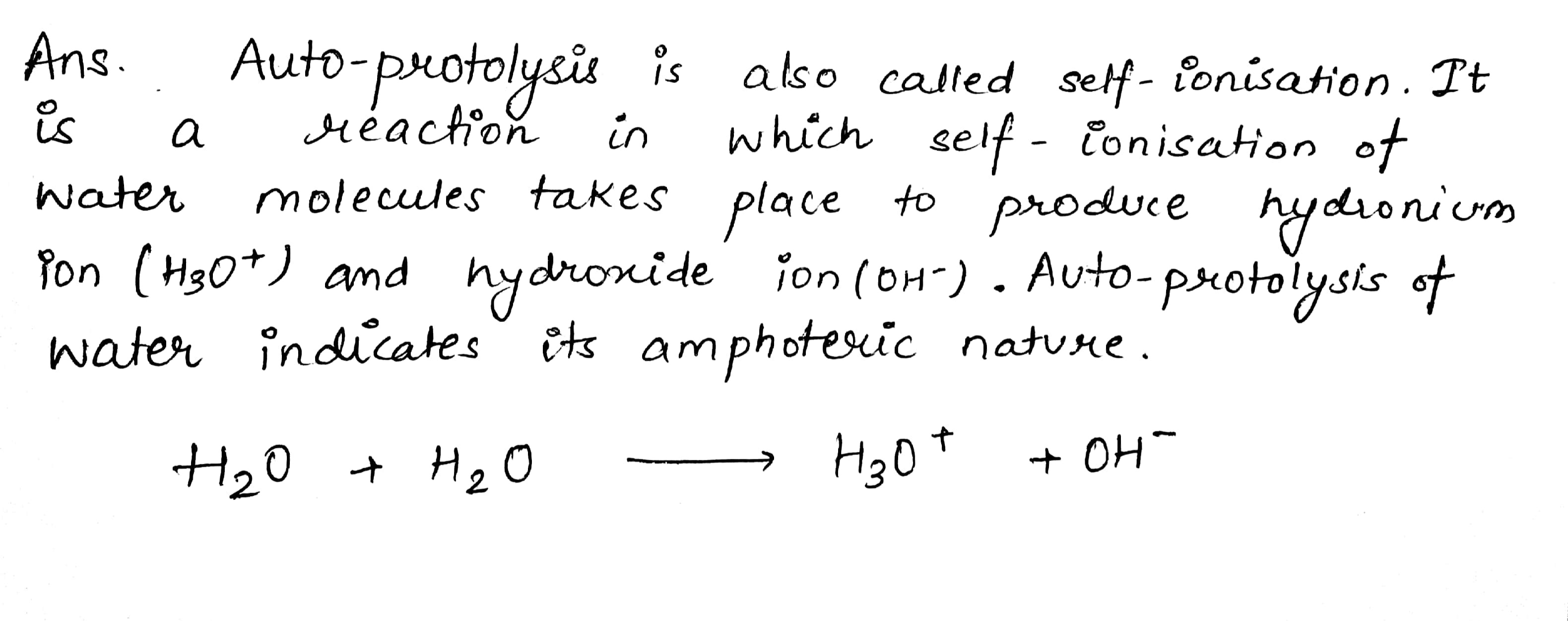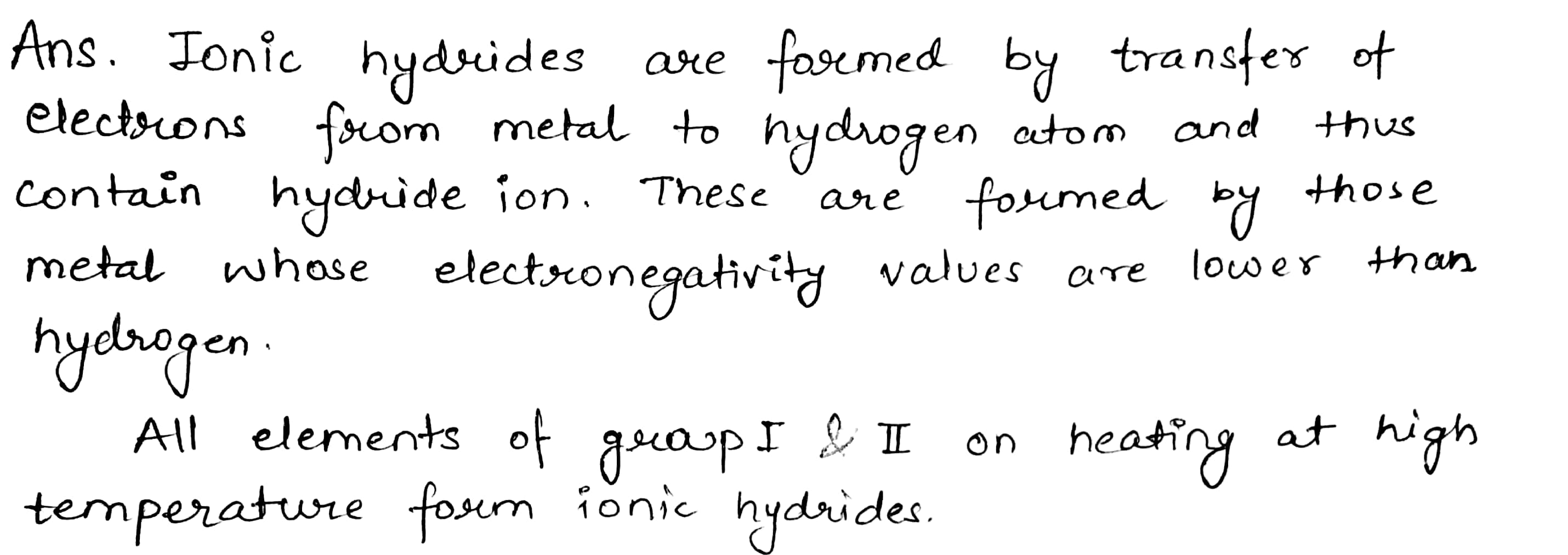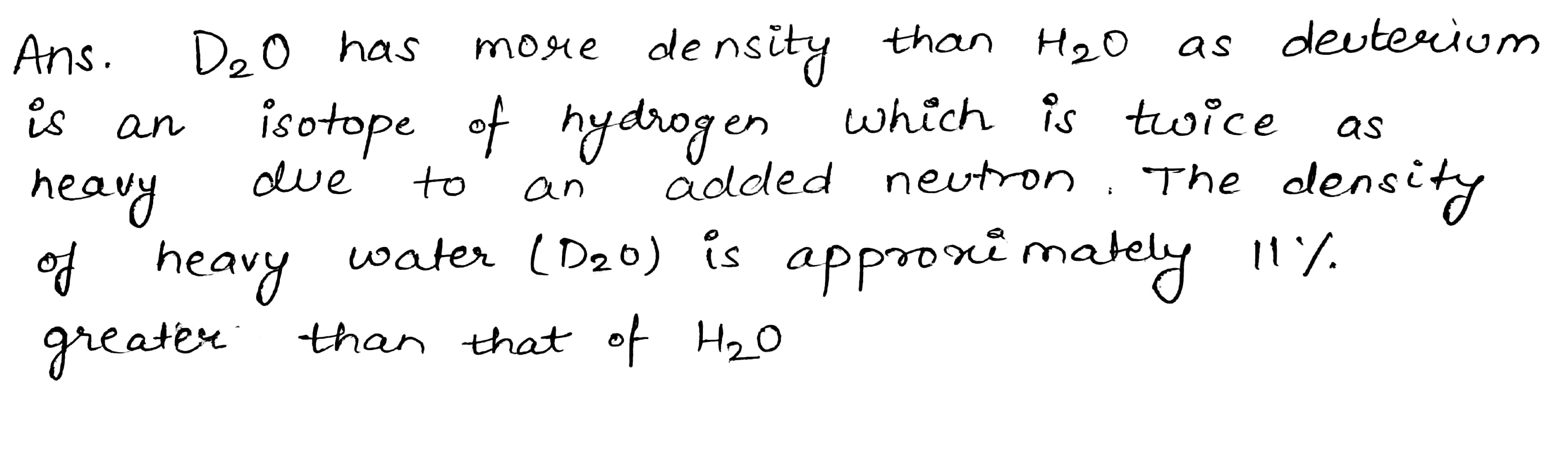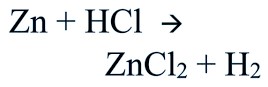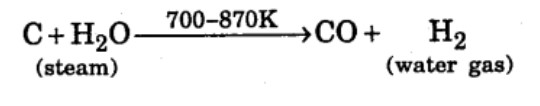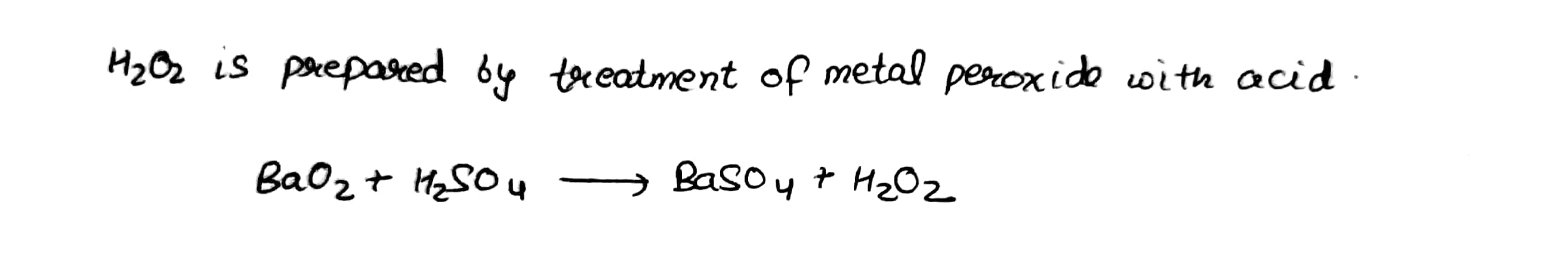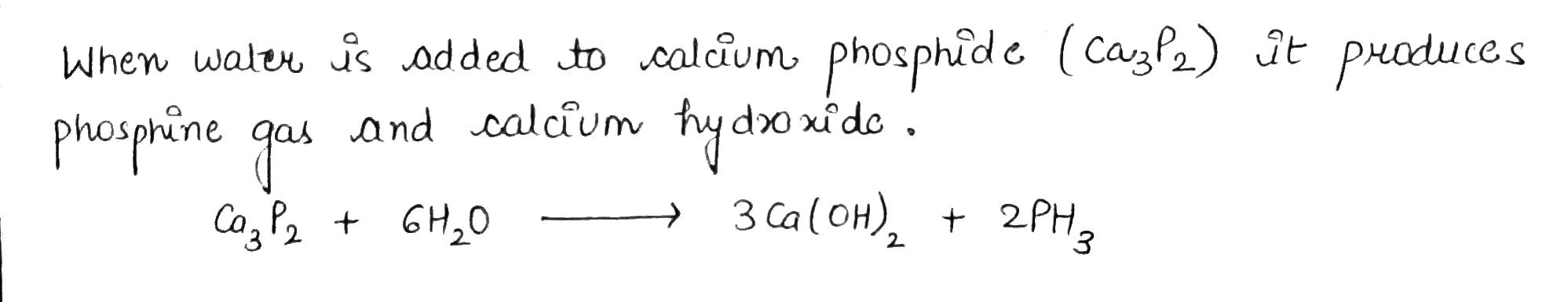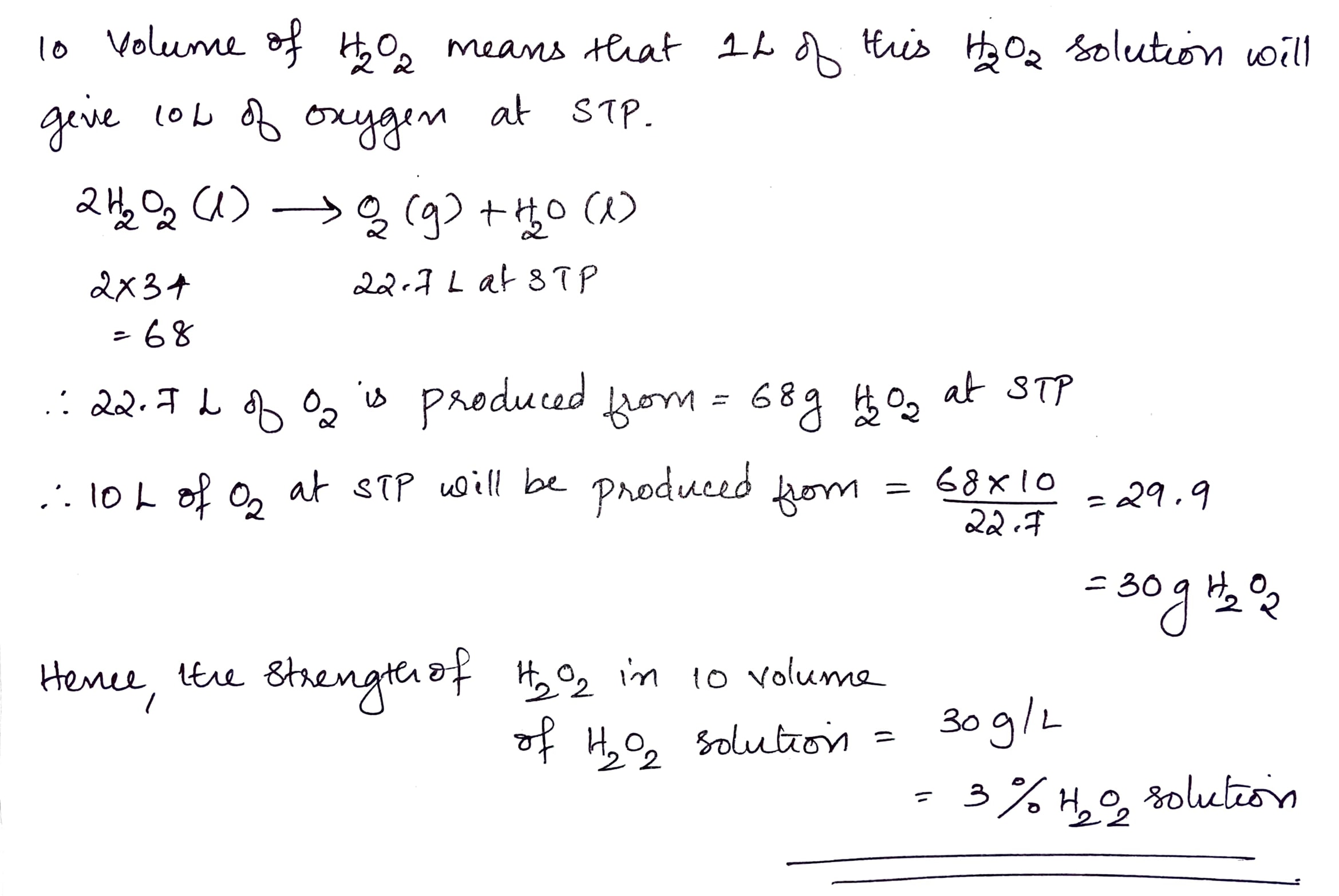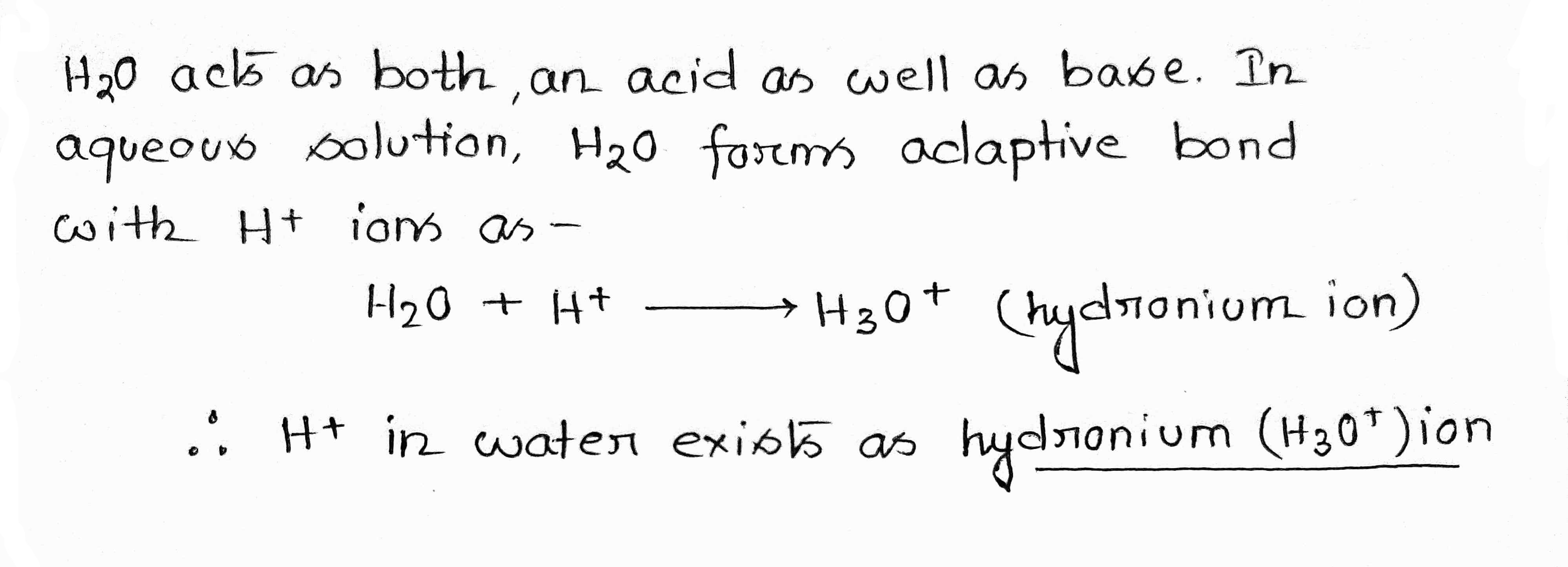Hydrogen - Class 11 Medical Chemistry - Extra Questions
State three uses of water vapour in the air.
How would you confirm that a colourless liquid given to you is pure water? (AS1)
Molecular mass of water is _______ a.m.u.
Name three metals which do not react with water, under normal conditions.
Water exists in a liquid state over a wide range of temperature 0oC to 100oC. Explain.
What is the chemical composition of water? Give its molecular formula.
What is the difference between 2H or H2.
In which group hydrogen is placed on the basis of electronic configuration?
H2O2 solutions are stored in dark-colored plastic or wax-coated glass vessels often with negative catalysts such as urea or sodium stannate added as stabilizers.
State whether the given reaction is True or False?
As many as _________ different combination of water are possible from 1H1,1H2,1H3 and 8O16,8O17 and 8O18.
Which gas is usually liberated when an acid reacts with a metal ?
Give the properties of water responsible for controlling the temperature of our body.
________ is oxidation number of hydrogen in NaH.
Explain the resemblance of hydrogen to the other members of its group.
The properties of water are different from the properties of the elements of which it is formed. Discuss.
What is the effect on litmus if it is introduced in pure water ?
Which property of water enables it to modify the climate ?
What is the composition of water? In what volume are its two constituent combined?
State the position of hydrogen in the periodic table.
Comment on the dual position of hydrogen in the periodic table
Why is aluminium metal not used in the lab preparation of hydrogen?
Hydrogen is collected by downward displacement of water and not of air, even though it is lighter than air. Explain why?
What is the physical state of water at 2570C?
Why does ice float on water ?
What is the physical state of water at 100∘C?
What is the physical state of water at 0∘C?
Explain why apparatus for laboratory preparation of hydrogen should be air tight and away from a naked flame?
Why is sodium metal not used in the lab preparation of hydrogen?
Deionised water is obtained in ion exchange method.
If this is true enter 1, if false enter 0.
In exchange process, H2S gas is passed through cold water, the hydrogen atoms in H2S exchange with deuterium from D2O present in water. Thus, H2S becomes D2S.
If this is true enter 1, if false enter 0.
How can the production of dihydrogen, obtained from coal gasification, be increased?
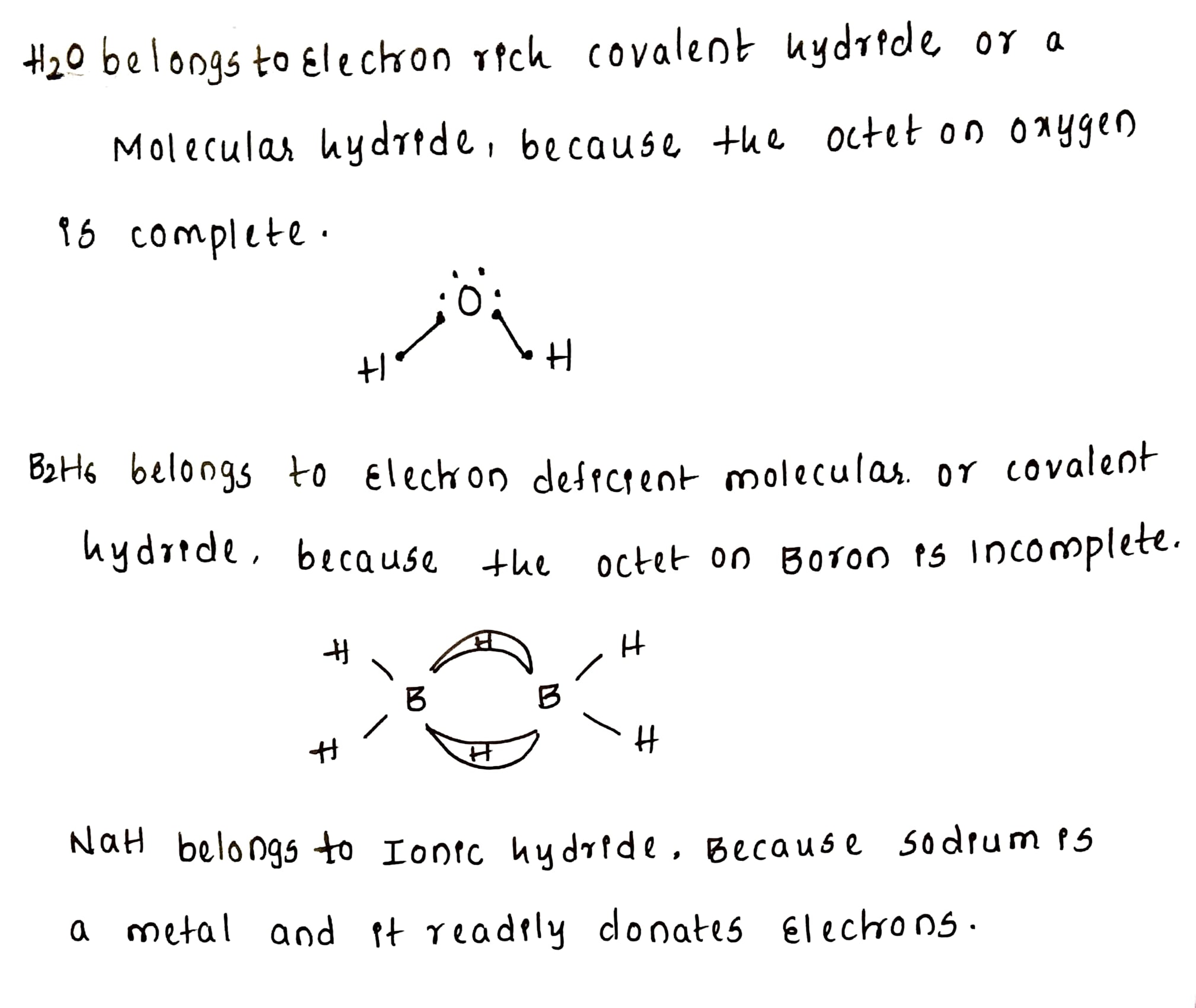
What do you understand by (i) electron-deficient, (ii) electron-precise, and (iii) electron-rich compounds of hydrogen?
What characteristics do you except from an electron-deficient hydride with respect to its structure and chemical reactions?
Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Saline hydrides are known to react with water violently producing fire. Can CO2, a well known fire extinguisher, be used in this case? Explain.
Complete the following chemical reaction and classify into (a) hydrolysis, (b) redox and (c) hydration reaction. AlCl3(g)+H2O(l)→
What do you understand by the term 'auto-protolysis' of water? What is its significance?
What do you understand by the term non-stoichiometric hydrides? Do you expect this type of the hydrides to be formed by alkali metals? Justify your answer.
Arrange the following.(i) CaH2, BeH2 and TiH2 in order of increasing electrical conductance.
(ii) LiH,NaH and CsH in order of increasing ionic character.
(iii) H−H,D−D and F−F in order of increasing bond dissociation enthalpy.
(iv) NaH,MgH2 and H2O in order of increasing reducing property.
(ii) LiH,NaH and CsH in order of increasing ionic character.
(iii) H−H,D−D and F−F in order of increasing bond dissociation enthalpy.
(iv) NaH,MgH2 and H2O in order of increasing reducing property.
Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why?
Complete the following chemical reaction and classify the into (a) hydrolysis, (b) redox and (c) hydration reactions.CaO(s)+H2O(g)→
How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Do you expect the carbon hydrides of the type (CnH2n+2) to act as Lewis acid or base? Justify your answer.
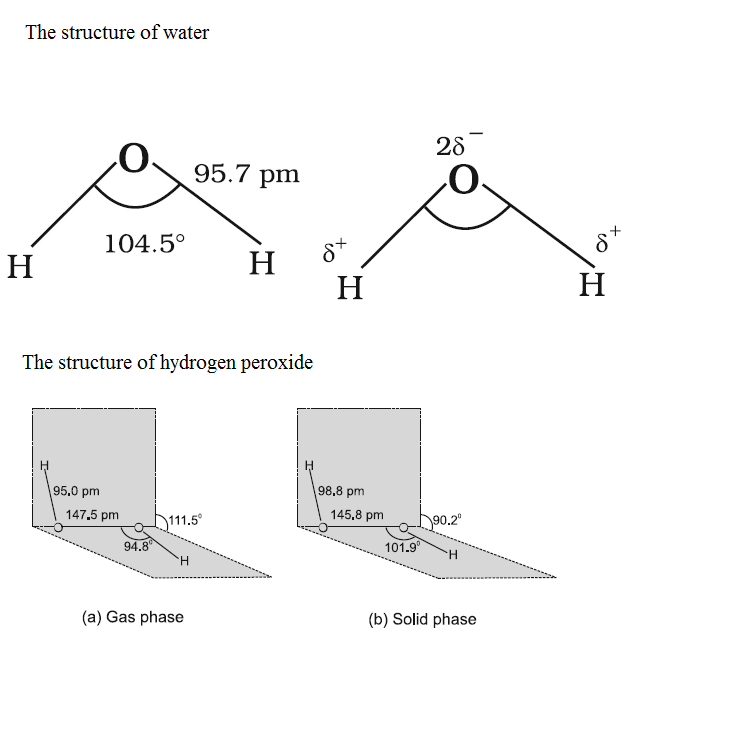
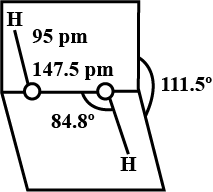
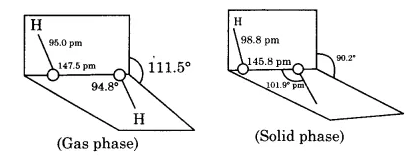
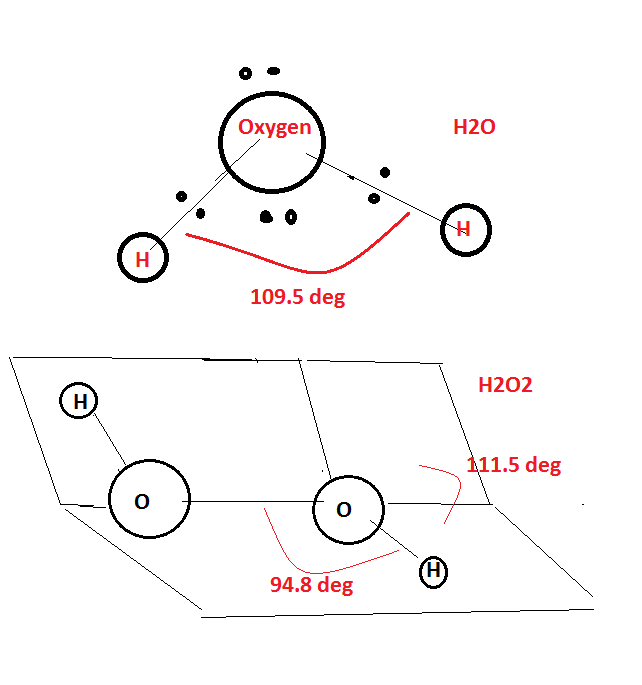
Compare the structures of H2O and H2O2.
What is the difference between the terms hydrolysis and hydration?
Explain syngas in detail.
Complete the following chemical reaction and classify into (a) hydrolysis, (b) redox and (c) hydration reactions. Ca3N2(s)+H2O(l)→
Write chemical reactions to show the amphoteric nature of water.
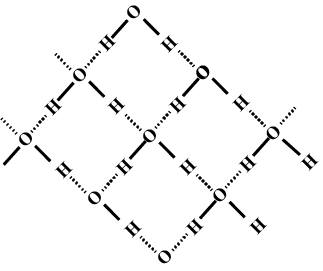
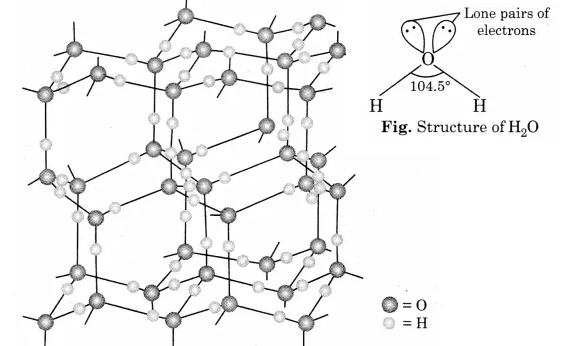
Describe the structure of the common form of ice.
How aquatic animals survive is cold countries when temperature fall below 0∘C?
As oxygen is ______ soluble in water, air dissolved in water contains a higher percentage of oxygen (30−35%) than ordinary air (21%).
Pure water is a _______ conductor of electricity.
Match the Column-I with Column-II:
Magnesium reacts with steam to form ________ and ________.
As we go up the mountains, the boiling point of water decreases. Why?
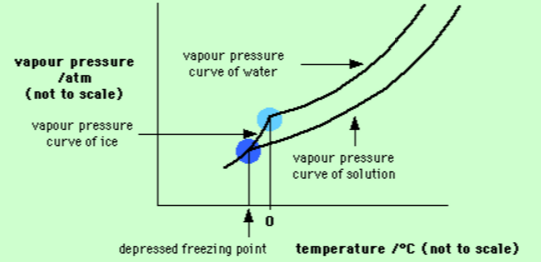
Water containing impurities doesn't freeze at 0. Explain?
Water bodies like sea, rivers etc., which are deep appear blue. Why?
Water is tasteless, but we experience different tastes of water in different places. Why?
Write the uses of dihydrogen gas:
Describe an experiment to prove that water is transparent.
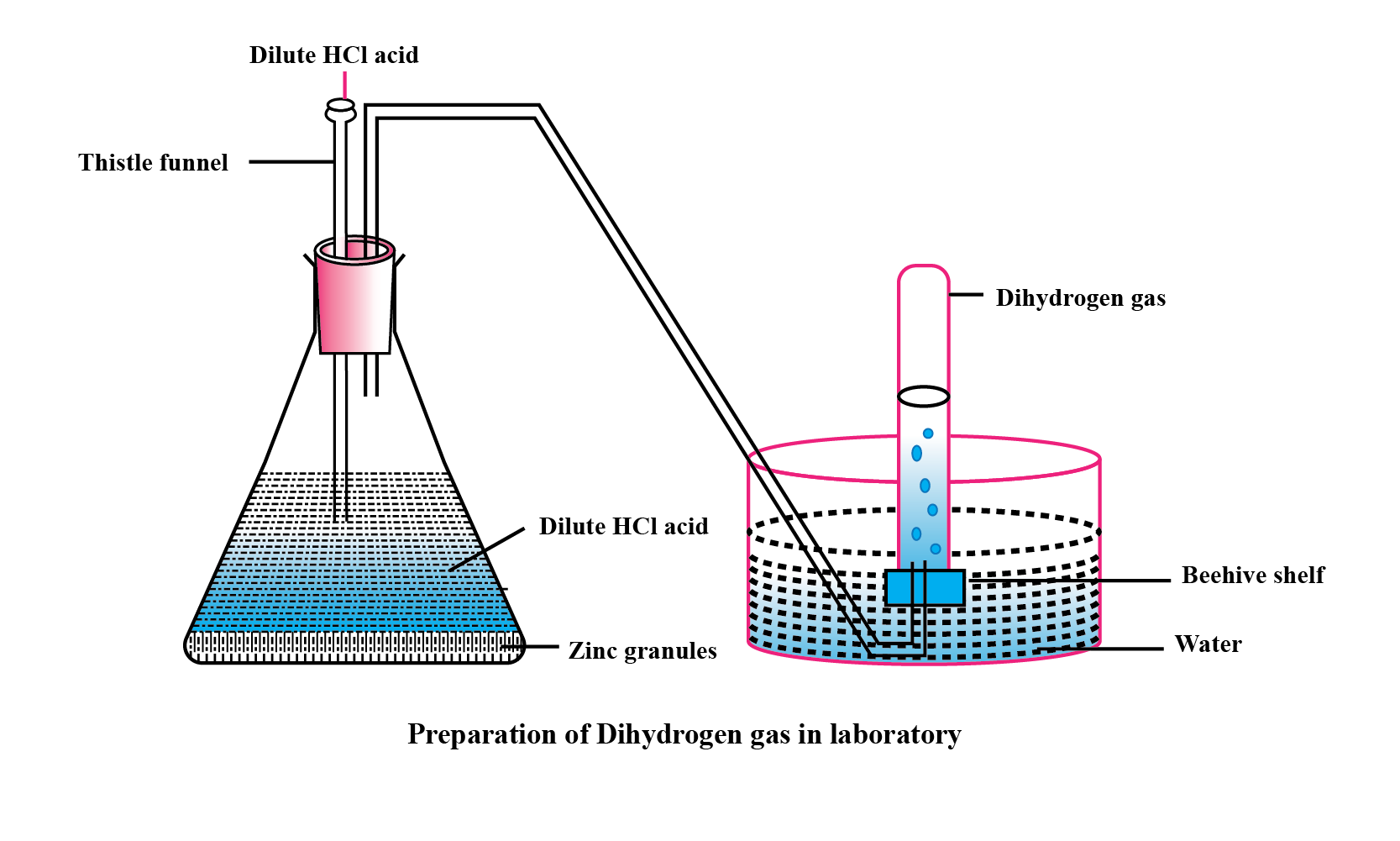
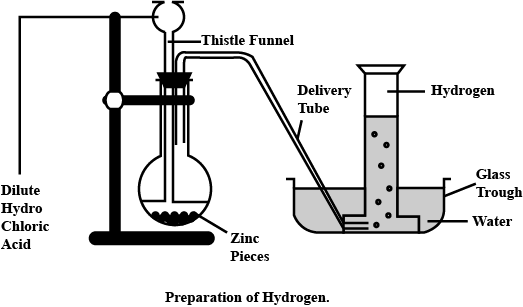
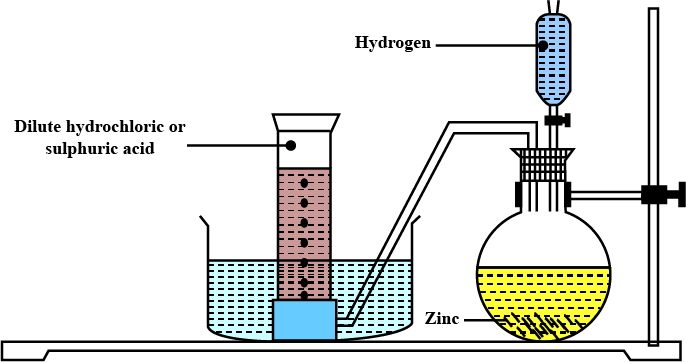
Explain the method of preparation of hydrogen gas in laboratory with chemical equation and diagram?
Excess of KI and dil.H2SO4 were mixed in 50 mL H2O2. The liberated I2 required 20 mL of 0.1 N Na2S2O3. Find out the strength of H2O2 in g per litre.
How many moles of H_2O_2 are present in 2 litres of 6.8% w/v H_2O_2 solution?
Calculate percentage of water of crystallization in : Mg\ SO_7H_2O [Atomic masses of Mg= 24, S=32, O=16, H=1]
Consider the reaction between Zn and dilute hydrochloric acid forming zinc chloride and liberating hydrogen.
Explain the method of preparation of hydrogen gas in laboratory with chemical equation and diagram.
Why Hydrogen although lighter than air, is not collected by the downwards displacement of air?
Why is Hydrogen placed in group I of the modern periodic table ?
Why is distilled water considered to be the purest form of water?
Why does water have higher boiling point than alcohol? At what temperature in kelvin, it can be change into solid state and into vapours?
Why does hydrogen resemble both group I and group 17 elements ?
Why ice is lighter than water?
Water is a good fire extinguisher. But it is not used extinguish electrical fires and oil for?
Explain the position of hydrogen in the periodic table on the basis of its electronic configuration.
Physical properties of ice, water and steam are different. What is the chemical composition of water in all the three states?
In order to decompose 9 g water 142.5 kJ heat is required. Hence, the enthalpy of formation of water is:
When water boils the temperature remains constant. Give reasons.
2H_2 is needed to make one molecule of water, here '2H_2' means:
How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Hydrogen is not a metal but it has been assigned aplace in the reactivity series of metals.Explain.
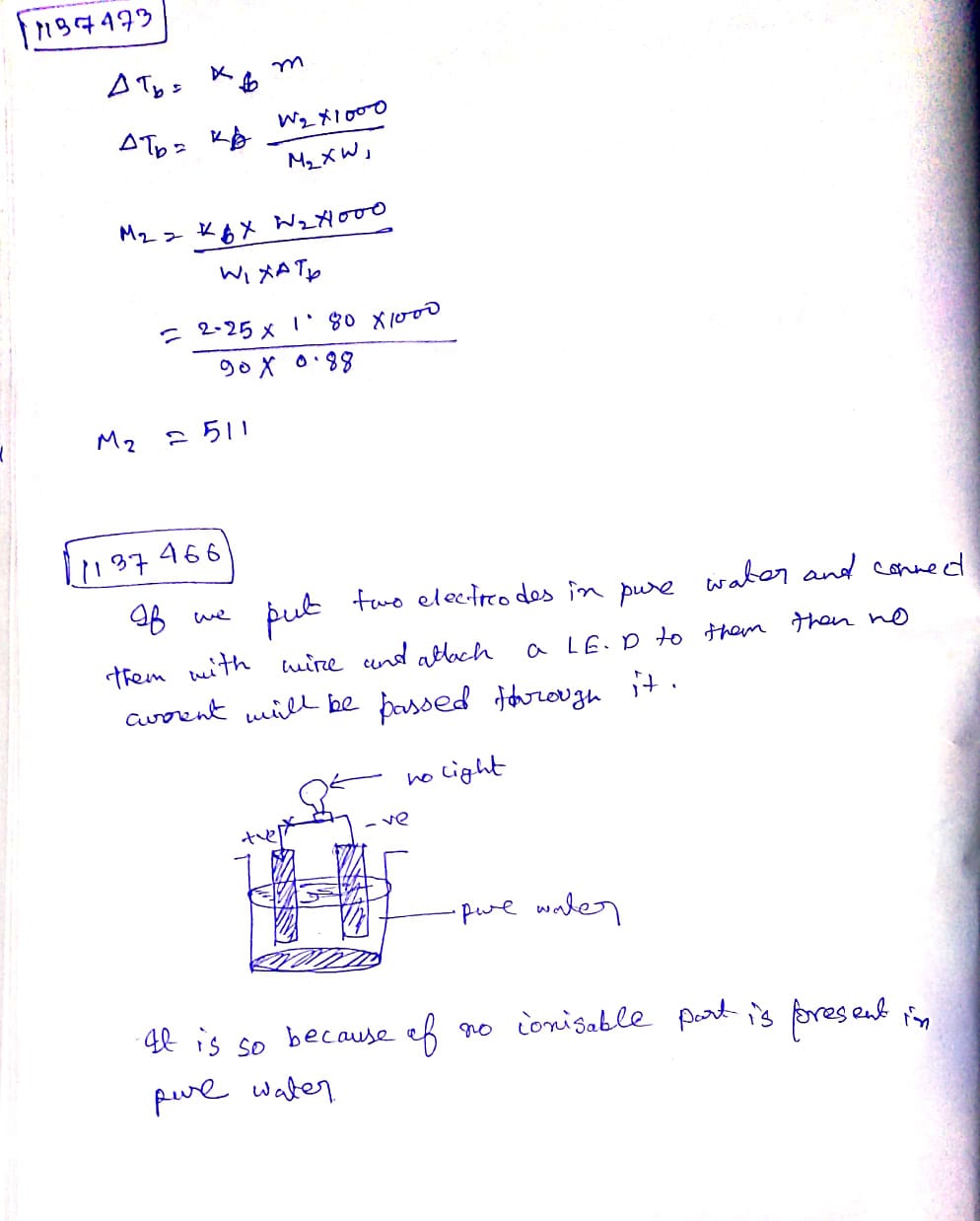
How can you prove that distilled water is a non-electrolyte? Explain with the help of well labelled diagram.
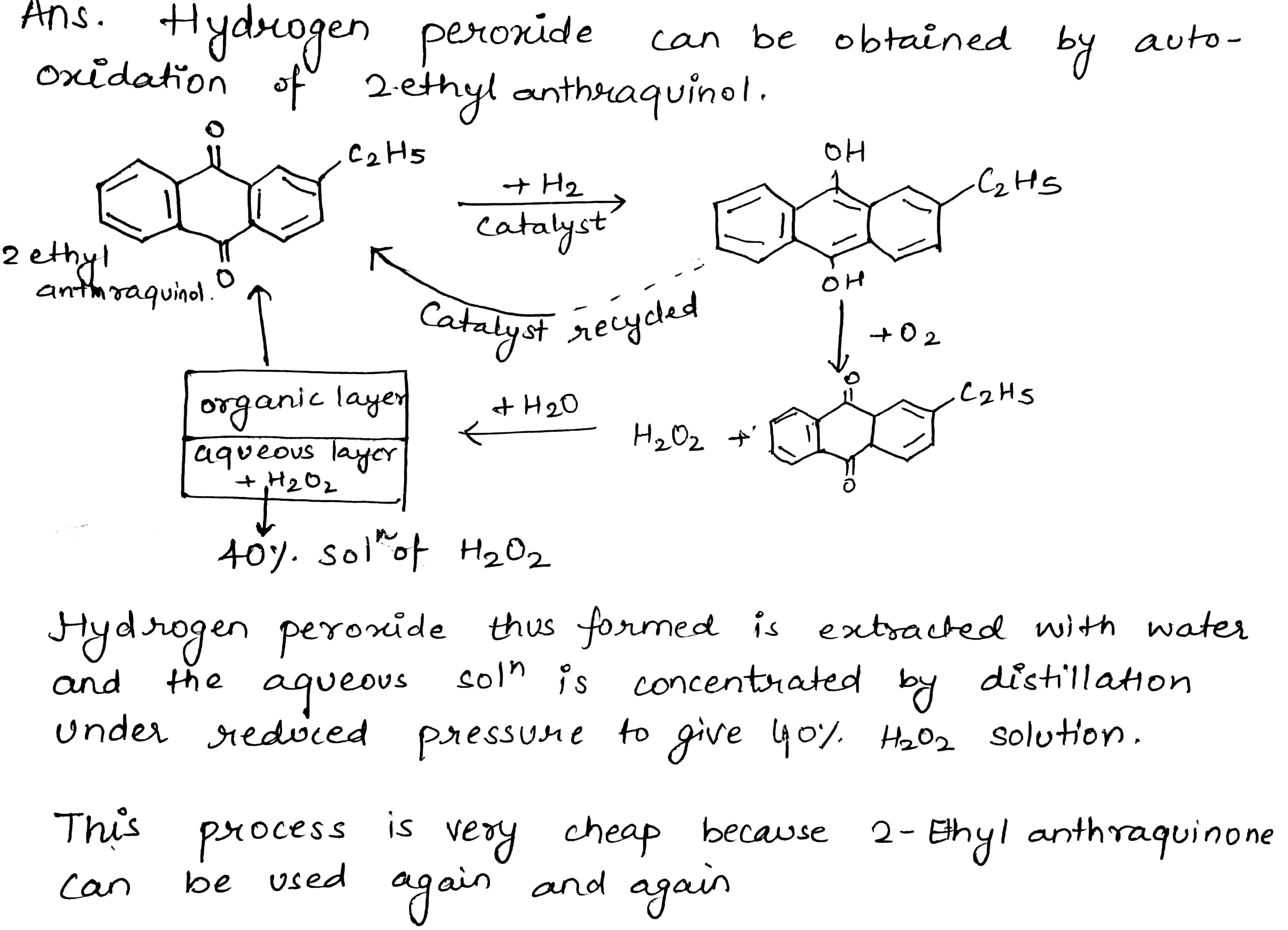
Explain the laboratory method for preparation of hydrogen peroxide.
Among PCl_3, CH_{3}^{+}, NH_{2}^{-} and NF_3, ......... is least reactive towards water.
Answer the following:(a) Name a process by which hydrogen gas is manufactured.
(b) Give equation for the reaction.
(c) How is hydrogen seperated from carbon dioxide and carbon monoxide?
As many as...... different combination of water are possible form
_{1}H^{1}, _{1}H^{2},_{1}H^{3} and _{8}O^{16},_{8}O^{17},_{8}O^{18}
Tripe point of water is
What is the physical state of water at :
(a) 25^oC
(b) 0^oC
(c) 100^oC ?
Wrte any two measures that you would suggest for the better management of water resources
Fill in the blanks :
............... is the purest form of natural water.
Fill in the blanks :
Water has .............. density and .............. volume at 4^0C.
Explain why H_{2} and O_{2} do not react at room temperature.
These metal combines H to give white crystalline ionic hydrides of the general of the formula MH.
The tendency to form their hydrides, basic character and stability decreases from Li to Cs since the electropositive character decreases from Cs to Li.
How hydrogen atom is different from atoms of all other element ?
What is potable water? Write its characters.
Give reason to explain why it takes longer to dry wet clothes in humid weather?
Water is liquid at room temperature but not hydrogen sulphide. Why?
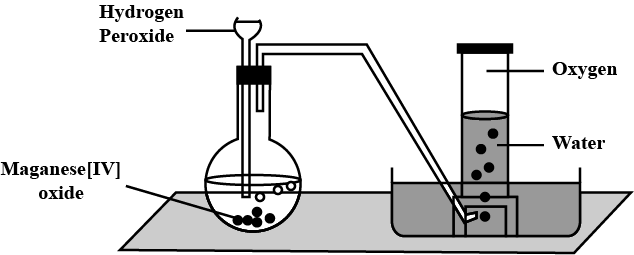
Taking hydrogen peroxide, how would you prepare oxygen in the laboratory ?
Why is water not used as solvent synthesis of Grignard reagent?
Why does hydrogen show dual nature ?
Is it possible for the atom of an element to have one electron, one proton or no neutron? If so, name the element.
An ice cube at 0^oC is placed in some liquid water at 0^oC, the ice cube sinks - why?Discuss the three types of Covalent hydrides
Describe the different types of hydrides which are formed.
The harmful substances dissolved in water are ..........
Suggest reasons for and against the inclusion of hydrogen in the main groups of the periodic table.
Give the name and formula of the compounds as indicated in the following statement.
A compound of calcium and hydrogen which is used as a portable source of hydrogen for filling balloons.
Why is the ionization enthalpy of hydrogen higher than that of sodium?
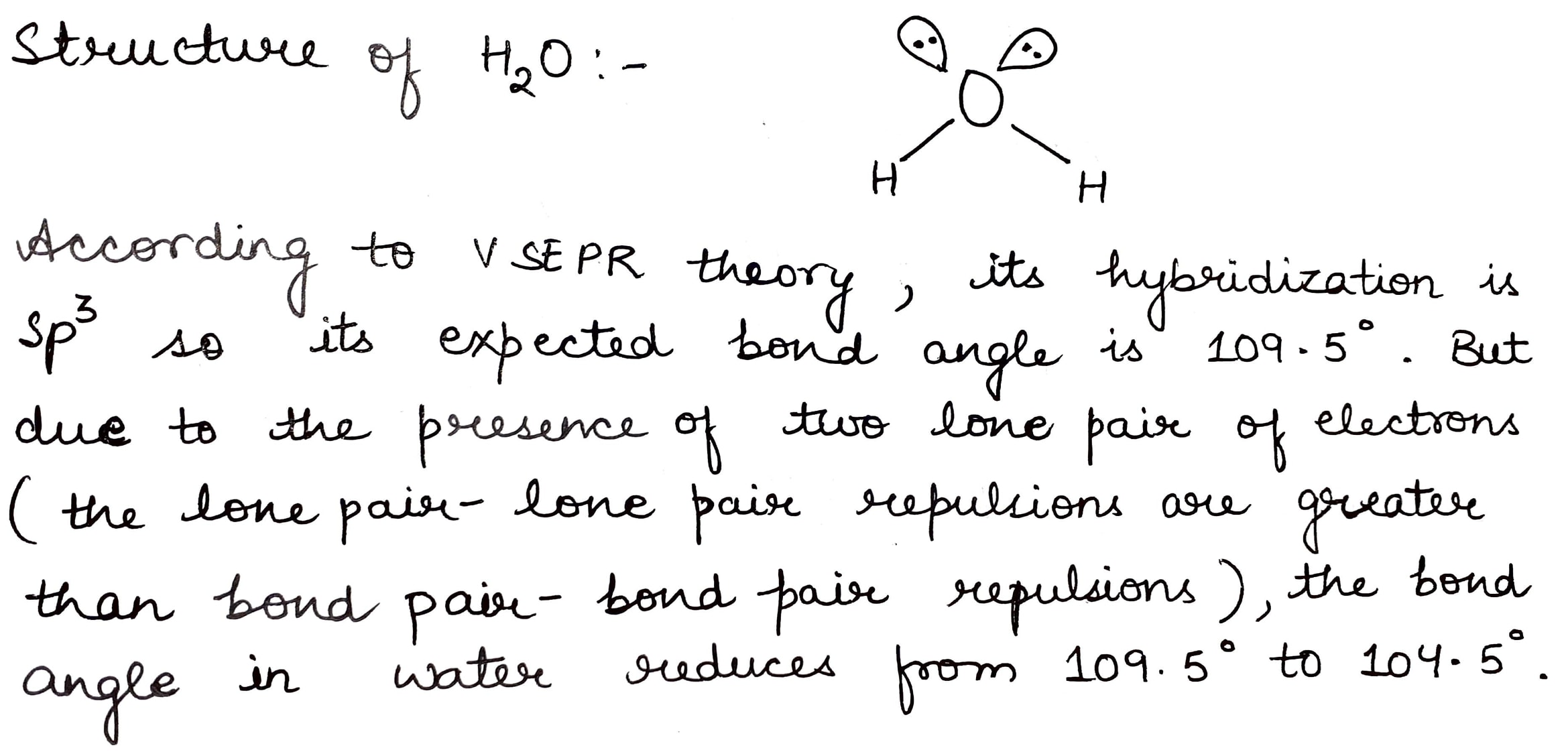
Why is water molecule polar?
What is deionised water? Can distilled water be called as deionised water?
Give three characteristics in which hydrogen resembles halogens.
Which is heavier, ice or water?
Answer the following:
What is autoprotolysis of water?
Give the pH of D_2O and H_2O at 298\ K.
Answer the following :
Name the chemical compound used in the preparation of H_2O_2 by auto- oxidation.
Give an example in which H_2O acts as reducing agent.
Answer the following :
What type of hydrides are formed by the elements having low electronegativity?
Answer the following :
Why D_2O has more density than H_2O?
Mention the condition in which the following statement is true.
Hydrogen can be prepared from water at ordinary temperature.
Give three characteristics in which hydrogen resembles alkali metals.
Answer the following :
In which group of the periodic table, hydrogen is placed?
What happens when water reacts with the following compounds?
Antimony chloride (SbCl_3), bismuth chloride (BiCl_3)
Antimony chloride (SbCl_3), bismuth chloride (BiCl_3)
What happens when?
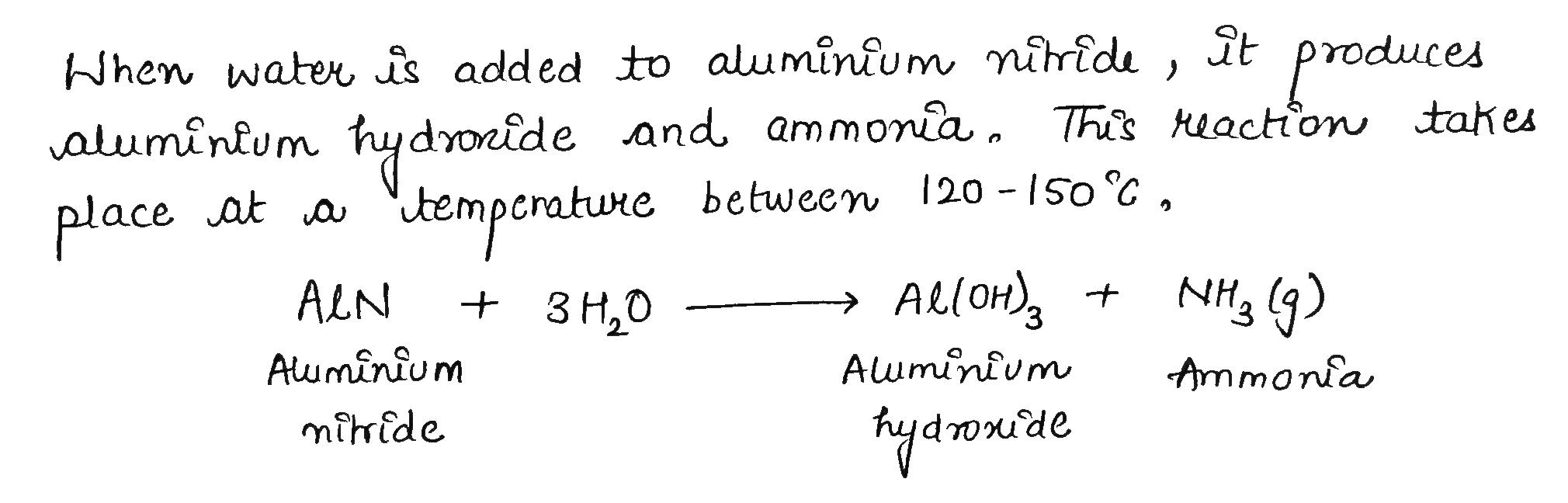
Water is added to aluminium nitride.
What happens when water reacts with the following compounds?
Red hot iron
Answer the following:
What is plumbosolvency?
Answer the following question:
What happens when F_{2} reacts with water?
"Hydrogen occupies a unique position in Modern Periodic Table". Justify the statement.
A sample of water under study was found to boil at 102^{\circ}C at normal pressure. Is the water pure? Will this water freeze at 0^{\circ}C? Comment.
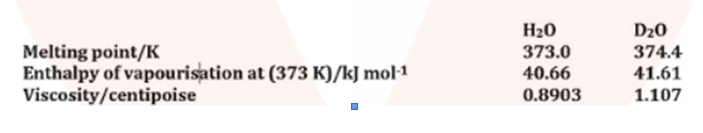
Melting point, enthalpy of vapourisation and viscosity data of H_2O and D_2O is given below:
On the basis of this data explain in which of these liquids intermolecular forces are stronger?
Complete the following reactions
(i) O_{2}^{2-}+H_{2} O \rightarrow
What is the fraction of the mass of water due to neutrons?
Write the balanced chemical equation for hydrogen peroxide catalyzed by manganese dioxide.
Give a balanced chemical equation of the reaction for hydrogen gas preparation in the laboratory.
Complete the following reactions
(ii) O_{2}^{-}+H_{2} O \rightarrow
Name:
A chemical called oxygenated water.
What is the effect on boiling point of water when
(a) pressure is increased
(b) impurity is added
Draw a neat and well-labelled diagram for the laboratory preparation of hydrogen.
Name the chemicals required to prepare hydrogen gas in the laboratory.
Why is oxygen collected by downward displacement of water ?
Answer in brief: What is mineral water?
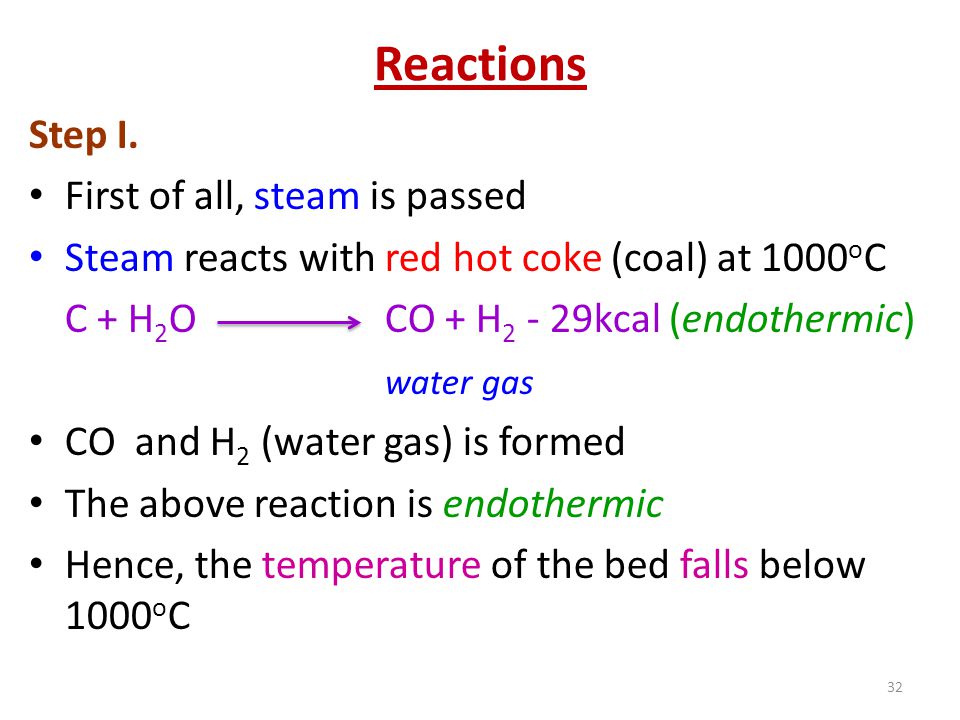
Name the gas produced when steam is passed over hot coke.
Why is hydrogen kept in the metal activity series?
Give any three important uses of water.
Name two gases dissolved in natural water.
What is the smallest particle of water? Describe its structure.
Water has __________ density and ___________ volume at 4^{0}C.
Water molecules in loose chemical combination with other substances is called .........................
Why is hydrogen placed with alkali metals?
How are natural springs of water formed?
Why sea water does not freeze at 0^{0}C?
What nature does hydrogen show ?
Compare hydrogen and halogens on the basis of:
Physical state
Name a liquid that is an oxidizing as well as reducing agent.
Why should a glass bottle completely filled with water never be kept in a freezer?
Explain the various properties of water.
Justify position of Hydrogen in the periodic table.
Compare hydrogen with alkali metals on the basis of:
Ion formation
Apparatus for laboratory preparation of Hydrogen should be air right be air tight and away from a naked flame.
Compare hydrogen and halogens on the basis of:
Valency
How is pure H_2 obtained?
Why ethyl alcohol has lower boiling point than water?
What are the ways in which water molecules are bonded to an anhydrous salt to form a hydrate?
Which type of bond is present in the water molecule?
Halima picks up a small stone from the ground and puts it in the water in a dish. Does the shape of the stone change ?
Write the molecular formula of water.
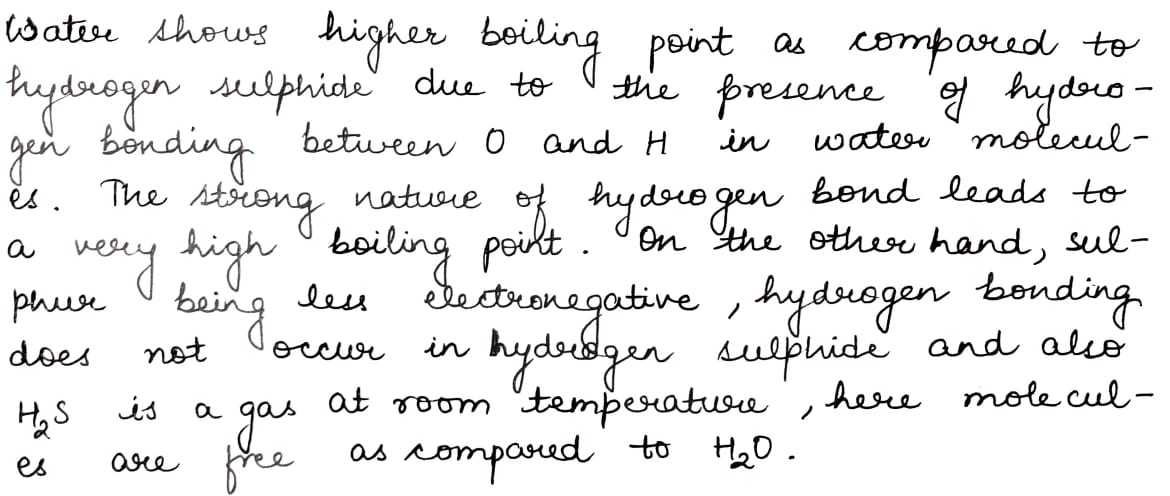
Why does water have a high boiling point and a high melting point as compared to H_2S?
Riya pours some water from her bottle into another bottle. Does it change the shape of the water?
Concentrated sulphuric acid cannot be used for drying H_2. Why?
Complete the reaction :
Fe(s) + H_2O(g) \rightarrow \;?
Why can dilute solution of hydrogen peroxide not be concentrated by heating?
In a reaction of F_2 and H_2O, what is the role of water?
Dilute solution of hydrogen peroxide cannot be heated strongly for its concentration. Explain.
Which type of oxide is water?
Find the volume strength of the 2N H_2O_2 solution.
Discuss the position of hydrogen in the periodic table is not justified.
What is water gas and how it is obtained?
How would you prepare dihydrogen from water by using a reducing agent?
What is the structure of H_2O_2? Draw a schematic diagram indicating the shape of the molecule clearly.
How would you prepare very pure H_2 in the laboratory?
How would you prepare dihydrogen from a substance other than water?
In what respects does hydrogen resemble alkali metals? How does it resemble halogen?
Describe the structure of the common form of ice.
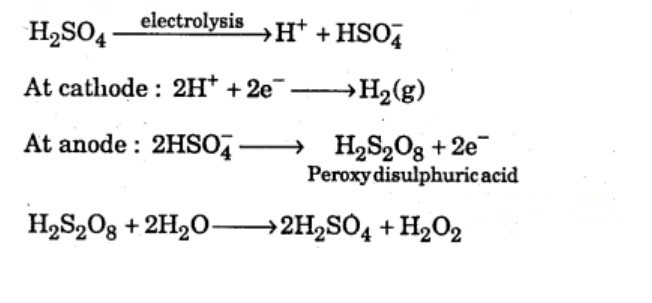
How is H_2O_2 manufactured?
Sample of hydrogen peroxide solution is 1.5 M. If it is to be labelled ‘X’ volume; what is the value of ‘X’.
Explain with examples :- Polymeric hybrids.
- Polymeric hybrids.
Explain with examples Complex hydrides.
Describe the different methods by which the concentration of Hydrogen peroxide.
Calculate the volume of 10 volume H_2O_2 required to neutralize 200 mL of 2N KMnO_4 in acidic medium.
Calculate the normality of 20 volume solution of H_2O_2.
30 mL of H_2O_2 solution after acidification required 30 mL of N/10 KMnO_4 solution for complete oxidation. Calculate the percentage and volume strength of H_2O_2 solution.
Write balanced equations for the following:
Hydrated Barium peroxide reacts with orthophosphoric acid.
Reaction of steam on hydrocarbon or coke at high temperature in the presence of catalyst yields a mixture of gases. Write a reaction to the above-mentioned process.
10 ml of given H_2O_2 solution contains 0.91\,g of H_2O_2. Express its strength in volumes.
Find the volume strength of the 1.6N H_2O_2 solution.
How is hydrogen peroxide prepared industrially? Explain why it is stored in colored wax-lined glass or plastic bottles?
Calculate the volume strength of a 3\% solution of H_2O_2.
Calculate the amount of Hydrogen peroxide presents in 10 ml of 25 volume solution of H_2O_2.
Write the similarities of hydrogen with alkali metals.
Ice floats on water. Give reason.
At what temperature, density of water is maximum.
How is the structure of hydrogen peroxide?
What is the reason of high boiling point of water?
Write the similarities of hydrogen with halogens.
What is hydride? How many types of hydrides are there? Explain example.
What do you meant by molecular association in water. Explain the structure of water with diagram. Explain the normal structure of ice.
It is justified to place hydrogen in a separate place in periodic table. Explain the statement.
Calculate molarity and normality of 20 volumes of H_2O_2. What is its mass percentage?
Which is the substance that absorbed hydrogen and oxygen from sugar in the ratio as in water?
Explain the following reaction with respect to the hydrogen peroxide
Formation of peroxide
Explain the laboratory method for preparation of dihydrogen.
List out the hydrogen compounds known to you
- The gas which is combustible and is formed through the electrolysis of water
What happens during the electrolysis of water? Complete its chemical equation given below
2H_2O\xrightarrow{electricity}............ + .............
Why is ice less dense than water and what kind of attractive forces must be overcome to melt ice?
Explain : (i) water has a maximum density at 277\, K. (ii) ice floats over water.
When water is heated at its boiling point or melting point, its temperature does not change.
a. What is meant by boiling point and melting point?
b. What are the boiling and freezing points of water?
c. Why is there no change in temperature?
Is it correct to say that hydrogen can behave as a metal? State the conditions under which such behavior can be possible?
A definite quantity of water and coconut oil are heated in separate test tubes using the same amount of heat.
a. In which case does the temperature increase slowly?
b. What is the reason for this?
c. Write any one practical application of this property.
The volume of O_2 gas at S.T.P. obtained by the decomposition of 1 c.c. of H_2O_2 solutionis known as its volume strength.
If this is true enter 1, if false enter 0.
Why do rivers and lakes not freeze easily ?
Hydrogen may be prepared in the laboratory by the action of a metal on an acid. How would you modify your apparatus in to collect dry hydrogen ? Which drying agent would you employ for this purpose ?
What is meant by demineralised water and how can it be obtained?
Do you expect different products in solution when aluminium(III) chloride and potassium chloride treated separately with (i) normal water (ii) acidified the water, and (iii) alkaline water? Write equations wherever necessary.
How can saline hydrides remove traces of water from organic compounds?
What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15, 19, 23 and 44 with dihydrogen? Compare their behaviour towards water.
A hydride is a compound containing hydride ion, {H}^{-}. Predict two elements whose hydrides would contain incomplete octets.
Do you expect different products in solution when aluminium (III) chloride and potassium chloride treated separately with (i) normal water (ii) acidified water, and (iii) alkaline water? Write equations wherever necessary.
The drain cleaner, Drainex contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20^o C and one bar will be released when 0.15 g of aluminum reacts?
The drain cleaner, Drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20C and one bar will be released when 0.15g of aluminium reacts?
The drain cleaner, Drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen at 20^{\circ}C and one bar will be released when 0.15g of aluminium reacts?
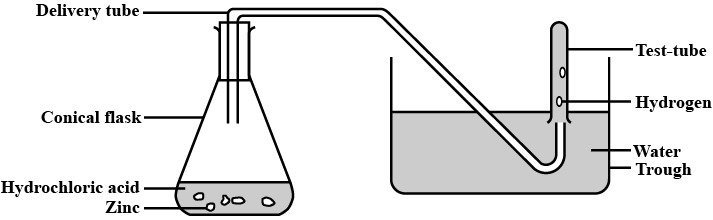
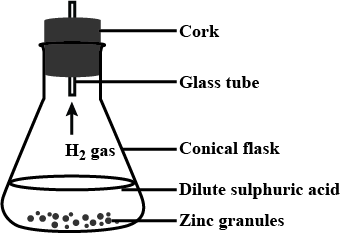
Observe the following activity and and answer the questions(a) Do you observe anything happening around the zinc granules ?
(b) Is there any change in its temperature ?
(c) Why is glass tube not dipped in dil H_2SO_4 ?
(d) How is H_2 gas collected by downward displacement or upward displacement of water ?
(e) Is H_2 gas soluble or insoluble in water ?
(f) Is H_2 gas heavier or lighter than air ?
When water is heated at a temperature x by a process called P. And when steam gets reconverted at the same temperature into water and the process is called as Q.(a) What is the temperature x in kelvin?(b) what is the process P & Q known as?(c) Name the energy released and absorbed during process P & Q respectively?
What are electrons deficient and electron rich compounds of hydrogen? Give examples.
Explain why on addition of 1 mol of NaCl to 1 litre of water, the boiling point of water increases, while addition of 1 mol of methyl alcohol to one litre of water decreases its boiling point.
What is negative catalyst? Which is the negative catalyst in the decomposition of H_2O_2?
Give examples of ionic hydride, molecular hydrides, interstitial hydride, electron precise hydride.
Comment on the reactions of dihydrogen with
(i) Chlorine
(ii) sodium, and
(iii) copper(ii)oxide
What do you understand by the term 'auto-protolysis' of water? What is its significance?
Compare the structure of H_{2}O and H_{2}O_{2}.
What types of elements form interstitial hydrides?
Why Nitric acid is not used for the preparation of hydrogen ?
Distinguish clearly between salt-like and covalent hydrides.
Why does elemental hydrogen react with other substances only slowly at room temperature?
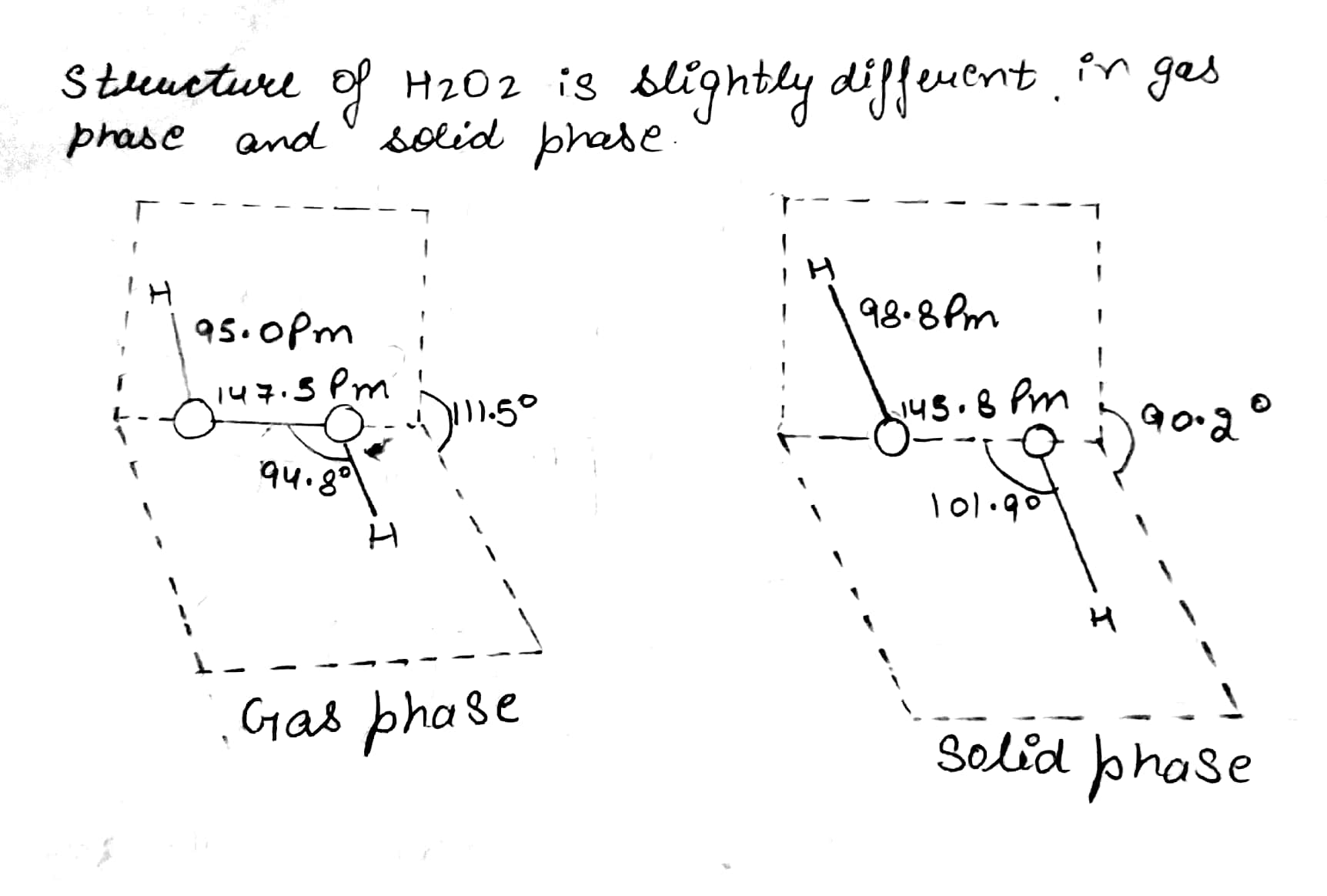
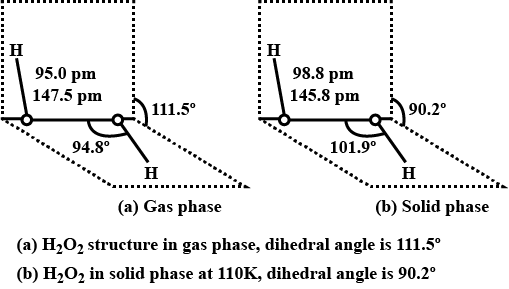
Draw the gas phase and solid phase structure of H_2O_2.
What are the metallic/interstitial hydrides? How do they differ from molecular hydrides?
Name the classes of hydrides to which H_2O, B_2H_6 and NaH belong.
If same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
Melting point, enthalpy of vaporization and viscosity data of H_2O and D_2O is given below:
| Properties | H_2O | D_2O |
| Melting point K | 373.0 | 374.4 |
| Enthalpy of vaporization at (373K)/kj\,mol^{-1} | 40.66 | 41.61 |
| Viscosity/centipoise | 0.8903 | 1.107 |
Give reasons: (a) Lakes freeze from top towards bottom.
(b) Ice floats on water.
Molecular hydrides are classified as electron deficient, electron precise and electron-rich compounds. Explain each type with two examples
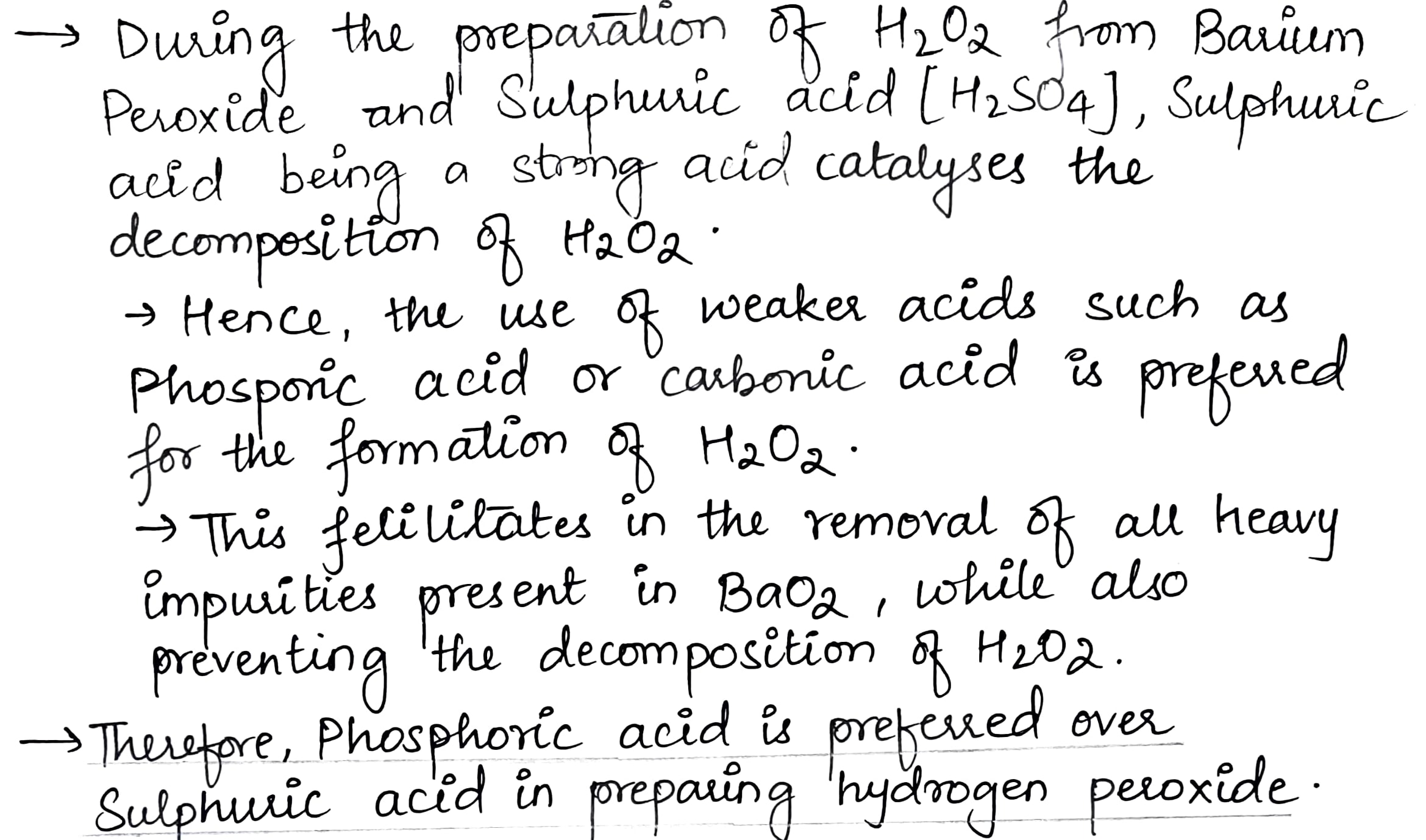
Phosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
Give reasons why hydrogen resembles alkali metals?
Why does water show higher boiling point as compared to hydrogen sulphide? Give reasons for your answer.
Give a method for the manufacture of hydrogen peroxide and explain the reactions involved therein.
An ionic hydride of an alkali metal has significant covalent character and is almost unreactive towards oxygen and chlorine. This is used in the synthesis of other useful hydrides. Write the formula of this hydride. Write its reaction with Al_2Cl_6
A colourless liquid 'A' contains H and O elements only. It decomposes slowly on exposure to light. It is stabilized by mixing urea to store in the presence of light.
Suggest a possible structure of A.
How will you account for 104.5^0 bond angle in water?
Which one is a covalent hydride?
BeH_2, NaH, CaH_2
Write short notes on:
Strength of hydrogen peroxide
Give reason
Presence of water is avoided in the preparation of H_2O_2 from Na_2O_2.
What is perhydrol? Give its composition and use
What happens when water is added to calcium phosphide?
What happens when?
Excess of water is added to a concentrated solution of antimony chloride.
Excess of water is added to a concentrated solution of antimony chloride.
Why does the hardness of water render it unfit for washing purposes?
Phosphoric acid is preferred over sulphuric acid in preparing hydrogen peroxide from peroxides. Why?
Compare hydrogen with alkali metals on the basis of:
Reducing -power
(i) Draw the gas phase and solid phase structure of H_2O_2.
(ii) H_2O_2 is a better oxidising agent than water. Explain.
Name the classes of hydrides to which H_2O, B_2H_6 and NaH belong.
For laboratory preparation of hydrogen, give the following fully labelled diagram
Compare hydrogen and halogens on the basis of:
Reduction with oxygen
For laboratory preparation of hydrogen, give the following materials used
For laboratory preparation of hydrogen, give the following chemical equation
For laboratory preparation of hydrogen, give the following method of collection
In what respect does hydrogen differ from
Alkali metals
Compare hydrogen with alkali metals on the basis of:
Reduction with oxygen
Compare hydrogen with alkali metals on the basis of:
Oxygen formation
In what respect does hydrogen differ from
Halogens
Compare hydrogen and halogens on the basis of:
Ion formation
Which gas is evolved when Mg_3N_2 (Magnesium nitride) is treated with H_2O? Give chemical reactions.
Anhydrous BaO_2 is not used for preparing H_2O_2. Why?
What is the strength of 10 volume $$H_2O_2$$.
Give an example of each of an ionic hydride and a covalent hydride.
When sodium hydride is electrolyzed, hydrogen is liberated at which electrode? Give an equation.
Give two examples of interstitial hydrides.
What is the strength of 20 volume H_2O_2.
Write the properties of Water.
Reaction of steam on hydrocarbon or coke at high temperature in the presence of catalyst yields a mixture of gases. What is a specific name given to the mixture of gases?
Reaction of steam on hydrocarbon or coke at high temperature in the presence of catalyst yields a mixture of gases. Name the gases produced.
Why do lakes freeze from the top towards the bottom ?
What do you understand by metallic hydrides?
Ionisation energy of hydrogen is much higher than those of alkali metals. In case of the former combines with the latter, which type of compound will be formed? Explain when H_2 reacts with C at high temperature, what type of hydride will be formed. Give the chemical equations.
Fill in blanks:
H+ ion in water exists as ……… ion called …………….
Reaction of steam on hydrocarbon or coke at high temperature in the presence of catalyst yields a mixture of gases. Write the reaction when the mixture of gases is reacted with steam in the presence of iron chromate as a catalyst.
The process \dfrac{1}{2}H_2(g) + e^– → H^– (g) is endothermic (\Delta H =+151kJmol^{-1}), yet salt like hydrides are known. How do you account for this?
Write two methods to reduce the surface tension of water.
The Boiling point of H_2O is higher than that of H_2S. Explain.
Soap decrease the surface tension of water.
a. What is surface tension?
b. How does the decrease in surface tension benefit washing of clothes?
Sodium fir in the laboratory should Do not be extinguished by Pouring Water .Why ?
Explain why beryllium forms a covalent hydride while calcium forms an ionic hydride.
100 ml each of coconut oil and water are taken in two beakers and kept in the freezer.
a. What difference can be observed in their volumes during freezing?
b. What do you infer from the observation?
c. When water is frozen in glass bottles, it is advised not to fill the bottles completely. Explain the reason.
In the following reactions assign for underlined atom for product of complete hydrolysis at R.T.
A. If product is oxy acid with —ic suffix.
B. If product is oxy acid with —ous suffix.
C. If product are two oxy acids one with —ic suffix and another one with —ous suffix.
D. If product is not oxy acid, neither with —ic suffix nor with —ous suffix.
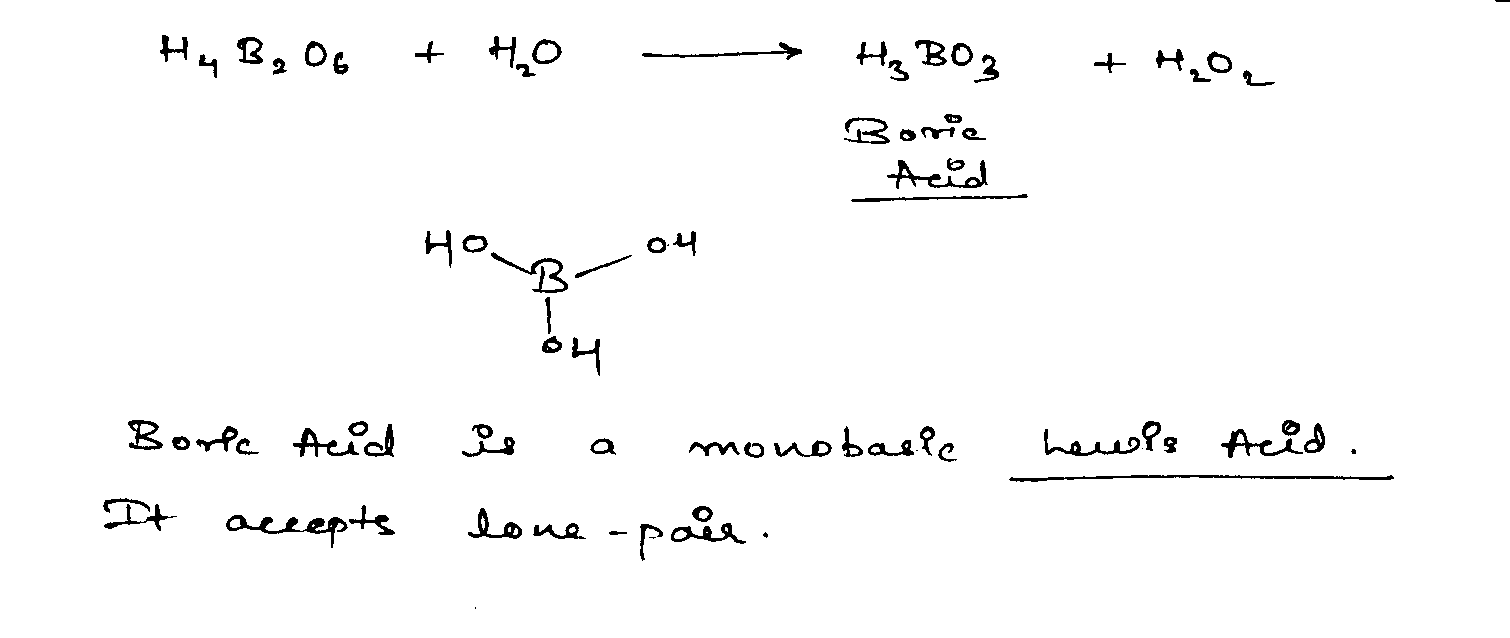
H_4\underline B_2O_6+H_2O \longrightarrow H_3BO_3+H_2O_2
Can marine species live in distilled water?
Discuss briefly the characteristics of salt like hydrides.
Explain why electrolysis of ordinary water occurs faster than heavy water.
How is dihydrogen obtained from
(i) water (ii) dilute acids (iii) alkalies?
In the reaction of F_2 with H_2O, water acts as a ................ .
The electrolysis of molten sodium hydride liberates ............... gas at the anode.
Ionic hydrides are frequently used to remove traces of water from organic compounds. What is the underlying basis of this process?
Complete the following reactions :
(i) CuO(s) +H_2(g)\rightarrow
(ii) CO(g) +H_2(g)\rightarrow
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions