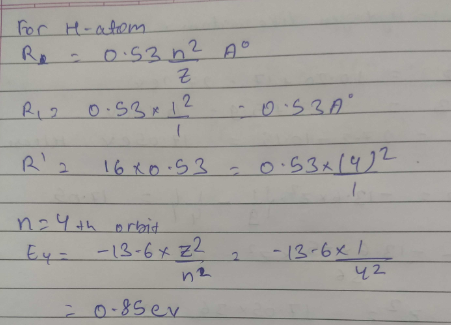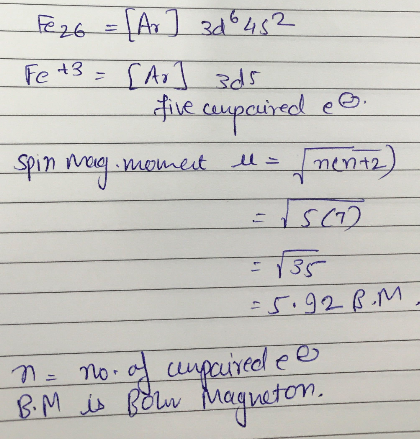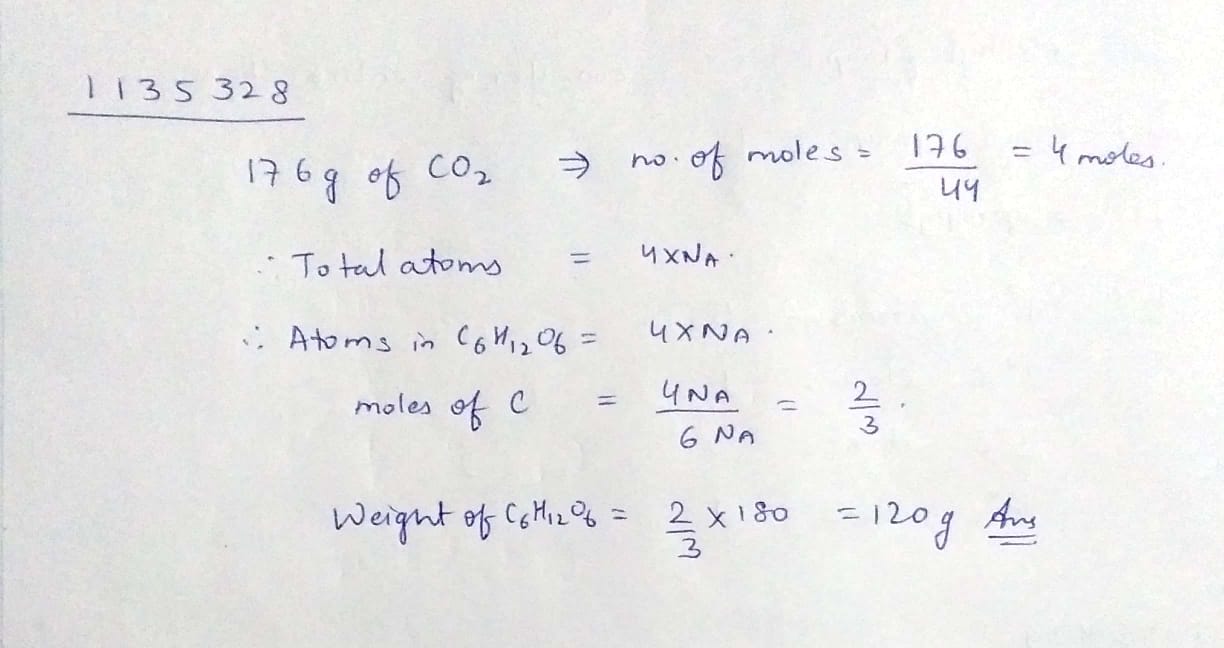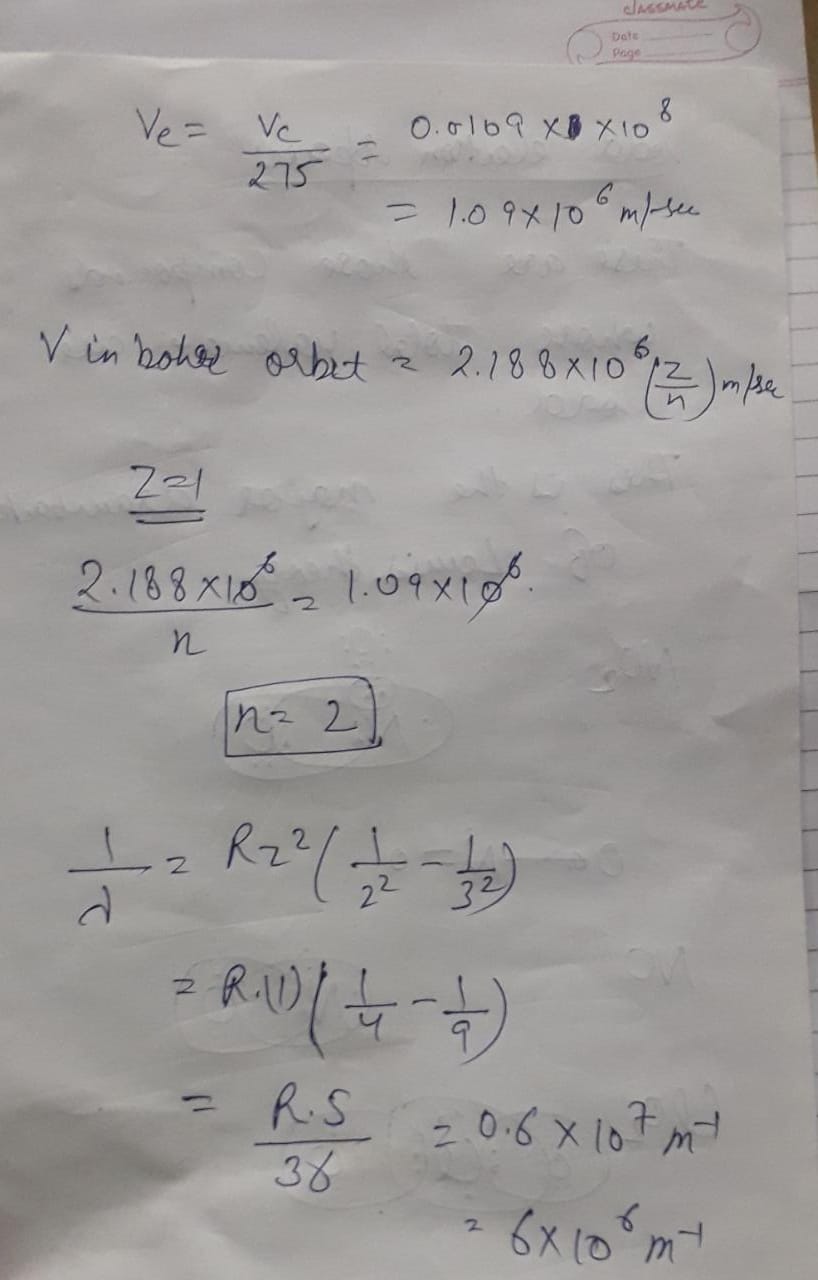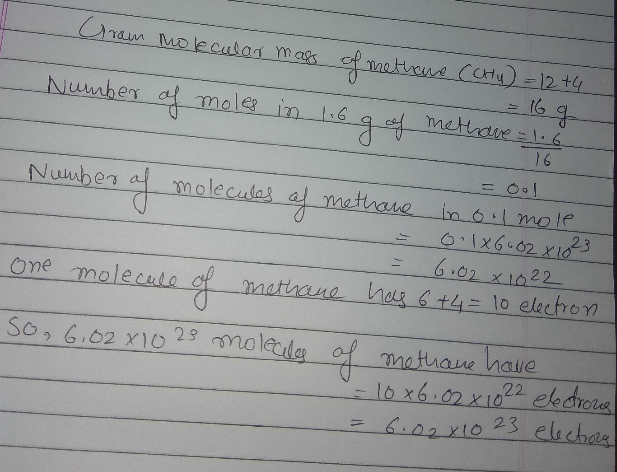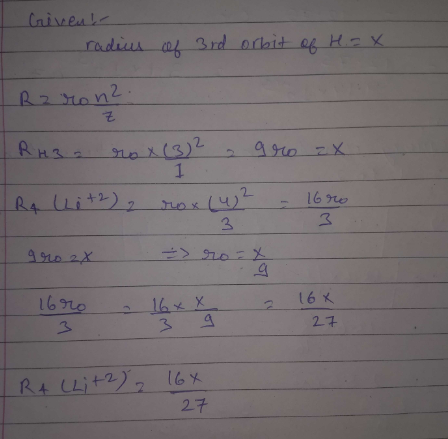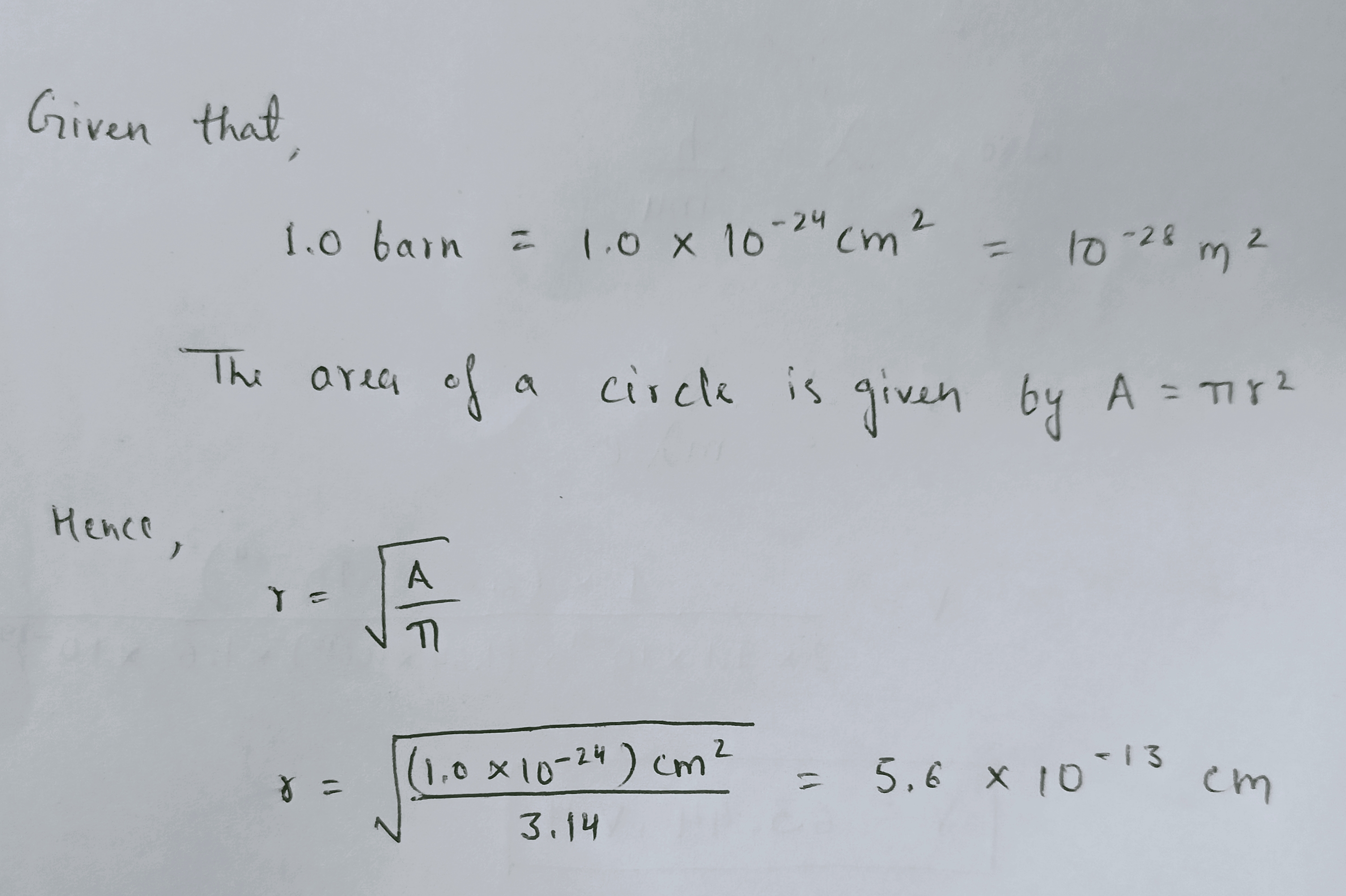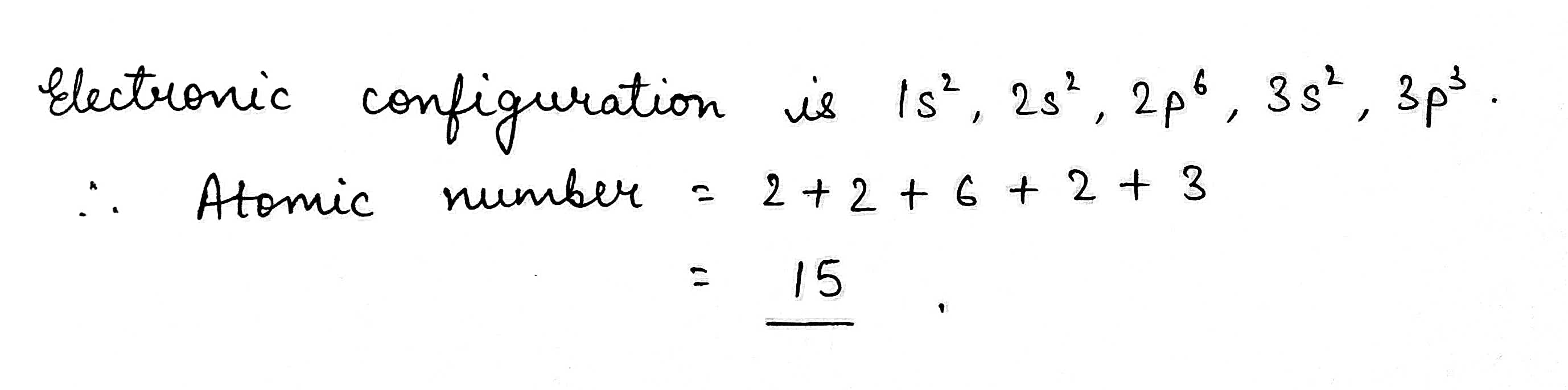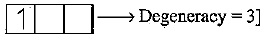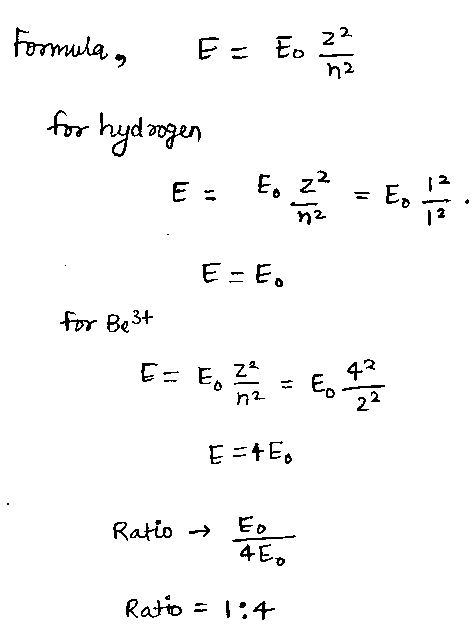Structure Of Atom - Class 11 Medical Chemistry - Extra Questions
Atoms of two elements P and Q have 5 electrons in 2nd shell and 3rd shell respectively. What could be the geometric representations of P and Q? What will be the atomic numbers of these elements?
A dipositive ion has an electronic arrangement 2,8,8. Find out the number of electrons, protons and neutrons in that element if its mass number is 40.
Write the two postulates Bohr's atomic model.
Name two processes which provide the best evidence for the motion of particles in matter.
Find the radius of nucleus of an atom having atomic mass number equal to(Take R0 = 1.3×10−15m)
Represent each of the following in terms of its symbols:
i) a proton ii) an electron
iii) a neutron
Out of 6s and 4f orbitals, which is filled first and why?
If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom ? and (II) what is the charge on the atom?
Calculate energy of electron which is moving in the orbit that has its radius. sixteen times the radius of first Bohr orbit for H−atom
The atomic numbers of two elements A and B are 11 and 12 respectively. Which element exhibits highest metallic property ? Write the molecular formula of the compounds formed when theses elements combine with the element 'Z' having atomic number 8.
An element belonging to 3d series of modern periodic table has spin magnetic moment =5.92 B.M.in +3 oxidation state. Determine the atomic number of element.
"The atomic number and mass number of sodium are 11 and 23 respectively".What information does this statement convey?
What is meant by the statement that "the atomic number of sodium is eleven"?
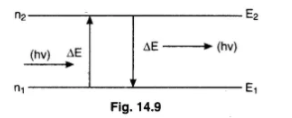
A hydrogen atom in ground state is heated by a photon exciting the electron to the 3rd excite state. The electron then drop to the 2nd orbit what is the frequency of radiation emitted and absorbed in the process.
The energy required to excite an electron of H-atom from first orbit to second orbit is
Name the three constituent of an atom and state their mass and charge of each. How are they distributed in an atom?
Define 'atomic number' and 'mass number'.
What are the postulates of Bohr's model of an atom?
If number of electrons in an atom is 8 and number of protons is also 8, then:
(i) What is the atomic number of an atom?
(ii) What is the charge on the atom?
(iii) Why does moon have very cold and very hot temperature even though it is present at the same distance from the sun as the earth is?
Mass of single atom of silver is 1.794×10−22. What is atomic mass of silver.
The electronic spectrum is characteristic of an element.
If true then enter 1, if false enter 0.
What do you mean by the dual nature of an electron?
An atom of an element has one electron in the valence shell and the two inner shells have 8 electrons each. Find the atomic number of that element.
The highest number of unpaired electrons are in Fe3+,Fe2+,Fe+ :
Define Isotopes.
An neutral atom has the same number of ......... as electrons.
An atom is made up of protons, neutrons and ..........
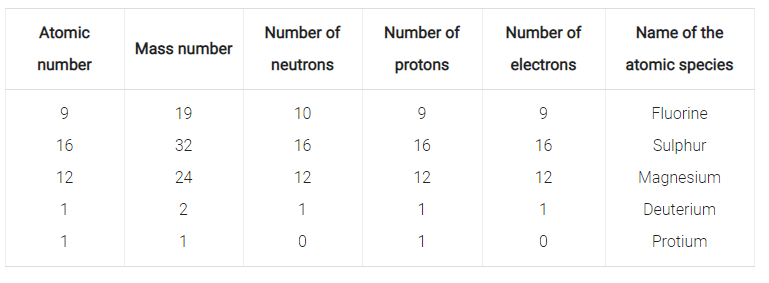
An atom of the element is 3517Cl. Complete the following table.
| Element | Mass number | Atomic number | No. of protons | No. of electrons | No. of neutrons |
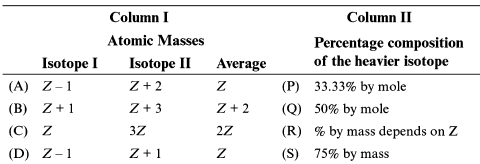
Match column I with column II and select the correct option from the codes given below:
Which of the following is (are) matter?Chair, air, love, smell, hate, almonds, thought, cold, cold-drink, the smell of perfume.
Compare the properties of protons, electrons and neutrons.
What are the characteristics of the particles of matter?
What are the limitations of J. J. Thomson's model of the atom?
Describe Bohr's model of the atom.
State Bohr's postulate of hydrogen atom which successfully explains the emission lines in the spectrum of hydrogen atom.
Use Rydberg formula to determine the wavelength of Hα line.
[Given: Rydberg constant R=1.03107m−1]
Column-I Column-II
According to Bohr's atomic model, electrons revolve in _______ orbits.
Three elements 'X', 'Y' and 'Z' have atomic numbers 7,8, and 9 respectively. Write the formula of the compound formed when 'X' combines with 'Z'.
On what basis did Bohr assume the concept of stationary orbits for an electron?
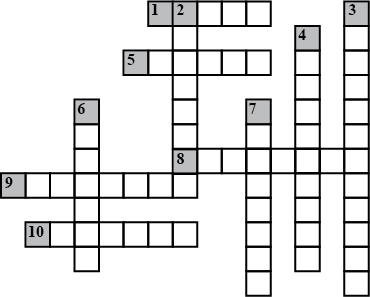
ACROSS
1 Maximum number of electrons in L shell
5 H+ ion
8 Negatively charged fundamental particles
9 Atoms of different elements with different mass numbers, but same number of neutrons
10 Atoms of different elements having same atomic numbers
DOWN
2 Atoms of same element having different mass number
3 Number of protons in an atoms
4 Proposed planetary model
6 Fundamental particle with no charge
7 Number of protons and neutrons
An atom of an element has one electron in the valence shell and the two inner shells have 8 electrons each. Find the atomic number of that element.
True or False
The radius of energy of Bohr's orbit is equal to integral multiples of 0.529.
___________ elements have same number of protons.
Filling the blanks................. can be classified chemically into pure substances and mixtures.
Fill in the blanks
According to Bohr's atomic model , electrons revolve around the nucleus in _________ orbits.
Shadow is not a matter because it has no mass and does not occupy _______.
Fill in the blanks with suitable words:
The particle of an atom that has no charge is ________.
Correct the following statements.a) Water boils at 1000 C under atmospheric pressure.
b) The liquid evaporates above its boiling point.
c) Solids have the largest interparticle space.
d) Gases have the strongest interparticle forces.
b) The liquid evaporates above its boiling point.
c) Solids have the largest interparticle space.
d) Gases have the strongest interparticle forces.
Which rule is violated in the electronic configuration 1s02s22p4?
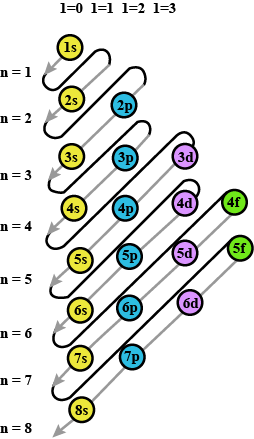
State Aufbau principle.
Fill in the blanks with suitable words:
The fundamental particle not present in a normal hydrogen atom is ________.
Give the main postulates of Bohr's model of an atom.
State the postulate of Bohr's theory regarding angular momentum of an electron.
State the postulate of Bohr's theory regarding emission off a photon.
Write Bohr's any two postulates for hydrogen atom.
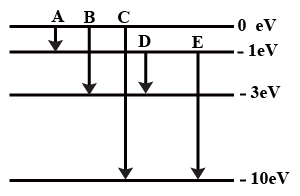
The energy levels of an atom of a certain element are shown in the given figureWhich one of the transitions A, B, C, D or E will result in the emission of photons of electromagnetic radiation of wavelength 618.75 nm?
Support your answer with mathematical calculations.
Write the statement of Bohr's Second postulate of quantisation. Determine the wavelength of first spectral line in the Lyman series of the hydrogen spectrum.[Rydberg constant R=1.097×107m−1].
Total energy of an electron orbiting around the nucleus of an atom is always negative. What is the significance of this?
a. What are Isotopes?
b. Write any three applications of Avogadros law?
What is meant by 'Quantization of charge'?
Write three postulates of Bohr. Mention two limitations of Bohr model.
Derive an expression for potential and kinetic energy of an electron in any orbit of a hydrogen atom according to Bohr's atomic model.
State the basic postulates of Bohr's theory of atomic spectra.
Determine the number of revolutions made by an electron in one second in the 2nd Bohr orbit of H-atom.
According to Bohr, 'Angular momentum of an orbiting electron is quantised'. What is meant by this statement?
Match the List 1 and List 2.
Write two limitations of Bohr's model.
The approximate radius of a H−atom is 0.05 nm, and that of a proton is 1.5×10−15m. Assuming both the hydrogen atom and the proton to be spherical, calculate a fraction of the space in an atom of hydrogen that is occupied by the nucleus.
(a) State Bohr's postulate to define stable orbits in hydrogen atom. How does de Broglie's hypothesis explain the stability of these orbits?
(b) A hydrogen atom initially in the ground state absorbs a photon which excites it to the n=4 level. Estimate the frequency of the photon.
Find out the wavelength of the electron orbiting in the ground state of hydrogen atom.
Write the postulates of Bohr's atomic model. What is the relationship between the radii of the nth and first bohr orbit of hydrogen atom?
State whether True or False.(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining. Therefore, it is neutral.
(c) The mass of an electron is about 12000 times that of a proton.
(b) A neutron is formed by an electron and a proton combining. Therefore, it is neutral.
(c) The mass of an electron is about 12000 times that of a proton.
Calculate the shortest and longest wavelength of Balmer series of H2 spectrum. Given R=1.097×107 m−1.
Give any two postulates of Bohr's theory of atomic model.
A hydrogen like atom (described by the Bohrs model) is observed to emit six wavelengths originating from all possible transitions between a graph of levels having energies between 0.85 eV and −0.544 eV (including both these values).
(i) Find the atomic number of the atom.
(ii) Calculate the smallest and longest wavelength emitted in these transitions.
(Take hc=1240 eV−nm and ground state energy of H−atom=−13.6 eV)
Write Bohr's postulates for the hydrogen atom model.
Calculate no. of ions, atoms, protons, electrons in 3g CO2−3.
Define matter.
One mole of an element contains 4.2×1024 electrons. What is the atomic number of the element?
Calculate the radius of Bohr's 3rd orbit in Li2+ ion
A binary compound between an unknown element E and hydrogen contains 91.27% E and 8.73%H by mass. If the formula of the compound is E3H8. Calculate the atomic mass of E.
Write electronic configurations of the elements (number in the brackets indicate atomic numbers)
oxygen (8)
What are the draw backs of Bohrs atomic model? Show that the circumference of the Bohr orbit for the hydrogen atom is an integral multiple of the de Broglie wavelength associated with the electron revolving around the orbit.
What is the energy of electron in an orbit which n=∞
In the Bohr's model, for unielectronic species following symbols are used.
Kn,z→ Kinetic energy electron in nth orbit with atomic number "z".
Calculate z in all cases.
Identify the following elements.
a) 25X55
b) 17X35
c) 20X40
In the Bohr's model, for unielectronic species following symbols are used.
rn,z→ Radius of nth orbit with atomic number "z".
Calculate z in all cases.
How does the ionization energy of an atom vary along a period and a group of the periodic table?
Isotopes are the atoms of ________ element having the ________ atomic number but ________ mass number.
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10g and 0.05 mole of X3Y2 weighs 9g, the atomic weights of X and Y are:
If radius of the nucleus is 3.5×−15 m then find the space or volume occupied by the nucleus.
Write the symbols of the first ten elements in the periodic table with the atomic numbers.
Consider Bohr theory for hydrogen atom. The magnitude of angular momentum, orbit radius and velocity of the electron in nth energy state in a hydrogen atom are l, r and v respectively. Find out the value of x if product of v, r and l is directly proportional to nx.
Difference between atomic number and mass number.
The kinetic energy of the e− in the 2nd Bohr orbit of a hydrogen atom is:(as in Bohr's radius)
The mass number of an atom is slightly less than the actual atomic mass. Give reasons.
Drawbacks of Bohr's atomic model :
Find the mass of 1.5055×1025 number of molecule of aluminium oxide.
The wavelength of β-line of the Balmer series is 4815oA . What is the wavelength of α-line of the Balmer series of the same atom?
Calculate the frequency of the electron in the 1st Bohr orbit in a H-atom.
State the postulates of Bohr's theory of hydrogen atom.
a) Calculate the energy associated with the first orbit of He+. What is the radius of this orbit?
b) What is the lowest value of n that allows ′g′ orbitals to exist?
What is meant by imperfect sheilding of e− by the nucleus?
Write the electron configuration of third element of group 15−(Z=33)
Name the Indian philosopher who proposed the theory matter.
What will the weight of C6H12O6 which contain same total number of atom as present in 176g of CO2
Give reason for the following:
Physical properties of isotopes of the same element are not identical.
Give reason for the following:
The mass number of an atom is slightly less than the actual atomic mass.
The mass number of an atom is slightly less than the actual atomic mass.
A 12.5 eV electron beam is used to excite a gaseous hydrogen atom at room temperature. Determine the wavelengths and the corresponding series of the lines emitted.
What is the value of magnetic moment of zinc in+2 oxidation state?(z=30)
The atomic number of an element X is 13.What will be the number of electrons in its ion X34
The potential energies of first, second and third Bohr's orbits of He +cation are E1, E2 and E3. Then What will be the correct sequence of these energies?
State Bohr's Postulates and write its drawbacks.
What point of Bohr's model of an atom can be used to arrive at this formula? Based on these points derive the above giving description of each step and each term.
The velocity of e− in a certain Bohr orbit of the hydrogen atom bears the ratio 1:275 to the velocity of light, What is the quantum no. "n" of the orbit and the wave no. of the radiation emitted for the transition from the quantum (N+1) to the ground state.
State Bohr's Model.
State bohr postulate of hydrogen atom that gives the relationship for the frequency of emitted photon in a transition.
Calculate the total number of electrons present in 1.6g of methane. Molecular wt. of methane= 16a.m.u
If the radius of 3rd Bohr;s orbit of H is x, then radius of 4th orbit of Li2 + ion would be
If the number of electrons is an atom 8 and the number of neutron is also 8 then
(i) what is the atomic number of an atom?
(ii) what is the charge on the atom?
What is matter?
Bohr's orbits are called stationary states. Why?
Radius of the first orbit of the electron in a hydrogen atom is 0.53˚A. Find the centripetal acting on the electron.
What are the limitations of J.J. Thomson's model of the atom?
Describe Bohrs model of the atom
State the postulates of Bohr atom model. Obtain the expression for the radius of the nth orbit of an electron based on Bohr's theory.
What is meant by radioactivity?
Write two difference between isobars and isotops.
(a) How many atoms are there in exactly 12 g of carbon-12 element?(C=12 u).(b) What name is given to this number?
(c) What name is given to the amount of substance containing this number of atoms?
(c) What name is given to the amount of substance containing this number of atoms?
Imagine removing one electron from He4 and He3. Their energy levels, as worked out on the basis of Bohr model will be very close. Explain why.
Define the following terms .
Atomic number
Using an expression for energy of electron, obtain the Bohr's formula for hydrogen spectral lines.
Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Match the following
State any two postulates of Bohr's theory of hydrogen atom.
An element has its electronic configuration as 2,8,2. Now answer the following question:
What is the atomic number of this element?
A bond order of 3 is computed for the diatomic molecule. It is possible that the bond may be made up of two σ and one π−bonds -Explain.
Calculate the effective neutron capture radius of a nucleus having a cross-section of 1.0 barn.
Determine the effective atomic number of the metal atom in the following:
[Cr(CO)6]
Give the atomic number of the inert gas in which the total number of d electrons is equal to the difference between the total number of p and s electrons.
Determine the effective atomic number of the metal atom in the following:
[Fe(CN)6]3−
The electronic configuration of an element is 1s2,2s2,2p6,3s23p3. what is the atomic number of the element in the periodic table?
An atom has the electron structure of 2,7.
(a) What is the atomic number of this atom?
(b) To which of the following would it be chemically similar? 7N, 15P, 17Cl, 18Ar
(c) Why would you expect it to be similar?
(c) Why would you expect it to be similar?
Which ancient Indian philosopher suggested that all matter is composed of very small particles ?
What name was given by him to these particles ?
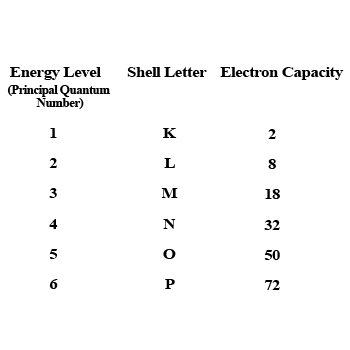
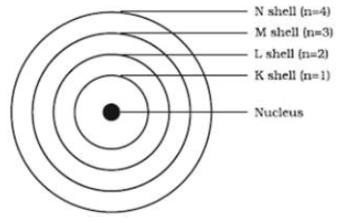
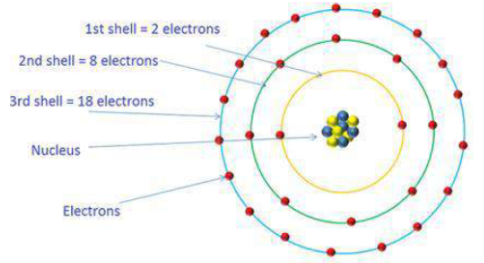
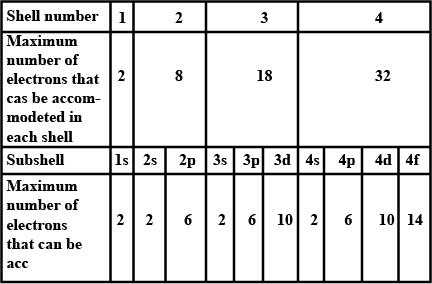
What is the maximum number of electrons an M shell of the atom can accommodate?
What is the maximum number of electrons that can go into N shell of an atom?
What is the maximum number of electrons which can be accommodated in the K-shell of an atom?
Name the scientists who described the arrangement of electrons in an atom.
What is the maximum number of electrons which can be accommodated in the L shell of an atom?
Why is the atomic number of the elements more important to a chemist than its atomic mass?
What is the maximum number of electrons which can be accommodated in:
(a) the innermost shell of an atom?
(b) the outermost shell of an atom?
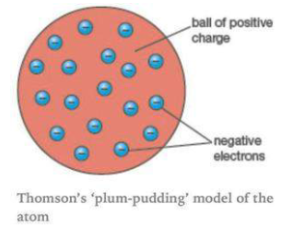
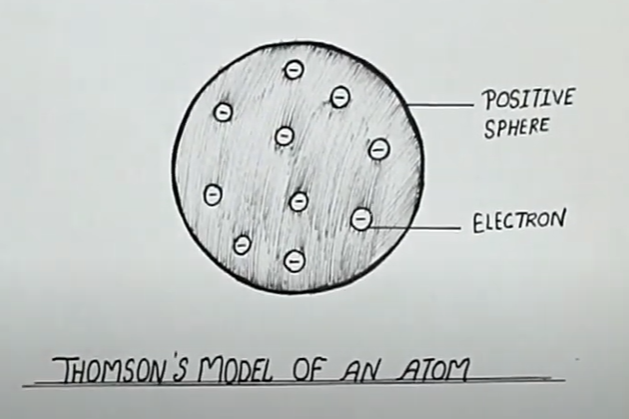
Describe Thomson's model of an atom.
Name the particles which actually determine the mass of an atom.
(a) Describe Thomson's model of the atom. Which subatomic particle was not present in Thomson's model of the atom?
(b) The mass number of an element isIt contains 7 electrons. What is the number of protons and neutrons in it? What is the atomic number of the element?
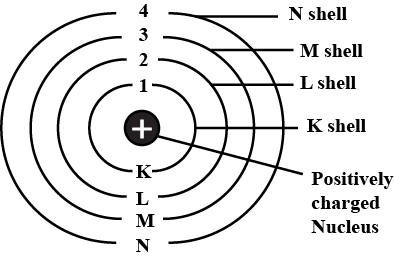
What are the various letters used by Bohr to represent electron shell in an atom?
(a) What is meant by (i) atomic number, and (ii) mass number, of an element? Explain with the help of an example.
(b) What is the relation between the atomic number and mass number of an element?
(c) If an element M has mass number 24 and atomic number 12, how many neutrons does its atom contain?
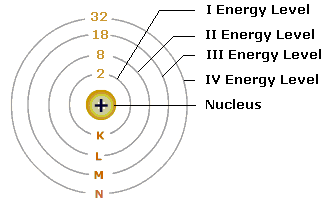
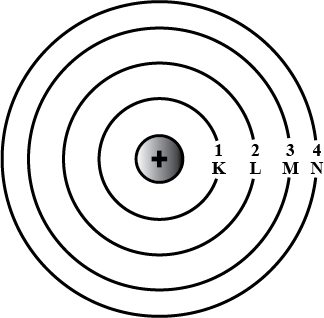
Draw the sketch of Bohr's model of an atom.
Explain Bohr-Bury scheme.
What is the usual symbol for (a) an electron (b) a proton, and (c) a neutron?
Identify the pair of isotopes from the following:
168X , 167X , 178X
168X , 167X , 178X
What is meant by man-made elements ?
Match the names of the Scientists given in List I with their contributions towards the understanding of the atomic structure as given in List II
There are 15 protons and 16 neutrons in the nucleus of an element . Calculate its atomic number and mass number .
Draw the sketch of Thomson's model of an atom.
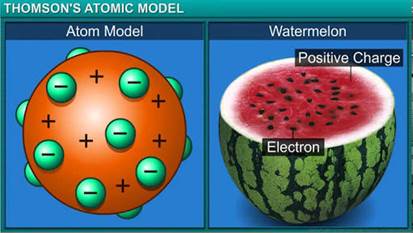
Why is Thomson's model of an atom compared with watermelon ?
Draw a sketch of Bohr's model of an atom with four shells .
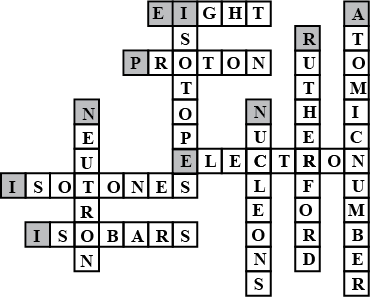
Complete the following crossword puzzle (Figure 5.1) Across:
(1) An element with atomic number 12.
(2) Highly reactive and soft metal which imparts yellow colour when subjected to flame and is kept in kerosene
(3) Metal used in making cans and member of Group 14.
(4) A lustrous non-metal which has 7 electrons in its outermost shell.
(5) The first element of second Period
(6) An element which is used in making fluorescent bulbs and is second member of Group 18 in the Modern Periodic Table
(7) A radioactive element which is the last member of halogen family.
(8) Metal which is an important constituent of steel and forms rust when exposed to moist air.
(9) The first metalloid in Modern Periodic Table whose fibres are used in making bullet-proof vests
Isotopes have same _______ but different ________.
Does the particle have an excess of electrons or a deficiency of electrons
An atom having atomic mass number 13 has 7 neutrons, What is the atomic number of the atom?
Give the following a suitable word/phrase.
Atoms of same element with same atomic number but a different mass number.
Define Atomic number.
How did Neils Bohr visualise the atom?
An excess of electrons produces a _____ charge.
State the number of atoms of each kind, present in
(a) C6H12O6
(b) H2SO4
(c) HNO3
(d) CaCO3
Also name these compounds.
Name three fundamental particles of the atom. Give the symbol with charge, on each particle.
On the basis of Thomson's model of an atom, explain how an atom as whole is neutral.
An element has 2 electrons in its N shell.
What is its atomic number ?
State one observation which shows that atom is not indivisible.
Name the three fundamental particles of an atom:
1224Mg and 1226Mg are symbols of two isotopes of magnesium
Compare the atoms of these isotopes with respect to:
composition of their nuclei
What is the atomic number of carbon?
Elements X, Y and Z have atomic number 6, 9 and 12 respectively. Which one :
has four electron in its outermost shell ?
Match the atomic numbers among, 4,8,10,15 and 19 with An element with 4 shells.
Write the names of the particles represented by the following symbols and explain the meaning of superscript and sub; script number attached.
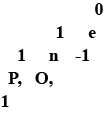
Match the atomic numbers, 4,8,10,15 and 19 with: Element which does not form ion.
Complete the statement by filling the gaps using the appropriate terms.
Electron ...... is complete in each hydrogen in a hydrogen molecule.
What is the name of Thomson's model?
Give reasons
Physical properties of isotopes are different
From the symbol 17Cl35 state atomic number of chlorine.
Name the species which has no electron.
Compare the properties of electrons, protons and neutrons.
List the three fundamental particles and their properties.
Name the scientist who discovered Neutrons.
Name the scientist who proposed about electrons.
State the postulates of the Bohr atomic model.
What is atomic number ?
How many maximum number of electrons can be present in first, second, third and fourth shell ?
Draw sketch of Bohr's model of an atom with three shells.
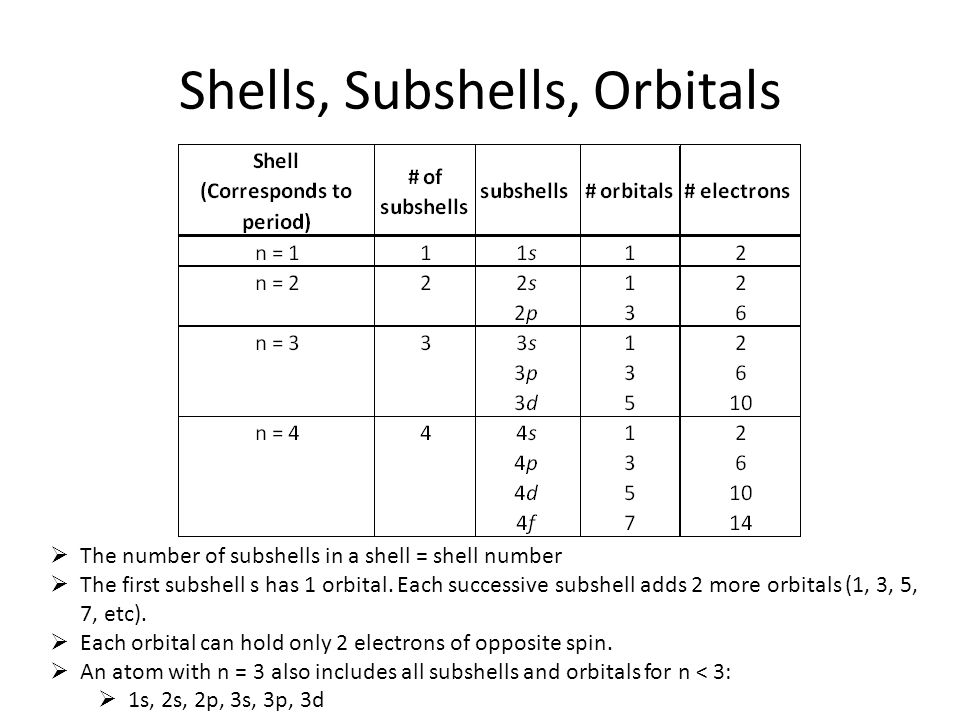
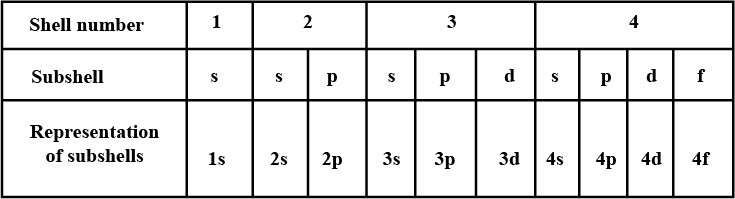
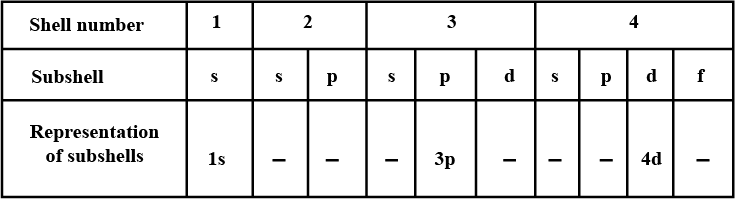
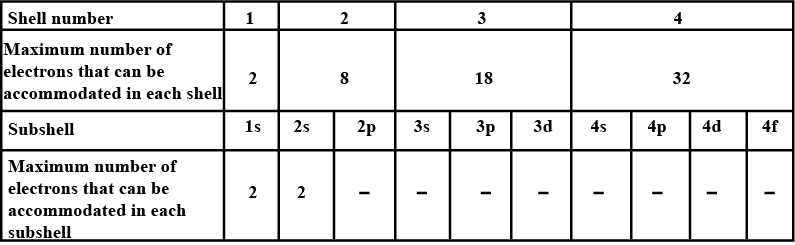
Complete the table of details about shells and subshells.
| Shell | K1 | L2 | M3 | N4 |
| Subshell | ||||
| No. of Electrons |
Arrange the following sub-shells in the in-creasing order of energy 5p,2s,4f,3s,4s,3d,6s
What are the fundamental particles of matter?
Write the atomic number of the element with outer configuration 4s1.
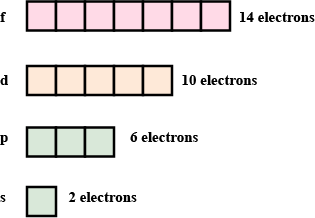
No of electrons in each shell.
| Shell | s | p | d | f |
| No. of Electrons |
Out of 4f and 4d which orbital will experience a higher effective nuclear charge?
An atom having mass number 40 and 20 neutrons in its nucleus. What is the atomic number of the element ?
How many electrons can be accomodated in an orbital ?
Write the atomic number at an element with outer configuration 3d3.
Complete the table
Write the sub-shell electronic configuration of this atom.
A compound of vanadium pentoxide (V20;) is used as catalyst.
How vanadium ion is represented?
How many shells are present in this atom?
Complete the subshell electronic configuration of He?
Certain subshell of an atom are given below 2s,2d,3f,3d,5s,3p
Which are the subshell that are not possible?
Complete the table
What is the maximum number of the electron that can be accommodated in the 's'?
Write names of subshells in accordance with increasing energy level,
What may be the maximum number of electrons to be filled in the 'p' subshell?
Certain subshell of an atom is not possible from given below 2s, 2d, 3f, 3d, 5s, 3p
Give the reason.
Write a short note about Niels Bohr's atomic model.
Which are the subshells of each shell?
Which are the fundamental particles whose masses are mainly responsible for the mass of an atom?
How many 's' subshell electrons are in 1s^{2},2s^{2},2p^{6},3s^{2},3p^{2}
Give information about properties of isotopes.
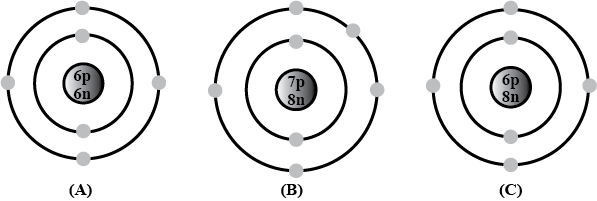
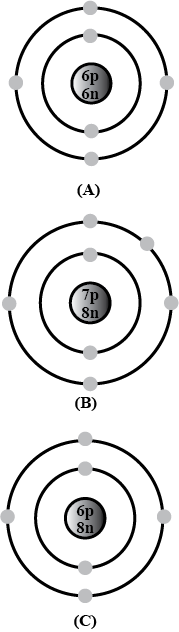
Bohr models of atoms A,\ B,\ C are given (Symbols are not real).
Write the atomic number, mass number, and electronic configuration of the atoms.
What is indicated by hydrogen and sodium spectrum?
Explain Bohr model of atom?
Which scientist and by which discovery invented neutrons?
Which of the subshell to which the last electron was added?
If number of electrons in an atom is 8 and number of protons is also 8, then
(i) what is the atomic number of the atom? and
(ii) what is the charge on the atom?
What is the difference between ground state and excited state ?
What is the difference between atomic mass and mass number ?
State Bohr's postulates for explaining the spectrum of hydrogen atoms. Derive a formula for total energy of electron in n orbit.
What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of metal other than gold?
Complete the following Table :
Isotopes of an element have:
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers
What was Thomson's model of atom ?
How many electrons in sulphur (Z = 16) can have n + l = 3 ?
What do you understand by stationary states?
Fill in the blanks:
The arrangement of different electromagnetic waves in order of their wavelengths is called ________.
Fill in the blanks:
The splitting of lines in the magnetic field is called ________.
The energy of separation of an electron is 30.6\ eV moving in an orbit of Li^{2+}. The number of waves made by the electron in one complete revolution in the orbit is:
The atomic mass of an element is 133 and number of neutrons is 41.8% more than number of protons. Find the number of unpaired electrons.
The average atomic mass of a sample of an element X is 16.2\ u. What are the percentages of isotopes _{ 8 }^{ 16 }{ X } and _{ 8 }^{ 18 }{ X } in the sample?
Electrons of energy 12.09 eV can excite hydrogen atoms. To which orbit is the electron in the hydrogen atom raised and what are the wavelengths of the radiations emitted as it drops back to the ground state?
State the postulates of Bohr atoms model. Obtain an expression for the radius of n^{th} orbit of an electron of hydrogen atom based on Bohr's theory.
Not considering the electronic spin, the degeneracy of the second excited state \left(n=3\right) of H-atom is 9, while the degeneracy of the second excited state of {H}^{-} is _______.
What is the distance of closest approach to the nucleus of an \alpha-particle which undergoes scattering by {180}^{o} in Geiger-Marsden experiment?
Match the electronic transitions of List-I with spectral properties of List-II.
In an atom first four shells (K, L, M and N) are completely filled. Then what is the total number of electrons in that atoms?
Radius of nucleus may be given as {R}_{N}={R}_{0}{A}^{1/3}, where A= mass number and {R}_{0}= constant. Show that the nuclear matter density is nearly constant. {R}_{0}=1.2\times {10}^{-15}.
Calculate the energy required to excite 1 L of hydrogen gas at 1 atm and 298K to the first excited state of atomic hydrogen. The energy for the dissociation of H-H bond is 436\ kJ\ mol^{-1}.
If each hydrogen atom in the ground state of 1.0 mol of H-atoms is excited by absorbing photons of energy 8.4 eV, 12.09 eV and 15.0 eV of energy, then the number of spectral lines emitted is equal to:
Suppose the potential energy between electron and proton at a distance r is given by -\frac{ke^2}{3r^3}. Use Bohr's theory to obtain energy of such a hypothetical atom.
In a hypothetical H-atom the mass of electron & its charge is double of what we consider calculate the total energy (in eV) of electron in the 1^{st} orbit of such a hypothetical H-atom?
What should be the kinetic energy of electron in the first orbit of hydrogen atom?
A sample of hydrogen atoms gas contains 100 atoms All the atoms are excited to the seventh state. The total energy released by all the atoms is \frac { 4800 }{ 49 } Ry( Where Ry = 13 eV) then discuss the following things
a)Maximum energy of the emitted photon will be less then \frac { 4800 }{ 49 } Ry
Using postulates of Bohr's theory of hydrogen atom, show that
(i) Radii of orbits increases as n^2 and
(ii) The total energy of electron increases as \dfrac{1}{n^2}, where n is the principal quantum number of the atom.
What transition of { Li }^{ +2 } spectrum will have same wavelength as that of second line of Balmer series in { He }^{ + } spectrum?
The potential energies of first, second and third Bohr's orbit of He^+ cation are {E_1}, {E_2} and {E_3} respectively. Write the correct sequence of these energies in increasing order.
The ratio of energy of ground state energy of hydrogen and first excited state energy of B{e^{3 + }} will be-
Find the ratio of maximum wavelength of minimum wavelength for the lines of Balmer series in hydrogen spectrum.
A hydrogen like atom (described by the Bohr's model) is observed to emit six wavelength, originating from all possible transition between a group of levels. These levels have energies between -0.85 eV and -0.544 eV(including both these value)
(a) Find the atomic number of the atom.
(b) Calculate the smallest wavelength emitted in these transition.
(use hc=1240 eV nm, E_1 H=-13.6 eV)
What is radial distribution function ? Draw this function for the 1s, 2s ,3s, 2p, 3p and 4 p orbitals in a hydrogen atom.
Determine the effective atomic number of the metal atom in the following:
[Ni(NH_3)_6]^{2+}
Determine the effective atomic number of the metal atom in the following:
[Co(CN)_6]^{4-}
Would the Bohr formula for the H - atom remain unchanged if proton had a charge \left[\dfrac{+4}{3}\right]e and electron a charge \left[\dfrac{-3}{4}\right]e , where e = 1.6 \times 10^{-19}C. Given reasons for your answer.
A deficiency of electrons produces a _____ charge.
Using Bohr model, calculate the electric current created by the electron when the H - atom is in the ground state.
Would the Bohr formula for the H-atom remain unchanged If proton had a charge (+4/3)e and electron had a charge (-3/4)e, where e = 1.6 \times 10^{-19} C? Give reasons for your answer.
State the postulates of the Bohr's model of an atom.
What is meant by radioactivity ?
Bohr models of atoms A,\ B,\ C are given (Symbols are not real).
Among these, which are isotopes? Why?
Explain the use of radioactive isotopes in the study of mechanism of photosynthesis in plants and hydrolysis of esters.
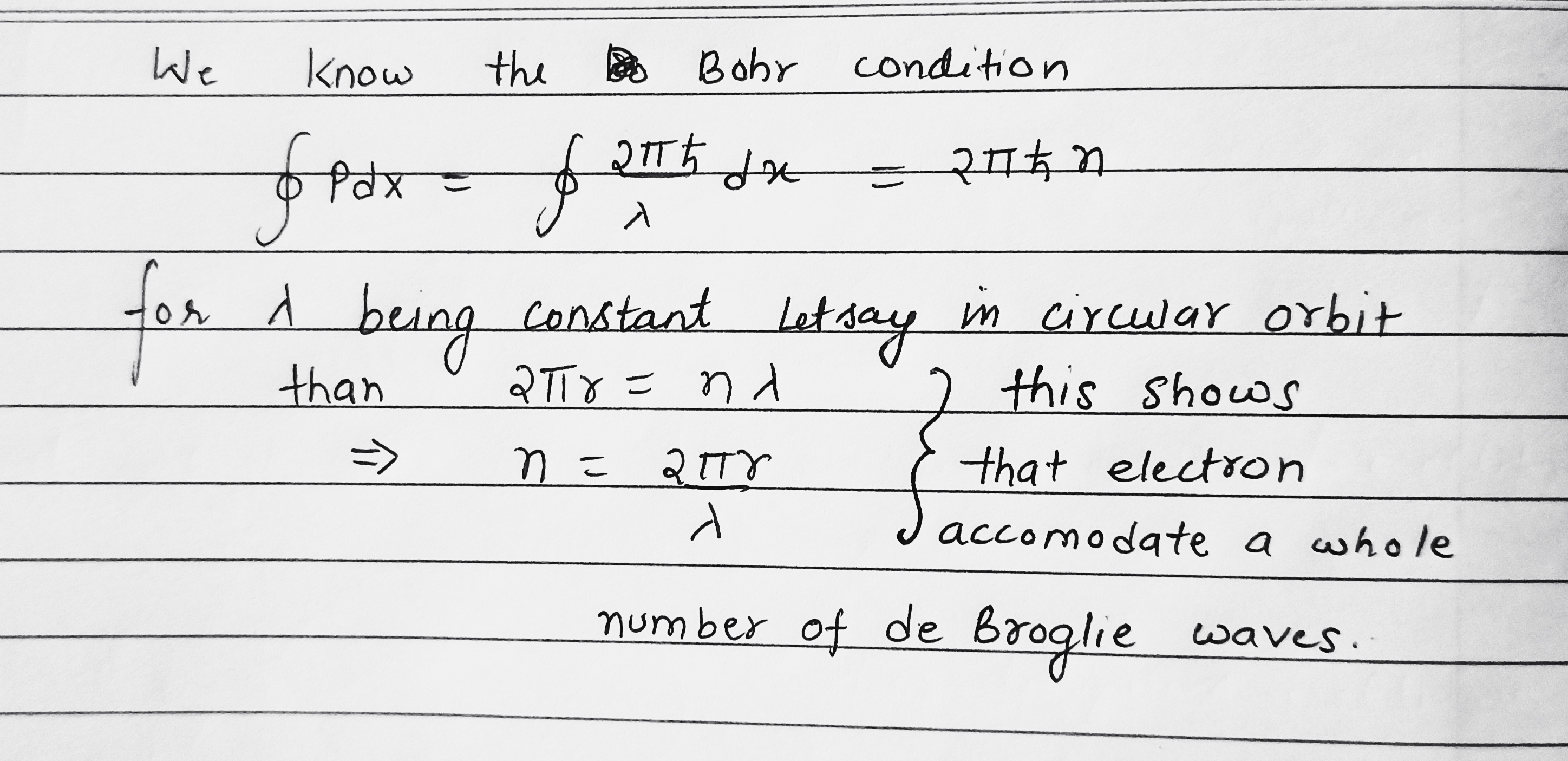
Describe the Bohr quantum conditions in terms of the wave theory: demonstrate that an electron in a hydrogen atom can move only along those round orbits which accommodate a whole number of de Broglie waves.
Making use of the Hund rules, find the number of electrons in the only partially filled subshell of the atom whose basic term is
(a) ^{3}F_{2} ; (b) ^{2}P_{3/2} ; (c) ^{6}S_{5/2}
What do you observe in the spectrum of NaCl ?
Using the Hund rules, write the spectral symbol of the basic term of the atom whose only partially filled subshell
(a) is filled by 1/3, and S = 1;
(b) is filled by 70\%, and S = 3/2.
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions