation number and find out ?
$$ \overset{0}{C} + 2 \overset{0}{Cl_2} \longrightarrow \overset{+4}{C} \overset{-1}{Cl_4} $$
Given below is the equation of the reaction between zinc and hydrochloric acid also indicating the oxidation number.
$$ \overset{0}{Zn} + 2 \overset{+1}{H} \overset{-1}{Cl} \rightarrow \overset{+2}{Zn} \overset{-1}{Cl_2} + \overset{0}{H} $$
Analyze and find the following:
The oxidation number of $$ \mathrm{N} $$ in $$ \mathrm{NI}_{3} $$ is $$\dots$$
Sone chemical equations are given below.
$$ C + O_2 \rightarrow CO_2 $$
$$ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O $$
$$ N_2 + O_2 \rightarrow NO $$
$$ CaCO_2 \rightarrow CaO + CO_2 $$
$$ H_2 + I_2 \rightarrow HI $$
$$ Fe + HCl \rightarrow FeCl_2 + H_2 $$
$$ CO_2 + C \rightarrow CO $$
Which among these are redox reactions ?
Match list-I (compounds) with list-II (oxidation states of nitrogen).
$${ { H }_{ 2 }O }_{ 2 }(aq)$$ reduces $$Cl{ O }_{ 4 }^{ - }(aq)$$ in acidic medium. If 1 mole of $${ { H }_{ 2 }O }_{ 2 }$$ reduces $$0.5$$ mole of $$Cl{ O }_{ 4 }^{ - }(aq)$$. What is the oxidation number of reduced species?(Answer is the modulus of the oxidation number)
Nitrogen forms five different compounds with oxygen. Match appropriate formulae for these compounds if the oxidation state of $$N$$ in them is $$+1,+2,+3,+4$$ and $$+5$$.
Match the compounds in Column I with the matter in Column II.
The number of redox reactions from the following equations are:
a. $${ H }_{ 2 }S + { Fe }^{ 3+ } \longrightarrow { Fe }^{ 2+ } + S\downarrow + { H }^{ \oplus }$$
b. $${ I }^{ \ominus } + I{ O }_{ 3 }^{ \ominus } + { H }^{ \oplus } \longrightarrow { I }_{ 2 } + { H }_{ 2 }O$$
c. $$Bi\left( s \right) + N{ O }_{ 3 }^{ \ominus } + { H }^{ \oplus } \longrightarrow N{ O }_{ 2 } + { Bi }^{ 3+ } + { H }_{ 2 }O$$
d. $${ I }^{ \ominus } + { O }_{ 2 }\left( g \right) + { H }_{ 2 }O \longrightarrow { I }_{ 2 } + \overset { \ominus }{ O } H$$
e. $$Cu\left( s \right) + { Au }^{ \oplus } \longrightarrow Au\left( s \right) + { Cu }^{ 2+ }$$
$$6$$ mol of $$Cl_2$$ undergo a loss and gain of $$10$$ mol of electrons to form two oxidation states of Cl. Write down the oxidation number of Cl having positive charge.
Match the following with their minimum and maximum oxidation number if any as well as with their respective variable oxidation number if any.
Match the reactions in column I with the molar ratio of their respective oxidants and reductant in column II.
Among the following elements, what is the total number of elements having the lowest oxidation state of zero?
a. $$Ta$$
b. $$Te$$
c. $$Tc$$
d. $$Ti$$
e. $$Tl$$
Match the reactions given in column I with average oxidation numbers given in column II.
Reactlon Oxidation state
What is the magnitude of summation of oxidation states of all oxygen in $$CrO_5$$?
Assign oxidation number to the underlined element in $$NaH_2\underline{P}O_4$$.
Arrange the following in the order of increasing oxidation number of nitrogen: $$NH_3, N_3H, N_2O, NO, N_2O_5$$.
Can the reaction $$Cr_{2} O_{7}^{2-} + H_{2}O \rightarrow 2CrO_{4}^{2-} + 2H^{+}$$ be regarded as a redox reaction?
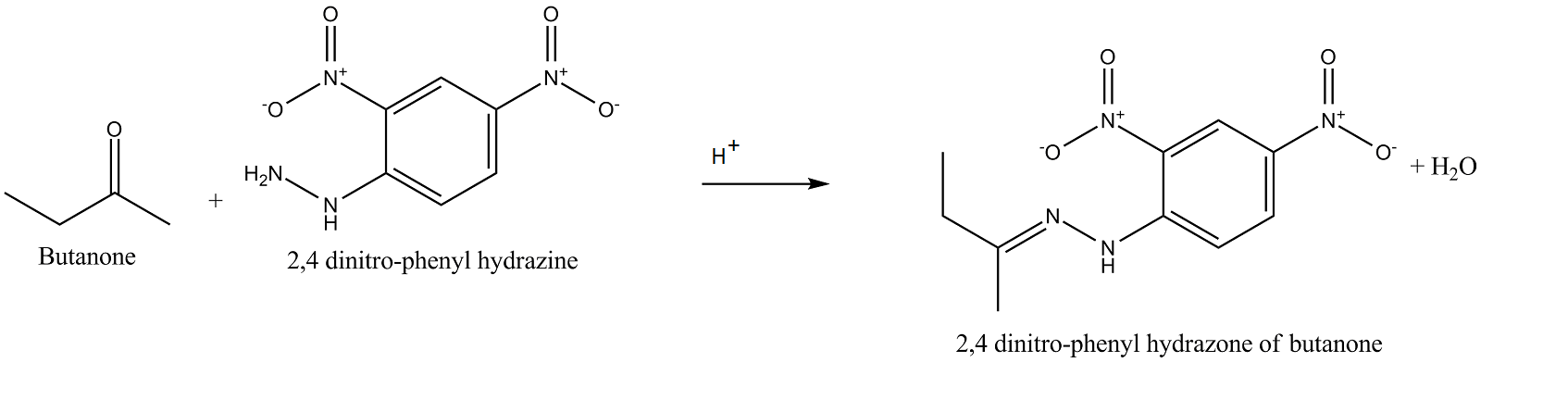
Complete and rewrite the balanced chemical equation.
Butanone$$+2, 4$$ dinitro-phenyl hydrazine $$\overset{H^+}{\rightarrow}?$$
In the following redox reaction $${ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }\ +\ { Fe }^{ 2+ }\quad \longrightarrow { Fe }^{ 3+ }\ +{ Cr }^{ 3+ } $$ 1 mol of $$Cr_2O_7^{2-}$$ oxidizes x mol of $${ Fe }^{ +2 }$$. Find x.
Dvide the following redox equations into oxidation half equation and reduction half equation.
$$a)P_{ (s) }+P{ O }_{ 4(aq) }^{ 3 }HP{ O }_{ 3(aq) }^{ 2 }\\ b)HNO_{ 2(aq) }\longrightarrow N{ O }_{ 3(aq) }^{ - }+NO_{ (g) }\\ c)I{ O }_{ 3(aq) }^{ - }+{ Re }_{ (s) }\longrightarrow { Re }{ O }_{ 4(aq) }^{ }+{ I }_{ (aq) }$$
$$AI{ F }_{ 3 }$$ is insoluble in a hydrous $$HF$$ but when little $$KF$$ is added to the compound it becomes soluble. On addition of $${ BF }_{ 3 }$$, $$AI{ F }_{ 3 }$$ is precipitated. Write the balanced chemical equations.
Why can $$MnO_2$$ be reduced by '$$Al$$' and not by carbon?
Give a clear idea about how $${ MnO }_{ 4 }^{ - }{ ,CrO }_{ 4 }^{ 2- },{ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$$ changes its oxidation state when it is in the acidic, basic and neutral medium.
What is oxidation state of manganese in manganate form?
When $$KMnO_4$$ acts as an oxidising agent and ultimately forms $$[MnO_4]^{-1}$$, $$MnO_2$$, $$Mn_2O_3$$, and $$Mn_2O^+$$, then the number of electrons transferred in each case respectfully is
Zinc oxide is white but it turns yellow on heating . Explain.
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ S+OH^{-}\to S^{2-}+S_{2}O_{3}^{2-}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ MnO_{4}^{-}+C_{2}O_{4}^{2-}+H^{+}\to Mn^{2+}+CO_{2}+H_{2}O$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the reaction:
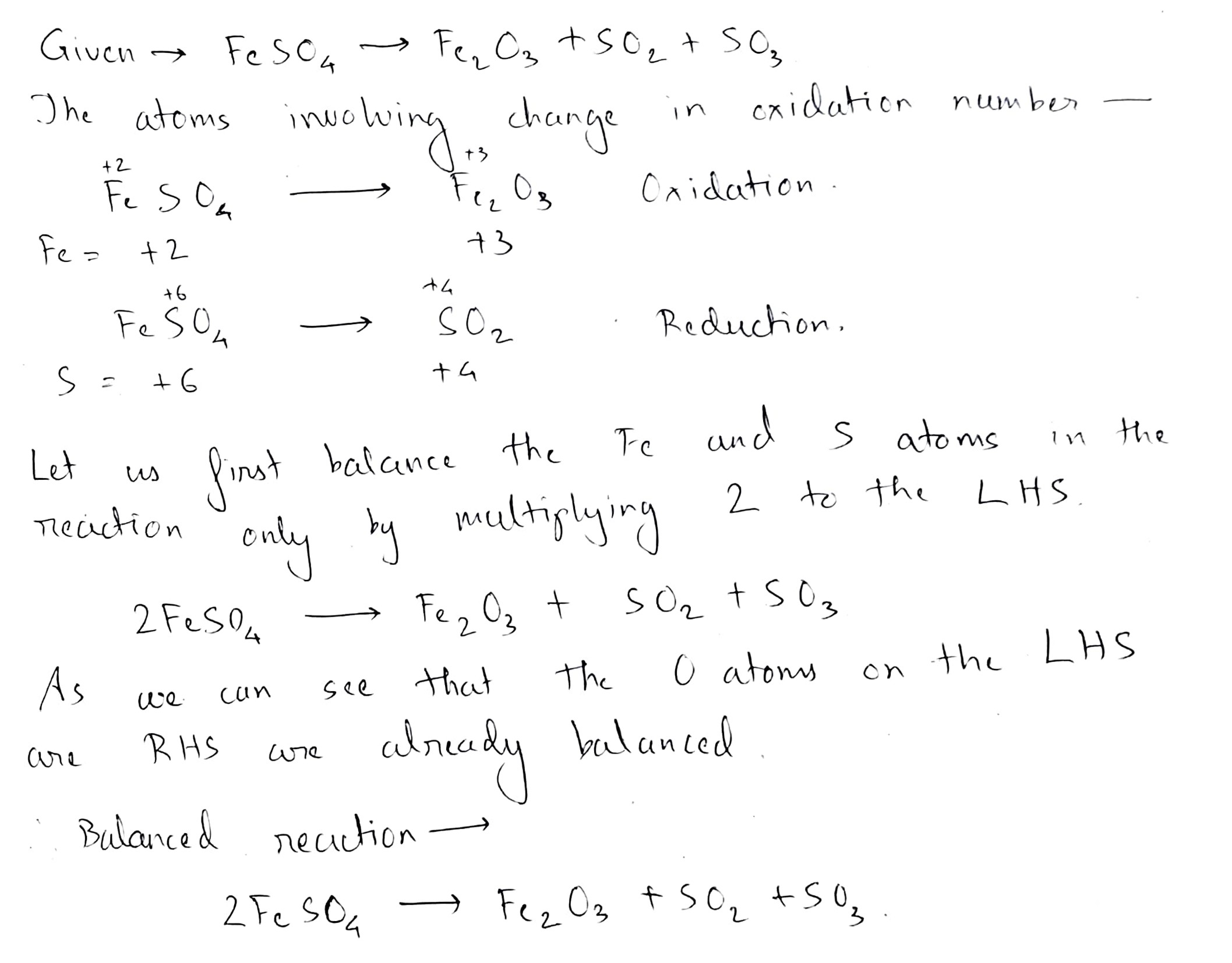
$$ FeSO_{4}\to Fe_{2}O_{3}+SO_{2}+SO_{3}$$.
Find the oxidation number of carbon in the following compounds:
$${ CH }_{ 3 }OH,\quad { CH }_{ 2 }O,\quad HCOOH,\quad { C }_{ 2 }{ H }_{ 2 }.$$
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation:
$$ Na_{2}SnO_{2}+Bi(OH)_{3}\to Bi+Na_{2}SnO_{3}+H_{2}O$$
Determine the ox. no of underlined atom in each of the following:
(a) $$K\underline { Cr } { O }_{ 3 }Cl$$
(b) $${ K }_{ 2 }\underline { Fe } { O }_{ 4 }$$
(c) $$Ba{ \left( { H }_{ 2 }{ \underline { P } O }_{ 2 } \right) }_{ 2 }$$
(d) $${ Rb }_{ 4 }Na\left[ H{ \underline { V } }_{ 10 }{ O }_{ 28 } \right] $$
(e) $${ Ba }_{ 2 }\underline { Xe } { O }_{ 6 }$$
(f) $${ Na }_{ 2 }{ \underline { S } }_{ 2 }$$
(g) $${ K }_{ 2 }\underline { Mn } { O }_{ 4 }$$
(h) $${ K }_{ 2 }{ \underline { Cr } }_{ 2 }{ O }_{ 7 }$$
(i) $$\underline { Mn } { O }_{ 4 }^{ - }$$
(j) $$\underline { S } { O }_{ 4 }^{ 2- }$$
(k) $$\underline { P } { O }_{ 4 }^{ 3- }$$
(l) $$\underline { C } { O }_{ 3 }^{ 2- }$$
(m) $$[\underline { Cu } { \left( { NH }_{ 3 } \right) }_{ 4 }]^{ 2+ }$$
(n) $$\underline { Ni } { \left( CO \right) }_{ 4 }$$
(o) $$\underline { C } { S }_{ 2 }$$
(p) $${ \left( { NH }_{ 4 } \right) }_{ 6 }{ \underline { Mo } }_{ 7 }{ O }_{ 24 }$$
(q) $${ \left[ \underline { Co } { F }_{ 4 } \right] }^{ - }$$
(r) $$\underline { Os } { O }_{ 4 }$$
(s) $${ Na }_{ 4 }\underline { Xe } { O }_{ 6 }$$
(t) $$K\underline { Cr } { O }_{ 3 }Cl$$
(u) $${ \underline { F } }_{ 2 }{ H }_{ 2 }$$
Arrange the following in order of:
(a) Increasing oxidation no. of $$Mn:Mn{ Cl }_{ 2 },Mn{ O }_{ 2 },Mn{ \left( OH \right) }_{ 3 },KMn{ O }_{ 4 }$$
(b) Decreasing oxidtaion no. $$X:HX{ O }_{ 4 },HX{ O }_{ 3 },HX{ O }_{ 2 },HXO$$
(c) Increasing oxidation no. $$I:{ I }_{ 2 },HI,HI{ O }_{ 4 },ICl,I{ F }_{ 3 },I{ F }_{ 5 }$$
(d) Increasing oxidation no. of $$N:{ N }_{ 2 },{ NH }_{ 3 },{ N }_{ 3 }H,{ NH }_{ 2 }{ NH }_{ 2 },{ NH }_{ 2 }OH,K{ NO }_{ 2 },K{ NO }_{ 3 },{ N }_{ 2 }O$$
What is the name of the reaction ?
$$ 2 CH_3 CH_2 CH_2SH \rightarrow CH_3CH_2CH_2 -S -S- CH_2CH_2CH_3 $$
whether condensation , oxidation reduction or polymerization ?
Select the nature or type of redox change in the following reactions:
(a) $$2{ Cu }^{ 2+ }\longrightarrow { Cu }^{ 2+ }+{ Cu }^{ 0 }\quad $$
(b) $${ Cl }_{ 2 }\longrightarrow Cl{ O }^{ - }+{ Cl }^{ - }\quad $$
(c) $$2KCl{ O }_{ 3 }\xrightarrow [ ]{ \Delta } 2KCl+3{ O }_{ 2 }$$
(d) $${ \left( { NH }_{ 4 } \right) }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }\longrightarrow { N }_{ 2 }+{ Cr }_{ 2 }{ O }_{ 3 }+4{ H }_{ 2 }O$$
(e) $$10Fe{ SO }_{ 4 }+2KMn{ O }_{ 4 }+8{ H }_{ 2 }{ SO }_{ 4 }\longrightarrow 2Mn{ SO }_{ 4 }+5{ Fe }_{ 2 }{ \left( { SO }_{ 4 } \right) }_{ 3 }+{ K }_{ 2 }{ SO }_{ 4 }+{ 8H }_{ 2 }O$$
(f) $$5{ H }_{ 2 }{ C}_{ 2 }{ O }_{ 4 }+2KMn{ O }_{ 4 }+3{ H }_{ 2 }{ SO }_{ 4 }\longrightarrow { K }_{ 2 }{ SO }_{ 4 }+2Mn{ SO }_{ 4 }+10{ CO }_{ 2 }+8{ H }_{ 2 }O\quad $$
Some chemical reactions are given below
$$Zn+2HCl \rightarrow ZnCl_2+H_2$$
$$2Mg+O_2 \rightarrow 2MgO$$
$$NH_4Cl \leftrightharpoons NH_3+HCl$$
What type of reactions are they all represent?
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions