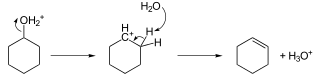
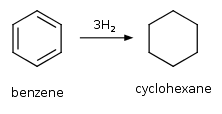
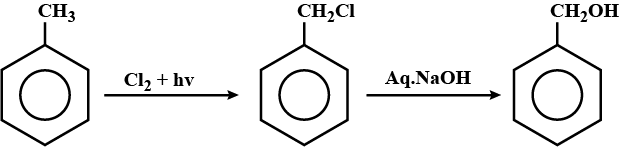
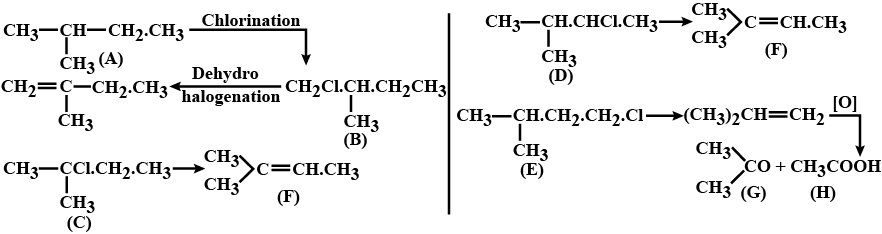
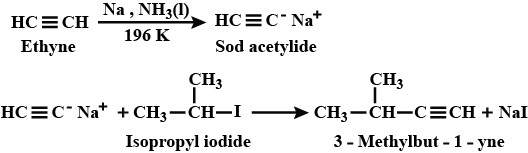
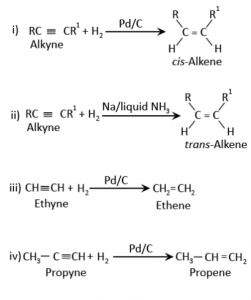
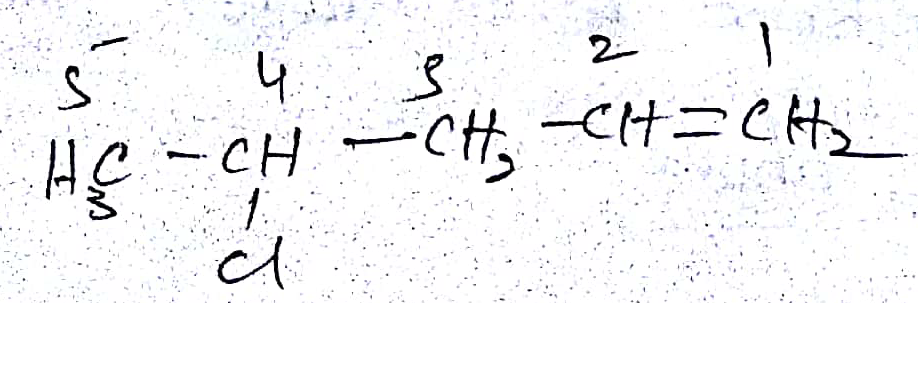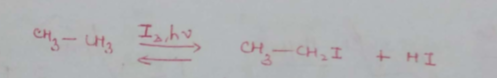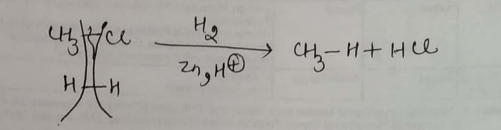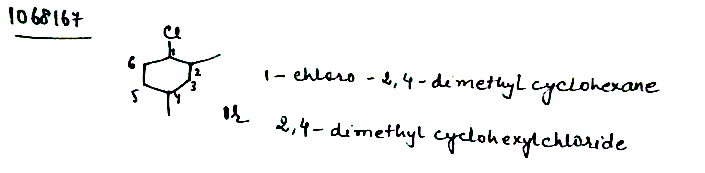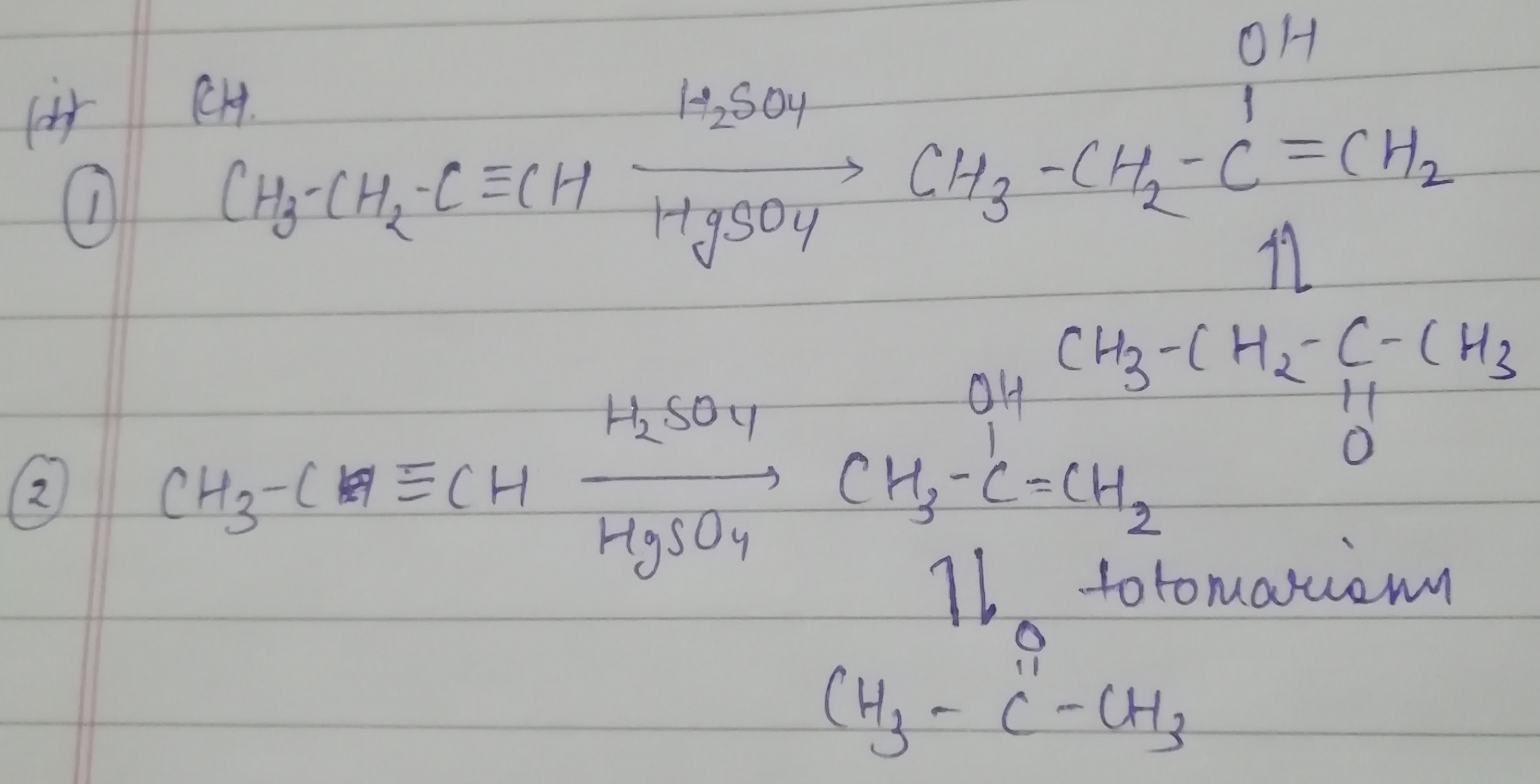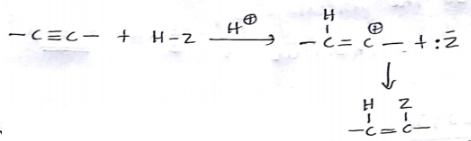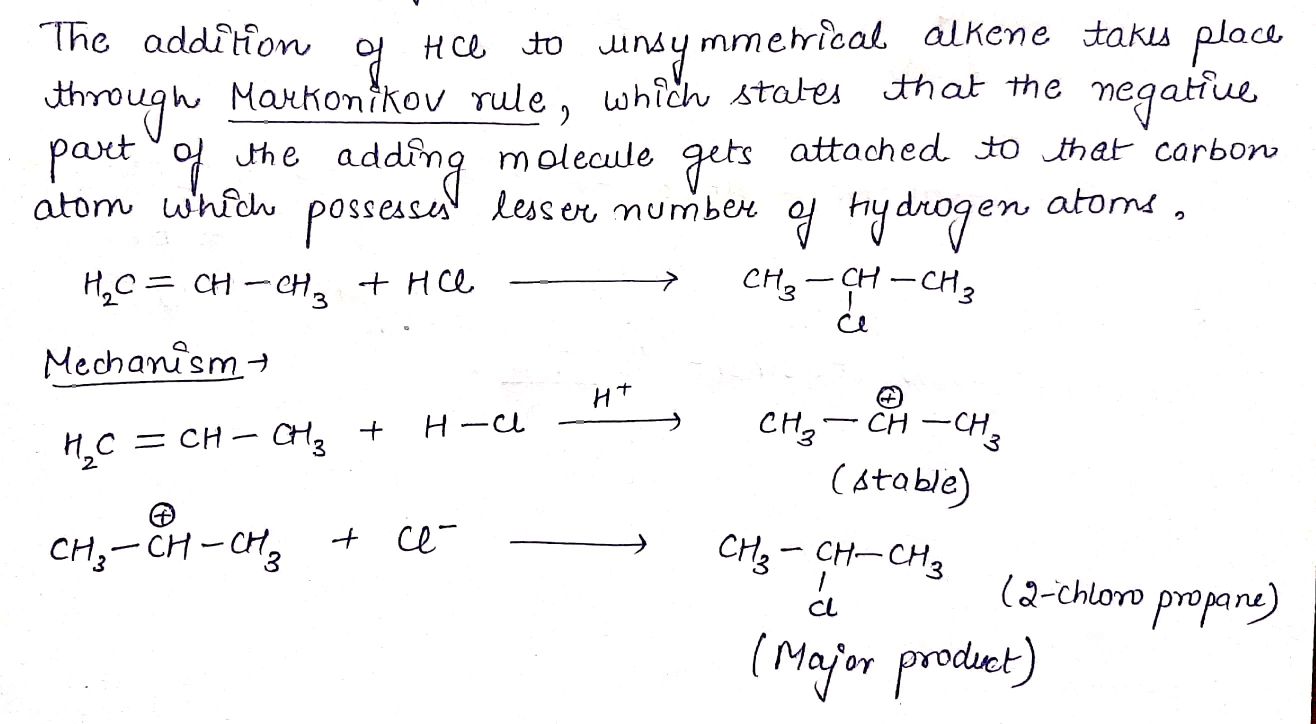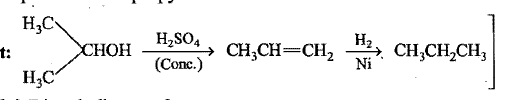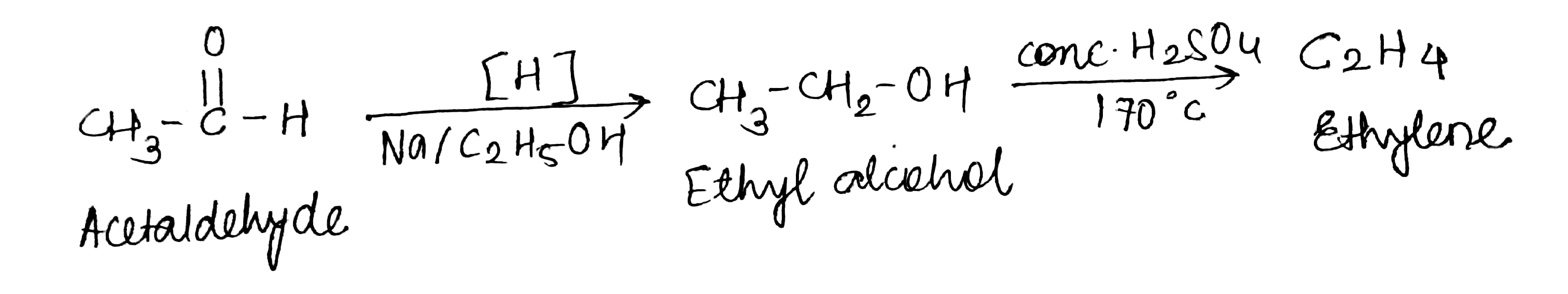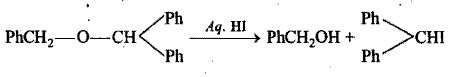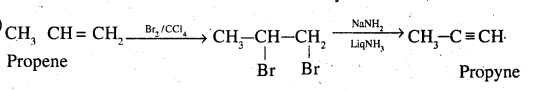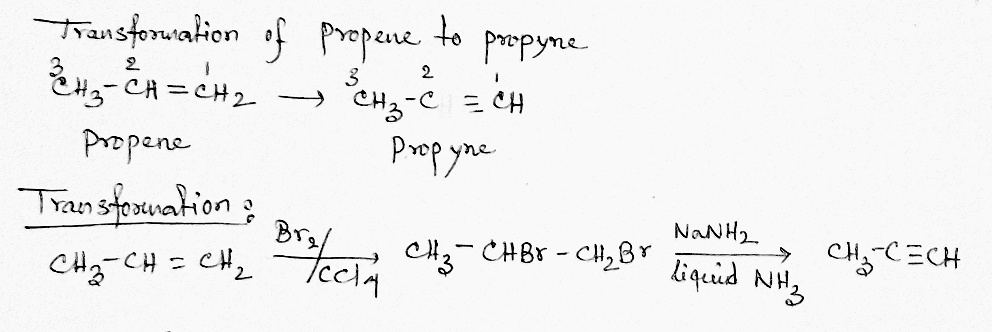Hydrocarbons - Class 11 Medical Chemistry - Extra Questions
IUPAC names for the following:
(i) (CH3)3CCH2C(CH3)3
(ii) (CH3)2C(C2H6)2
(iii) tetra-tert butylmethane
Give the IUPAC name of CH2=C(CH3)−CH2−Br.
CH3−CH=CH−C≡CHWhat is the IUPAC name of the given compound?
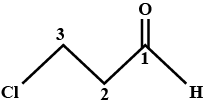
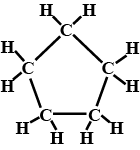
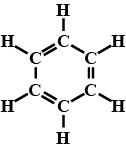
Give the IUPAC names  ?
?
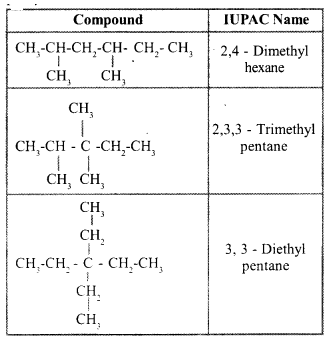
Write the IUPAC names of all the alkanes in Table 6.2.
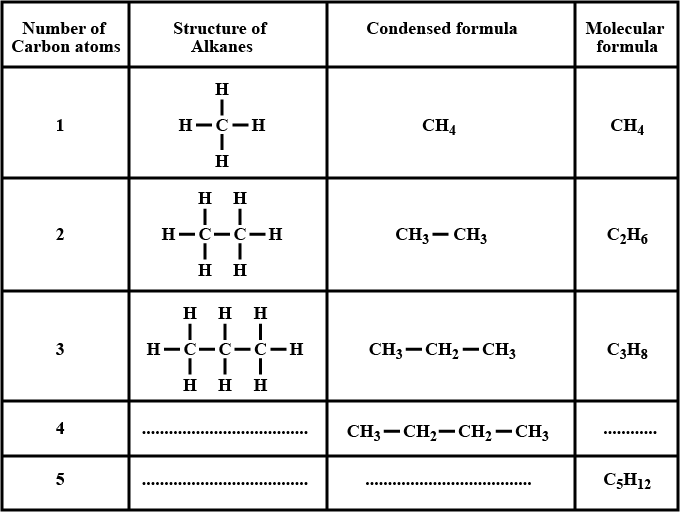
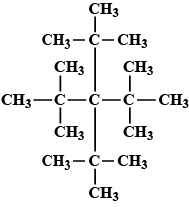
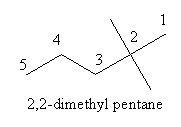
Write the IUPAC name of the following
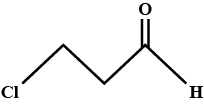
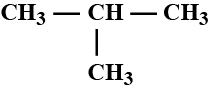
Write the IUPAC name of the compound given above.CH3−CH|CH3−CH|CH3−CH2−CH2−CH|CH3−CH3
Write the IUPAC name of the given chemical compound.
Carbide of calcium yields ethyne on reaction with H2O. If true enter 1, else 0.
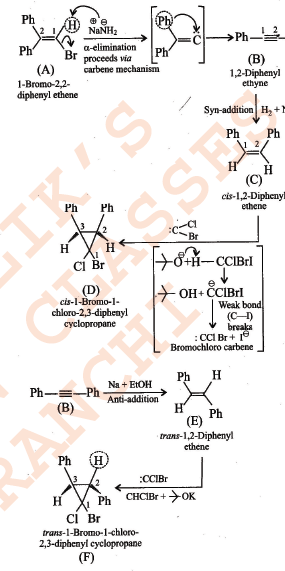
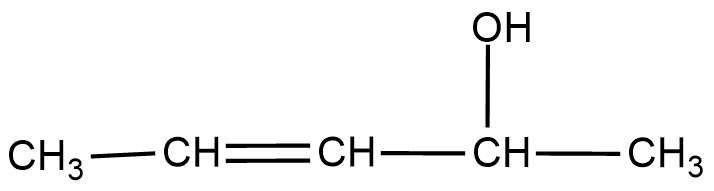
Assuming that cation stability governs the barrier for protonation in H -X additions, predict which compound in each of the pairs parts (I) & (II) will be more rapidly hydrochlorinated in a polar solvent.
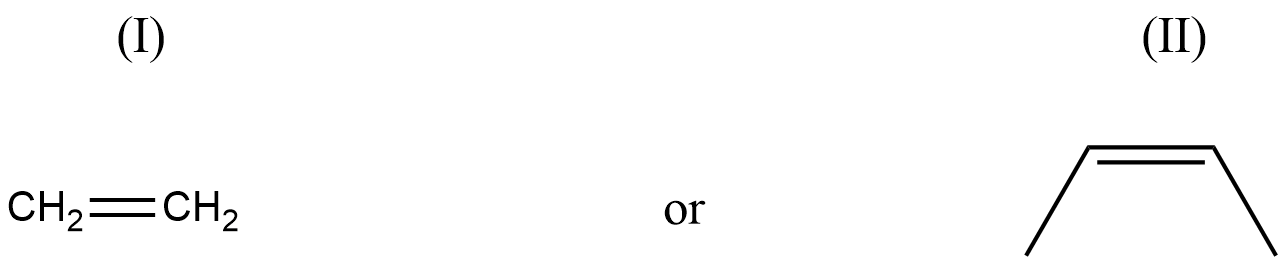
"Be2C and Al4C3 are called ethanides because they react with H2O yielding ethane."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
SN1 reaction undergoes through a carbocation intermediate as follows:
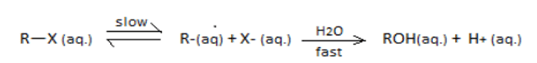
[R = t-Bu, iso-Pr, Et, Me] (X=Cl,Br,I)
The correct statements are:
I. The decreasing order of rate of SN1reaction is t−BuX>iso−PrX>EtX>MeX
II. The decreasing order of ionization energy is MeX>EtX>iso−PrX>t−BuX
III. The decreasing order of energy of activation is t−BuX>iso−PrX>EtX>MeX
Which among the following is the simplest example for polycyclic arenes?
a) Benzacephenanthrylene
b) Naphthalene
c) Pyrene
d) Dibenzo-anthracene
b) Naphthalene
c) Pyrene
d) Dibenzo-anthracene
When Mg2C3 undergoes hydrolysis, how many carbon atoms is/are present in product?
"Of the alkaline earth metals, only magnesium carbide gives CH4 on reaction with H2O."
Answer whether the above statement is true or false.
If true enter 1, else enter 0.
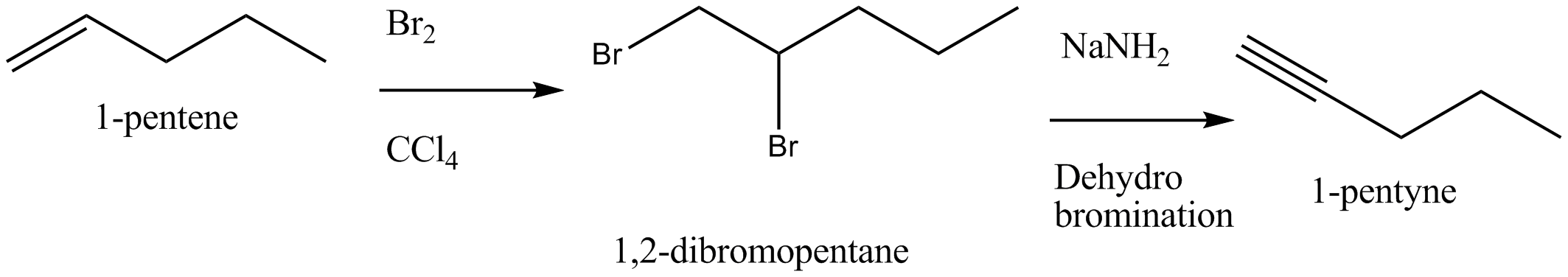
Bring about the following conversion 1-pentene →1− pentyne
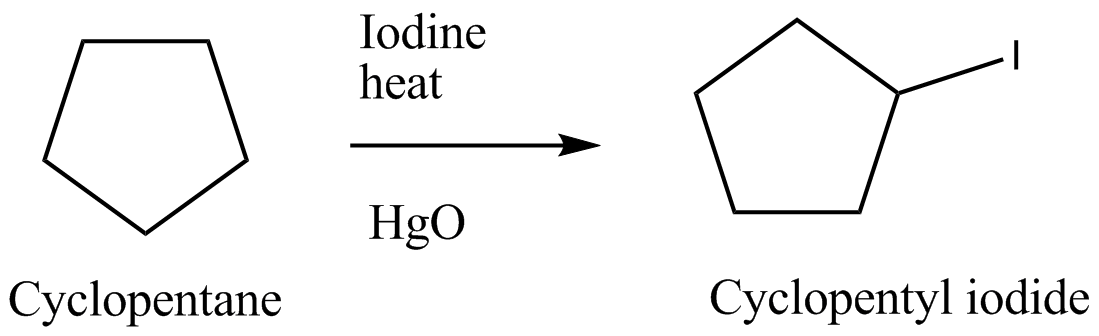
Suggest a sequence of reactions suitable for preparing following compound from the indicated starting material. You may use any necessary organic or inorganic reagents.Cyclopentyl iodide from cyclopentane occurs through the following reactions:
I2/Heat→HgO
I2/Heat→HgO
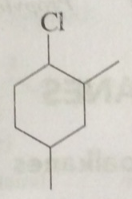
The IUPAC name of the following compound is:
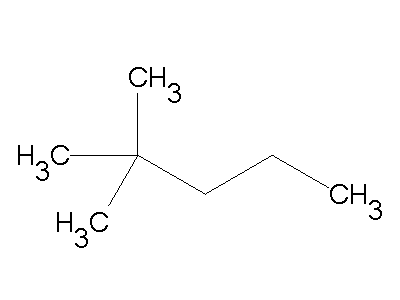
Give the correct IUPAC name:
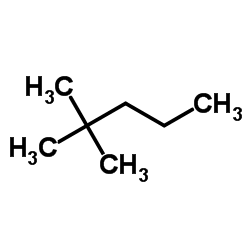
Write the IUPAC name of CH3−CH(Cl)−CH2−CH=CH2.
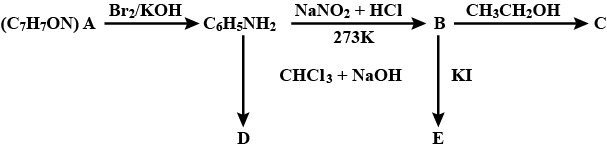
An aromatic compound 'A' of molecular formula C7H7ON undergoes a series of reaction as shown below. Write the structures of A, B, C, D and E in the following reactions.
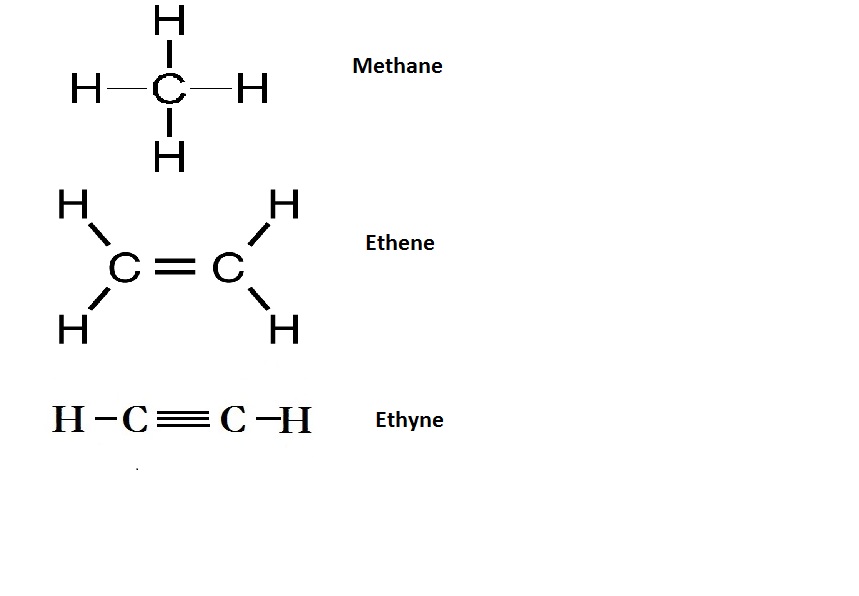
Why are certain compounds called hydrocarbons? Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction that converts alkenes into alkanes and also write a chemical equation to show the necessary conditions for the reaction to occur.
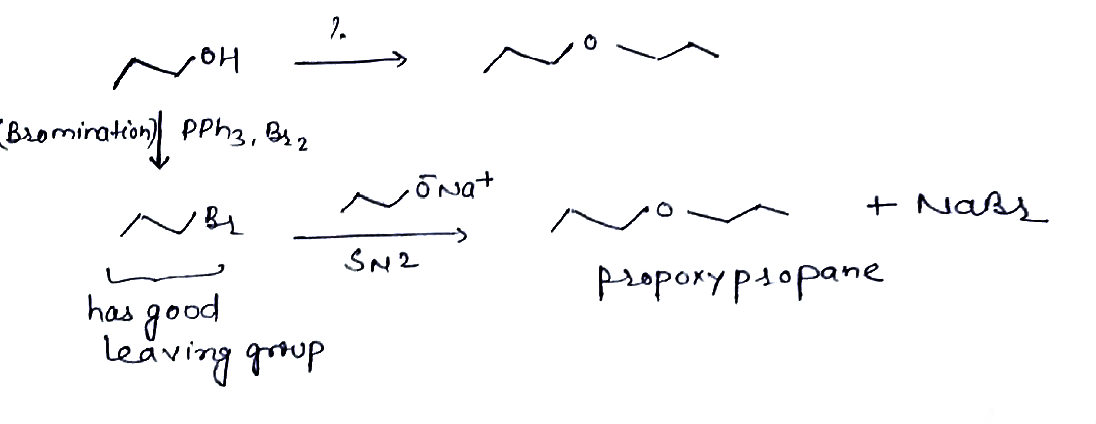
Give the following reaction:Propan−1−ol→Propoxy propane.
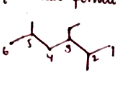
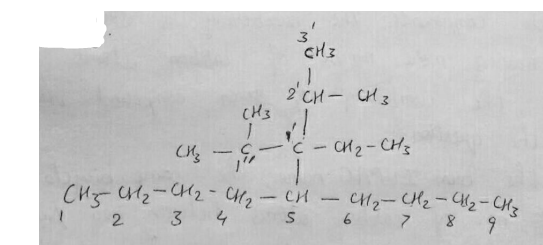
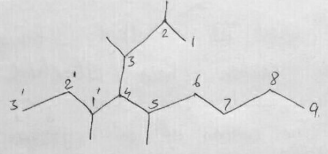
Consider the following alkane and answer the following questions.
(CH3)2CHCH2CH(C2H5)CH(CH3)CH3
(a) Write the complete structural formula of the alkane.
(b) What is its molecular formula?
(c) Write its bond line formula and give IUPAC name.
(d) How many methyl, methylene and methine groups are present in this alkane?
(e) How many ethyl and isopropyl groups are present in this alkane?
(f) How many carbon atoms are 1o,2o,3o,4o?
Consider the alkane- (CH3)2CHCH2CH(C2H5)CH(CH3)CH3
(i) What is its molecular formula?
(ii) How many 1o,2o,3o,4o carbon atom are present.
(iii) State the number of methyl, methylene and methane group in the alkane.
CS2+H2SCu→(A)[O]Cu tube→473K/100 atm(B).
Butylated hydroxy toluene is antioxidant why?
Match the column :
Give the IUPAC name of the following:
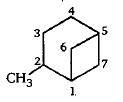
Give the IUPAC name of the following:
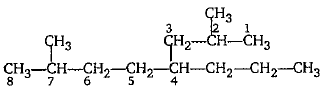
Give the IUPAC name of the following.
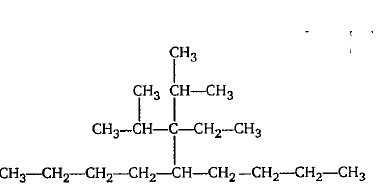
Give the IUPAC name of the following.
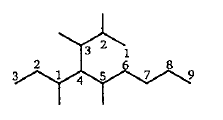
Find the final product of the reaction.
Why oxidizing agents like HNO3 and HIO3 are used in the iodination of alkane?
Convert Ethanol to But-1-yne.
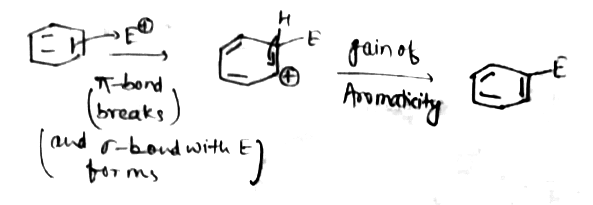
What is the role of sigma and pie bond in aromatic electrophylic substitution?
Explain Kolbe's electrolytic method.
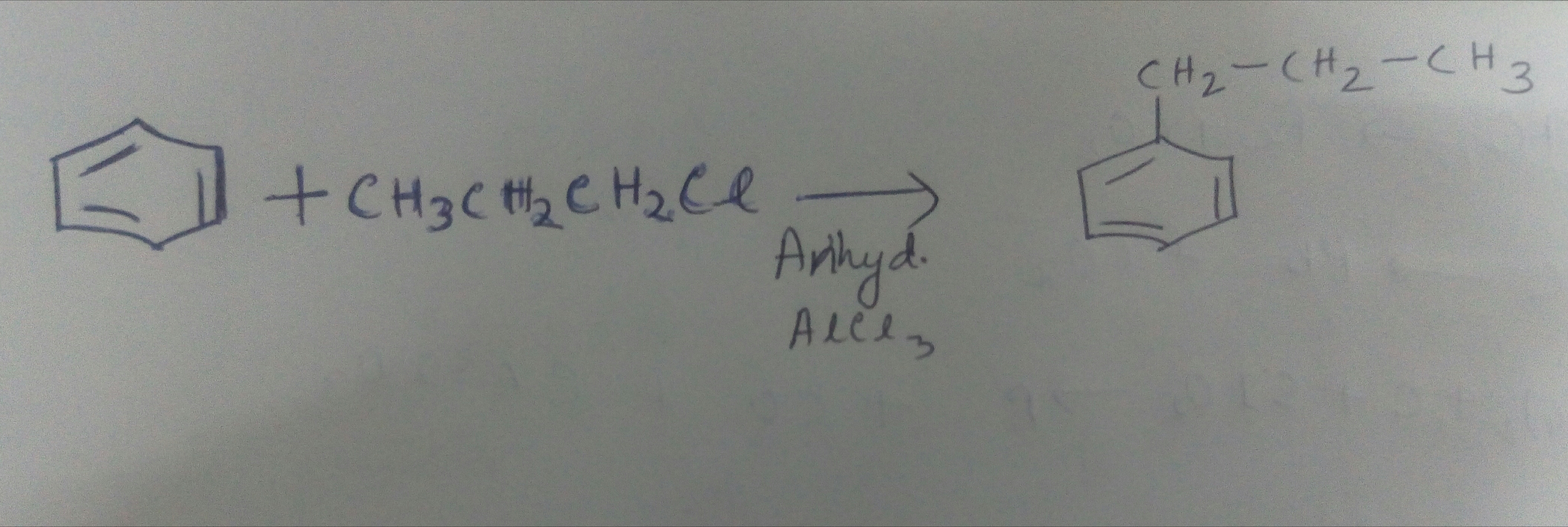
C6H6+CH3CH2CH2ClAnhydrous AlCl3→P
Identify P.
Alkyl halides (except fluorides) on reduction with zinc dilute hydrochloric acid give alkanes.Write the reaction involved for chloromethane to methane
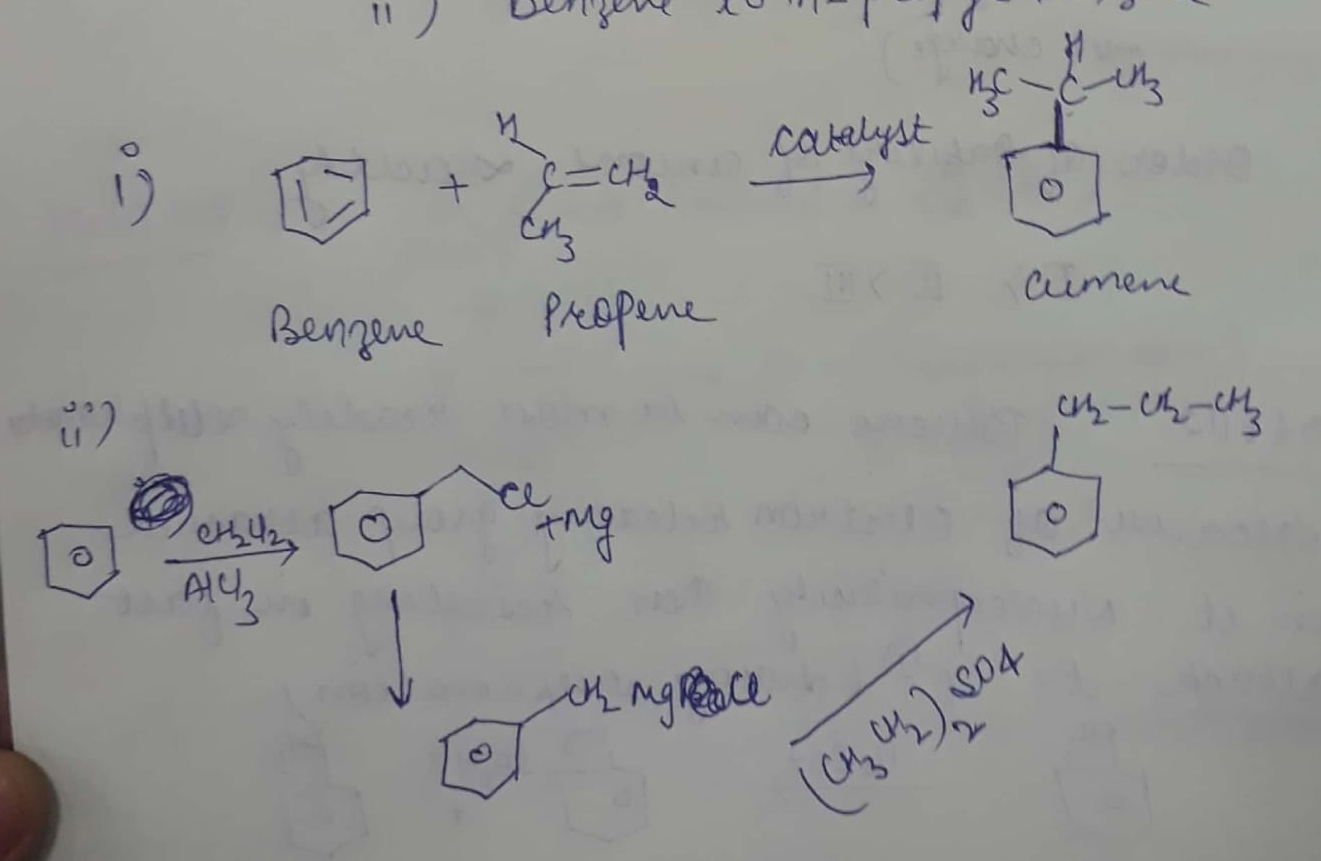
How will you synthesize
(i) cumene from benzene?
(ii) benzene to n-propyl benzene
Preparetion of Butane from ethene?
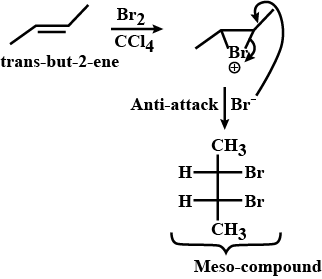
Trans-but-2-ene is treated with Br2 in CCl4 solvent. Write the product formed.
Given name for the following substituted alkanes:
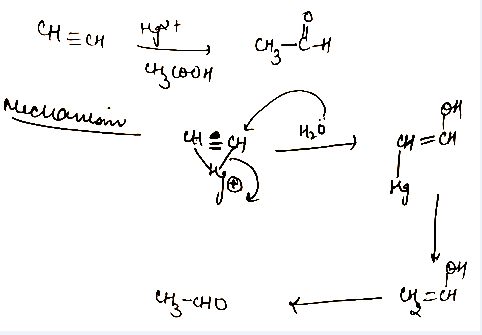
HC≡CHHg+2CH3COOHComplete the reaction.
(i) Give the hydration of 1-butyne.
(ii) Convert propyne to propyn-2-ol.
Predict the alkanes obtained by (i) the action of sodium metal on n-propyl chloride (ii)catalytic hydrogenation of isobutylene.
How will you prepare ethane from:
Ethyl Chloride
Comment on alkynes preparation and chemical properties of alkyne.
How will you prepare ethane from:
Ethanol
Predict the alkanes obtained by (i) the action of sodium metal on n-propyl chloride (ii) catalytic hydrogenation of isobutylene.
Consider the following alkane and answer the following question
(CH3)2CHCH3CH(C2H5)CH(CH3)CH3
(a) How many methyl, methylene and methine groups are present in this alkane?
(b) How many ethyl and isopropyl groups are present in this alkane?
(c) How many carbon atoms are 1o,2o,3o and 4o?
Identify 'A' and 'B' in the following reactions?
What will be the product of the given reaction?
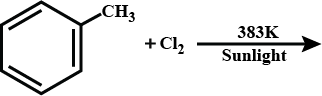
Name the carbon compound which heating with an excess of concentrated sulphuric acid at 3 k gives ethane.
Why are alkali metals normally kept under kerosene ?
How will you prepare propane from sodium salt of fatty acid?
What is the action of the following reagents on propyne?
i) Chlorine gas
ii) hydrogen iodide
Complete and balance the following equation:
CH4+Cl2Sunlight→
What happens when methane reacts with chlorine?
Name two catalysts that can be used in the hydrogenation of unsaturated compounds.
Propane from n- propyl alcohol
How will you prepare acetylene from:- (Give Balanced chemical equation)
Ethlidence dichlioride?
What effect does branching of an alkane chain has on its boiling point?
Carry out the following resonance:
Ethyl alcohol to ethane.
How will you convert ethene to ethane.
Why trans-isomers of alkene has higher melting point than its Cis-isomer?
Write the IUPAC names of the given compounds:
Fill in the blanks:
The carbon atoms of ethyne are ______ hybridized.
Write the IUPAC names of the given compounds:
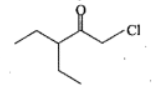
Write the IUPAC names of the given compounds:
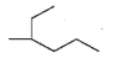
Fill in the blanks:
Carbon atoms in Ag2C2 (silver acetylide) are ________ hybridized.
Write the IUPAC names of the given compounds:
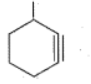
Write the IUPAC names of the given compounds:
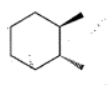
Write the IUPAC name of the given compounds:
Complete the following equations :
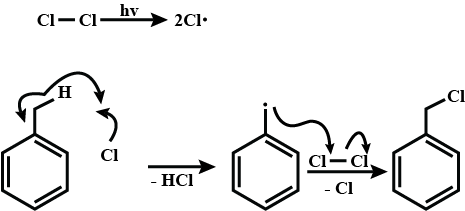
(CH3)3C−CH=CH2+HCl→(A).
(CH3)3C−CH=CH2+HCl→(A).
Complete the following equations :
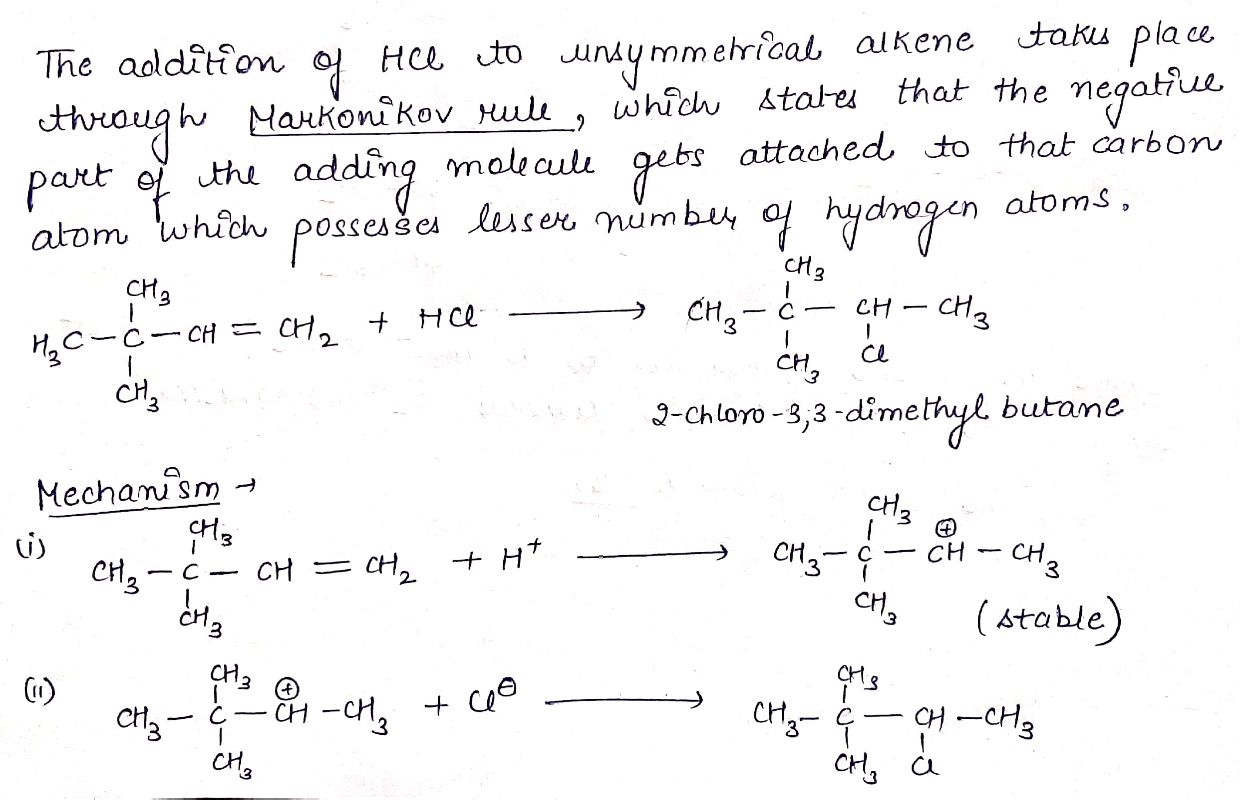
H2C=CH−CH3+HCl→(A).
H2C=CH−CH3+HCl→(A).
Complete the following equations:
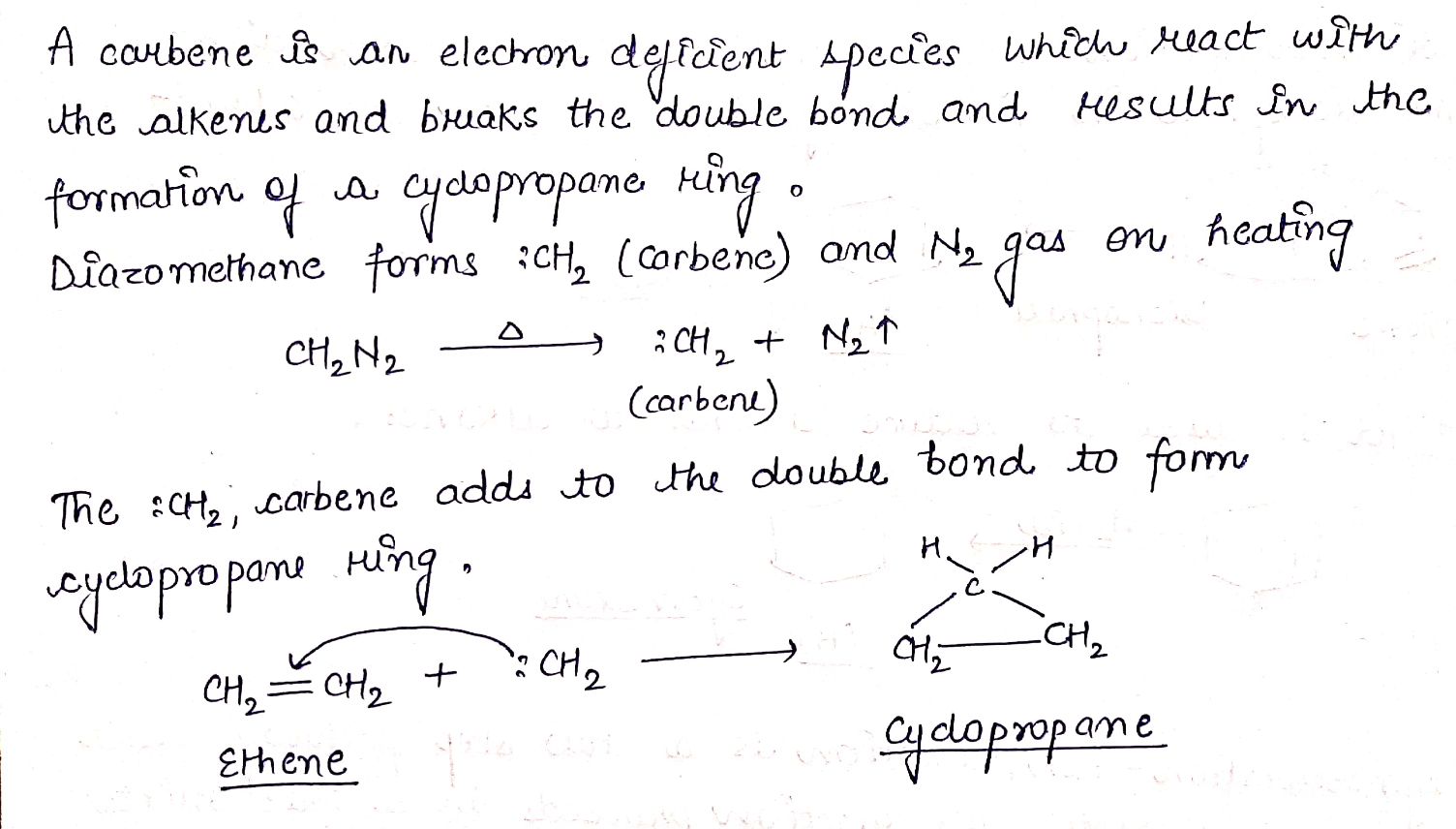
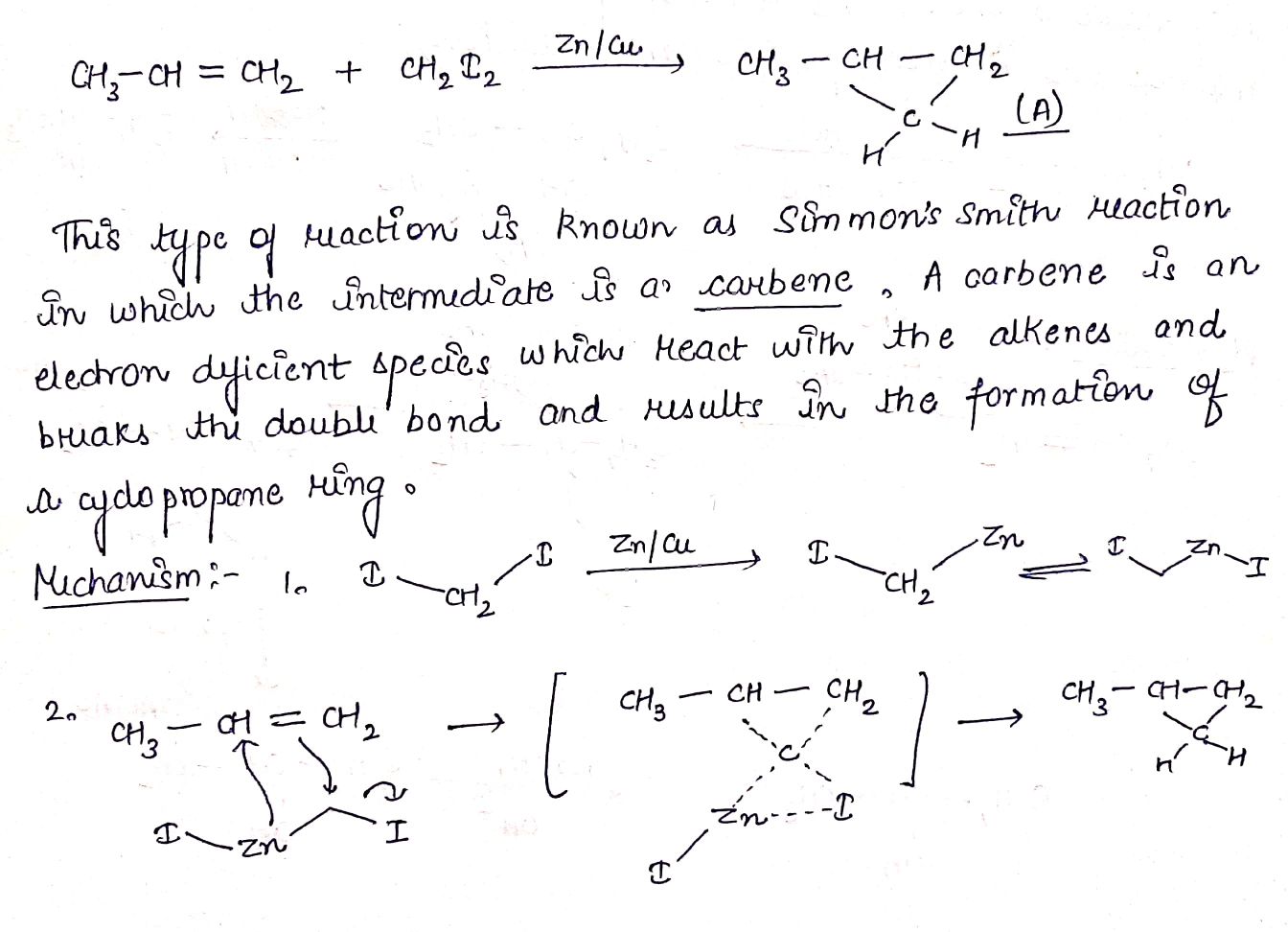
H2C=CH2+CH2N2→(A).
H2C=CH2+CH2N2→(A).
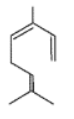
Give the IUPAC names for the given compound.
Give the IUPAC names for the given compound.
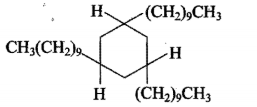
Complete the following equations :
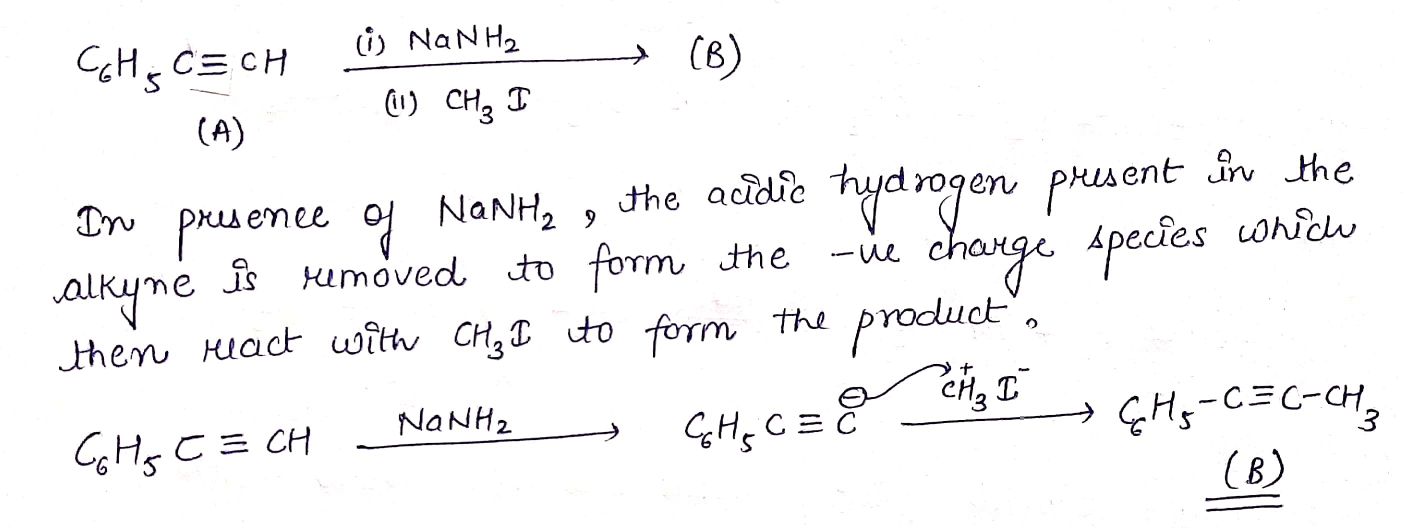
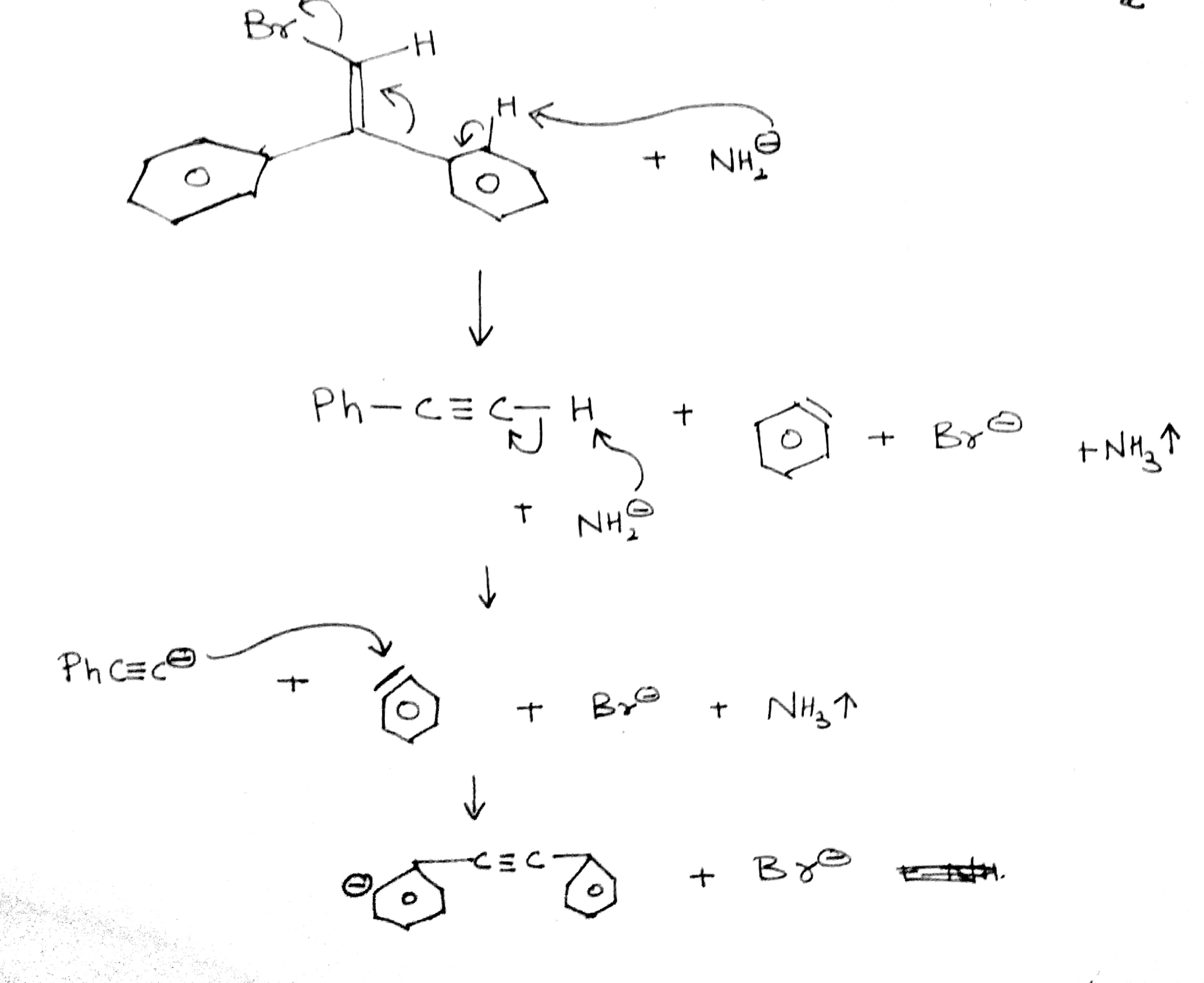
C6H5C≡CH(A)(i)NaNH2(3.0equiv.)→(ii)CH3I(B).
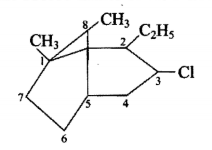
Give the IUPAC names for the given compound.
Write the various types of isomerism shown by alkenes. Give one example of each.
Give the IUPAC names of the given compounds:
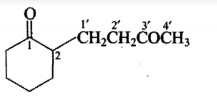
Give the IUPAC names of the given compounds:
Give the IUPAC names of the given compound.
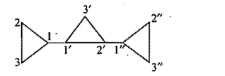
Give the IUPAC names of the given compounds:
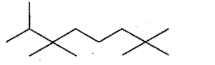
Give the IUPAC names of the given compounds:
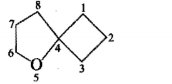
Give the IUPAC names of the given compounds:
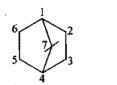
Give the IUPAC names of the given compounds:
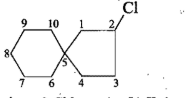
Give the IUPAC names of the given compounds:
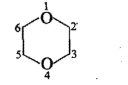
Fill in the blanks in the following:
All alkanes are ...... than water.
What happens when:
Water is added to aluminium carbide?
Fill in the blanks in the following:
Halogenation of alkanes does not occur in ......
Fill in the blanks in the following:
When ...... is strongly heated with sodalime C2H6 is obtained.
Calculate the total moles of major products are obtained when dry sodium propionate is heated with soda-lime.
Complete the following reaction:
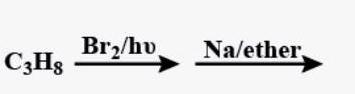
CH3BrNa→ether(A)hv→Br2(B)Na→ether(C)
What happens when:
Ethyl iodide dissolved in dry ether is treated with sodium metal?
How will you prepare the following?
Ethane from ethanol in one step.
Ethane from ethanol in one step.
Complete the following reactions:
(CH3)3C−ClCH3COO−→△ ?
(CH3)3C−ClCH3COO−→△ ?
How will you synthesise?
But−1−yne from acetylene.
How will you synthesize propane from isopropyl alcohol?
Identify the (A), (B), (C) and (D) in the following reactions sequence:
CH3COCH3LiAIH4→(A)HBr→(B)Alc.KOH→(C)
Fill in the blanks:
The ring structure of benzene was proposed by ______.
How will you synthesize cyclohexene from cyclohexane?
How will you obtain the following from propene?
1,2−Dichloropropane
1,2−Dichloropropane
How would you bring the following conversions?
Acetaldehyde to ethylene
How will you synthesise?
n−Hexane from propene.
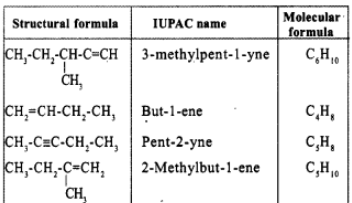
Write the IUPAC names of the following:
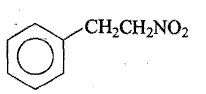
Answer the following:
Prove that benzene molecule has three double bonds.
Complete the following equations:
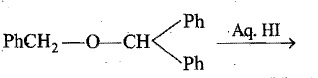
Name the final product of the following reaction:
Benzene is catalytically hydrogenated.
Name the final product of the following reaction:
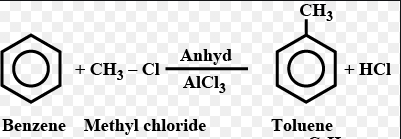
Benzene is treated with methyl chloride in the presence of anhydrous aluminium chloride.
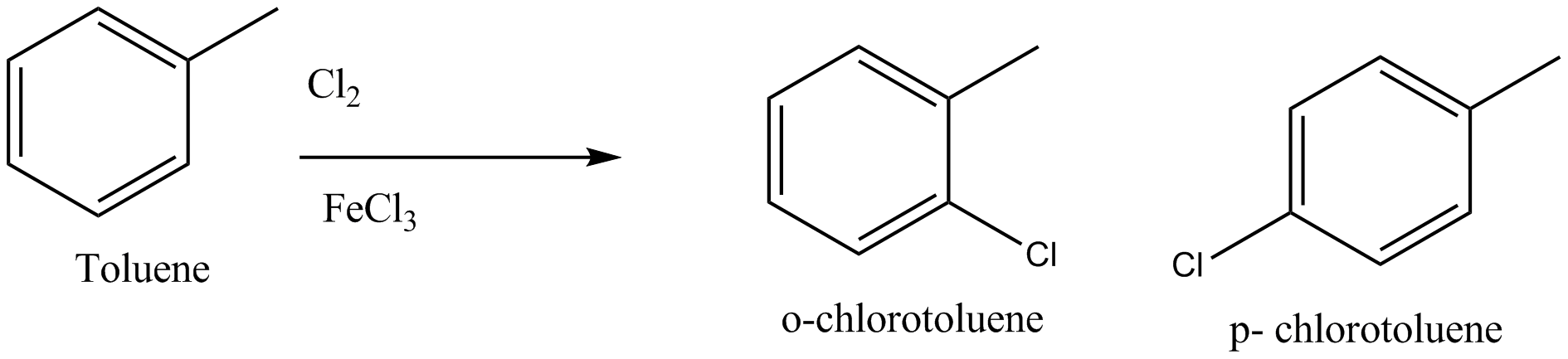
How will you obtain o− chlorotoluene from toluene?
Complete the following, giving the structures of the principal organic products:
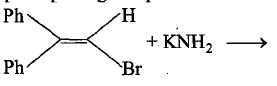
Number of possible fractions of B are:
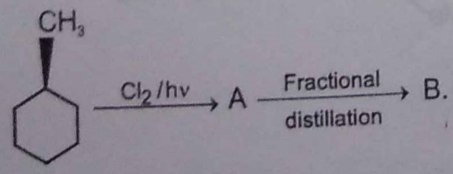
Identify the following compounds:
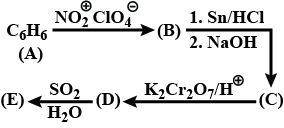
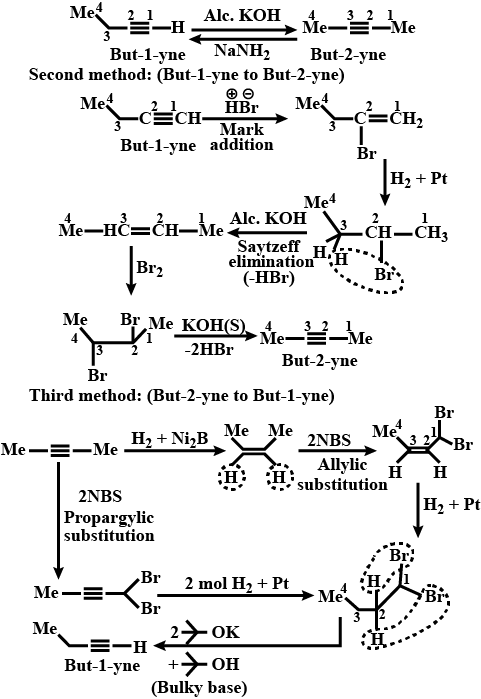
Convert but−1−yne to but−2−yne and vice versa.
Identity the products:
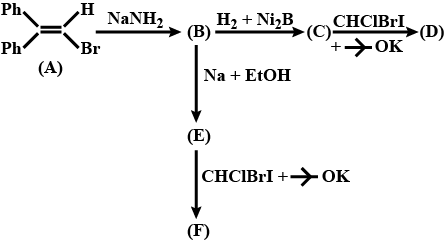
Complete the above reactions:
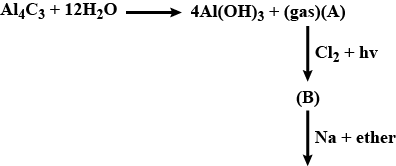
Convert the above
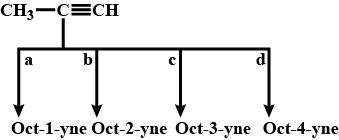
Give an example example branched chain compund.
What are the sources for alkynes ? Give the general formula of alkynes.
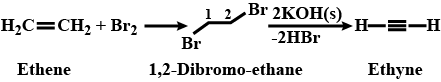
Outline the reaction sequence for the conversion of ethene to ethyne ( the number of steps should not be more than two).
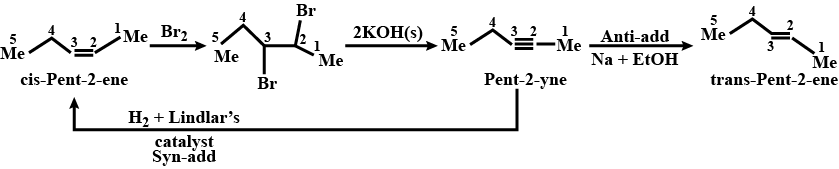
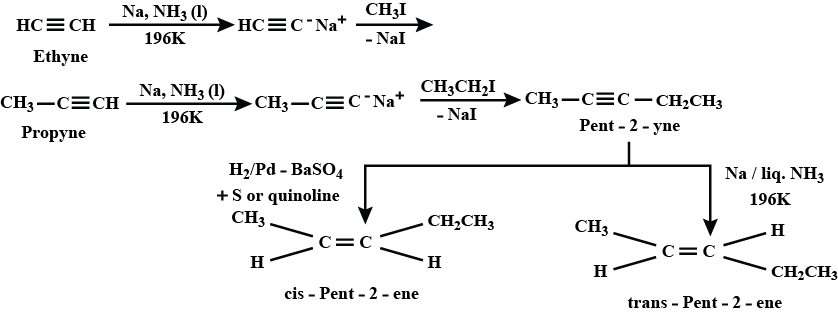
Convert (cis−or trans) pent−2−ene to pent−2−yne and vice versa.
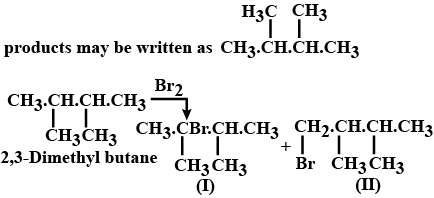
Which alkane having a molecular weight of 86, will form only two monobromo alkanes?
Give the IUPAC names of the following compounds
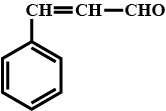
Give the trivial (common) names and the IUPAC names of the following:
C2H2
Give the trivial (common) names and the IUPAC names of the following:
C2H4
Name the compound prepared from:
"sodium propionate"
Write a balanced equation for the same
How ethane is prepared in the laboratory?
Name a compound of each type and draw the figure.
Cyclic compound with a single bond
Cyclic compound with a single bond
What will be the formula and structure of benzene?
Write: molecular formula of ethene ( ethylene)
Give the trivial (common) names and the IUPAC names of the following:
C3H6
Write IUPAC name of isobutane.
Write the:
Molecular formula of methane and ethane
How will you bring about the following conversions?
Propene to propyne
Write the rules of nomenclature of alkane.
Write the general formula for each of the following hydrocarbons and give one example for each.
Alkyne
Name the second member of the alkyne series.
Write the name of the following compounds?
(i)CH3−CH2−Br
(ii)H−H|C=O
(iii)H−H|C|H−H|C|H−H|C|H−H|C|H−CH=CH2
Write IUPAC names of following:
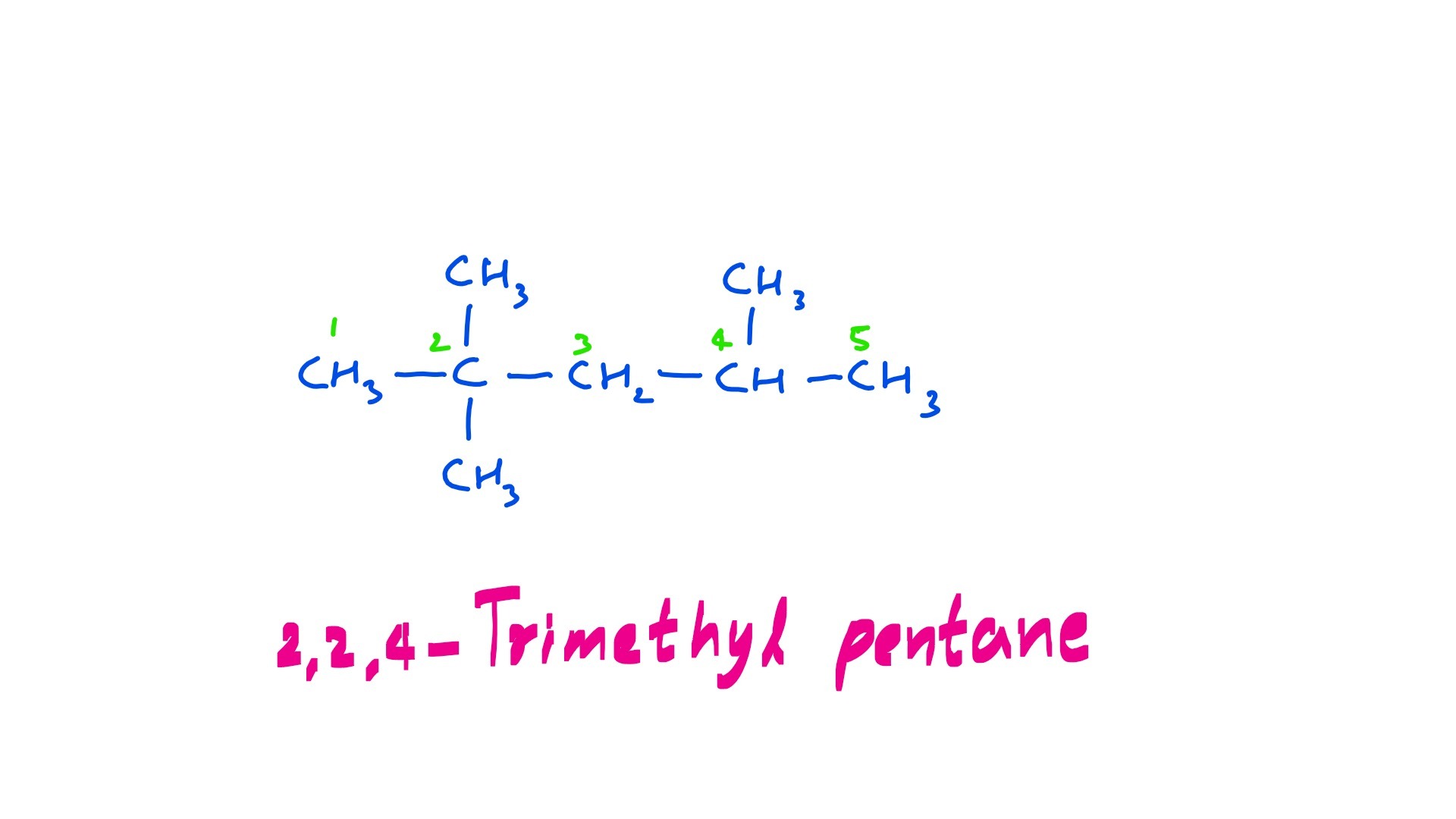
Iso-octane
Name the following compound.
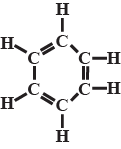
Write the formula of inorganic benzene.
Write down the IUPAC name of the given compound.
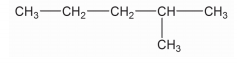
Write the IUPAC name of CH3−CH2−CH2−OH.
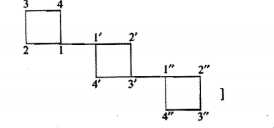
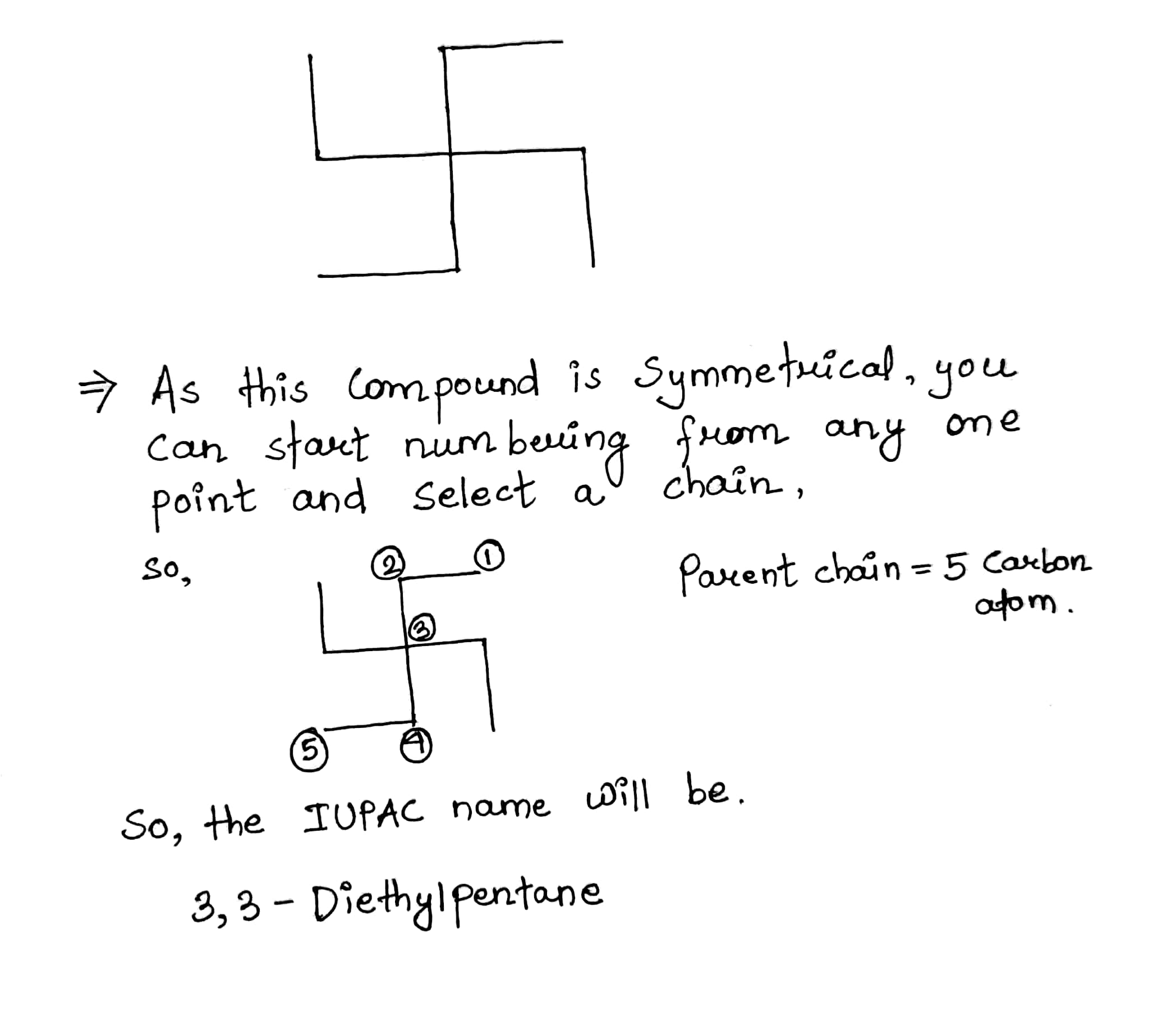
| Compound | IUPAC name |
| CH3−CH|CH3−CH2−CH|CH3−CH2−CH3 | .............. |
| ............. | 2, 3, 3-Trimethylpentane |
| .............. | 3, 3-Diethylpentane |
Write general formula of alkenes and explain general method for its preparation.
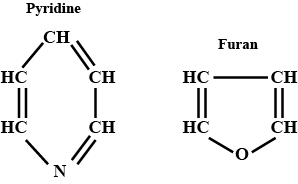
Write the name and structure of two aromatic hetrocyclic compounds.
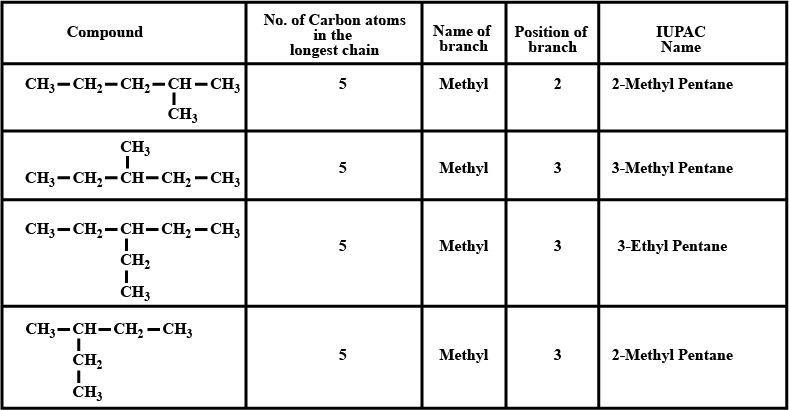
Number the carbon atoms in the main chain of the above compound given above. Put a tick mark against the correct position numbers of the branches.CH3−CH2−CH3|CH−CH2−CH|CH3−CH3
a. What is the IUPAC name?
b. Which is the second branch?
a. What is the IUPAC name?
b. Which is the second branch?
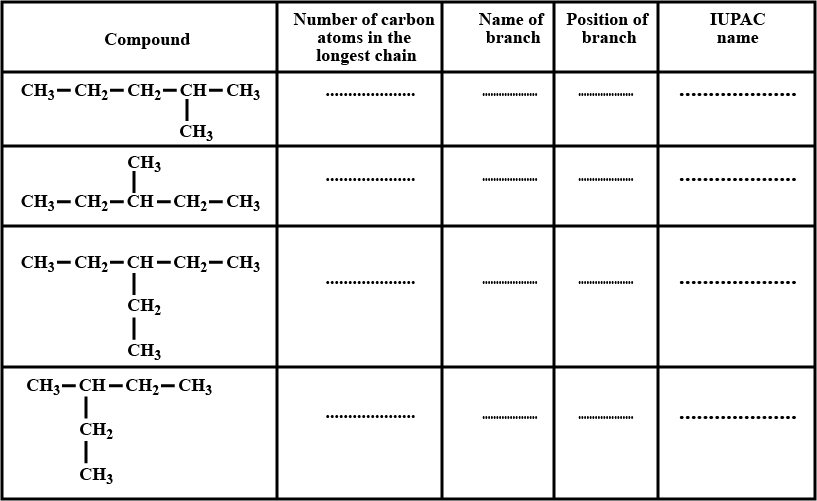
Complete the table given above.
Name the given compound.CH3−CH3|CH−CH2−CH−|CH3CH3
CH3−CH−CH3
|
CH3
a. Write the IUPAC name of this compound.
b. Write the molecular formula.
c. Write the structural formula of its isomer
d. Identify the type of isomerism in the above.
Write down the IUPAC name of the given compound.
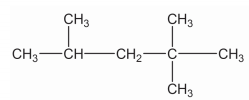
Match the above.
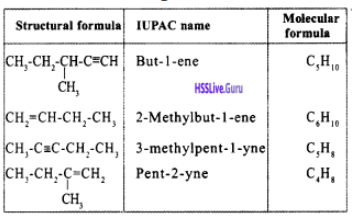
Write down the IUPAC name of the given compound.
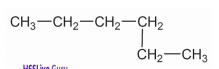
Write down the IUPAC name of the given compound.
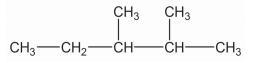
Write down the IUPAC name of the given compound.
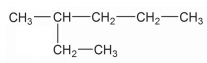
Write down the IUPAC name of the compound given above:
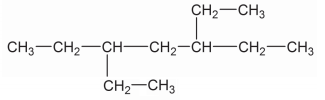
Write down the IUPAC name of the compound given above:
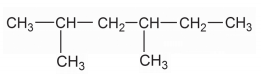
Write down the IUPAC name of the compound:
CH3−CH(CH3)−CH2−CH3
A hydrocarbon chain with molecular C7H16 is numbered in four different ways.
a. Which of the above is numbered correctly ?
b. What is the name of alkyl radical found as the branch ?
c. Write the IUPAC name of the compound.
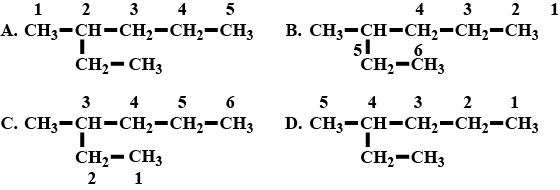
Give equations for the following :
Formation of acetylene.
Write the IUPAC name of the following organic compound:
CH3−CH−CH2−CH2−CH2−CH3
|
CH3
How can the following reactions be used to prepared alkanes?
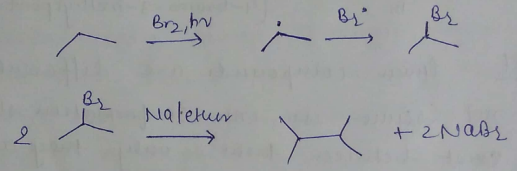
Kolbe's electrolytic method
Kolbe's electrolytic method
Write the name of the group: −CH2−CH3
Write the IUPAC name of the following organic compound:
CH3−CH2−CH2−C≡C−CH3
How can alkanes be prepared from an alkane?
Organic compounds which contain at least one benzene ring are called ....compounds.
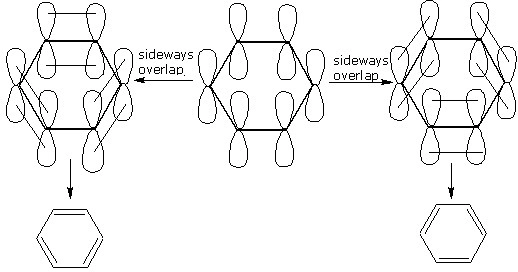
Discuss the orbital structure of benzene
Comment upon acidic character of terminal alkynes.
A ....chain alkane has usually a lower boiling point than corresponding ....chain alkane.
Coal is the chief source of..... hydrocarbons.
How will you distinguish between buta−1,3−diene and but−1−yne?
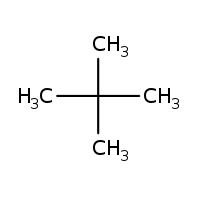
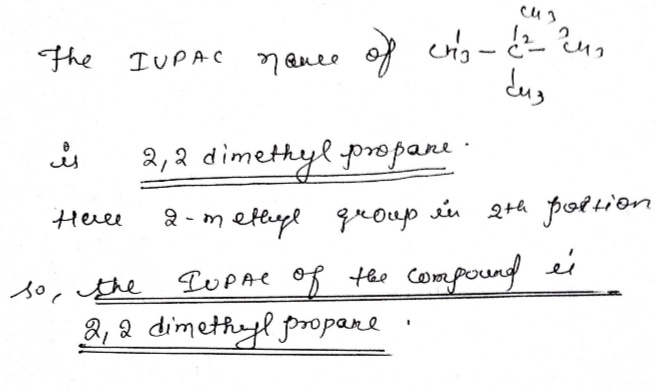
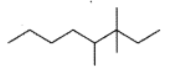
Give the IUPAC name of the lowest molecular weight alkane that contains a quaternary carbon.
How can the following reactions be used to prepared alkanes?
Sabatier and Senderen's reaction
Sabatier and Senderen's reaction
Give reasons for the following:
The boiling points alkanes decrease with branching.
Out of isopentane and neopentane,......has a higher boiling point because of the larger surface area.
Why is that the carbon-carbon bond distance in benzene is intermediate between carbon-carbon single bond and a carbon-carbon double bond.
What are alkynes? How are they prepared in the laboratory?
How will you demonstrate is that double bonds of benzene are somewhat different from that of olefins?
Discuss briefly the various physical properties and chemical reaction of alkanes.
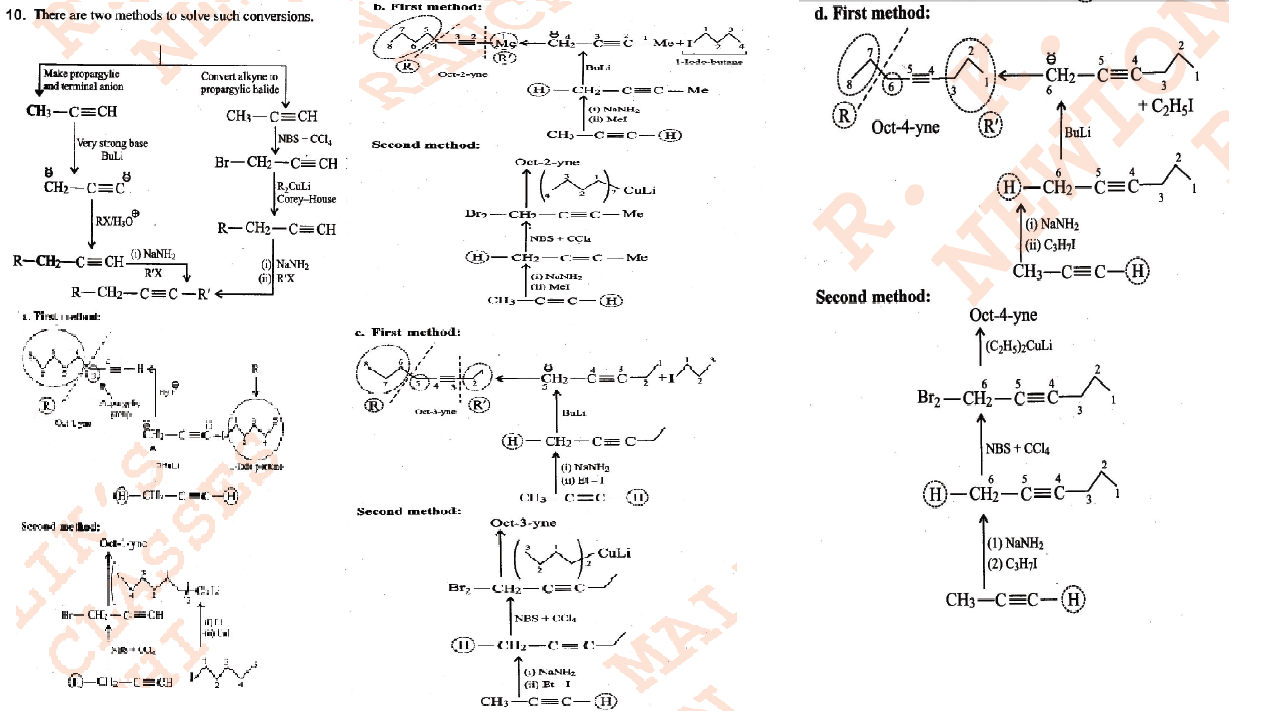
Devise a scheme for the synthesis of n-butane using CH3I as the only carbon source. Can you employ the reactions in your scheme to synthesis propane in fairly pure state? Explain.
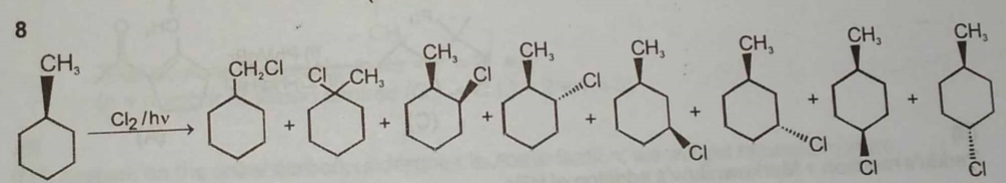
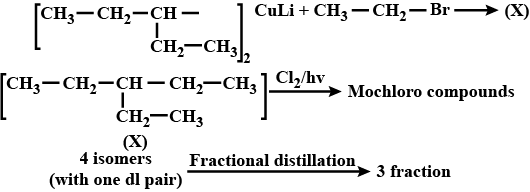
Lithium di(3-pentyl) cuperate on reaction with an alkyl bromide produced (X) C7H16. The mixture of a total number of monochloro isomers formed from (X) is subjected to fractional distillation. The number of fractions obtained is :
Calculate the number of 3∘-free radical formed by homolysis of C-H bonds of alkanes having molecular formula C6H14 .
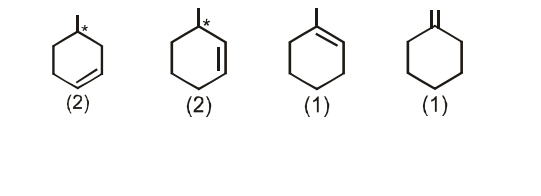
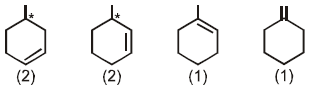
Number of alkenes (be sure to consider the stereochemistry of the alkene) which can be hydrogenated to form methyl cyclohexane are_________.
The number of alkenes (consider stereochemistry of the alkene too) that can be hydrogenated to form methyl cyclohexane is :
Free-radical chlorination of CH4 is nearly 11 times faster than CD4. What is the reason behind it?
CH3−H|C|H−D+Cl2→CH3CD|H−Cl+HCl]93%+CH3−CH2−Cl+DCl]7%What is the relative abstraction of H and D?
Which factors determine the reactivity of halogens in the substitution reaction?
Calculate the percentage of compounds obtained by monobromination of isobutane. The relative reactivity of 1,2,3 H atoms to bromination is 1:82:1600.
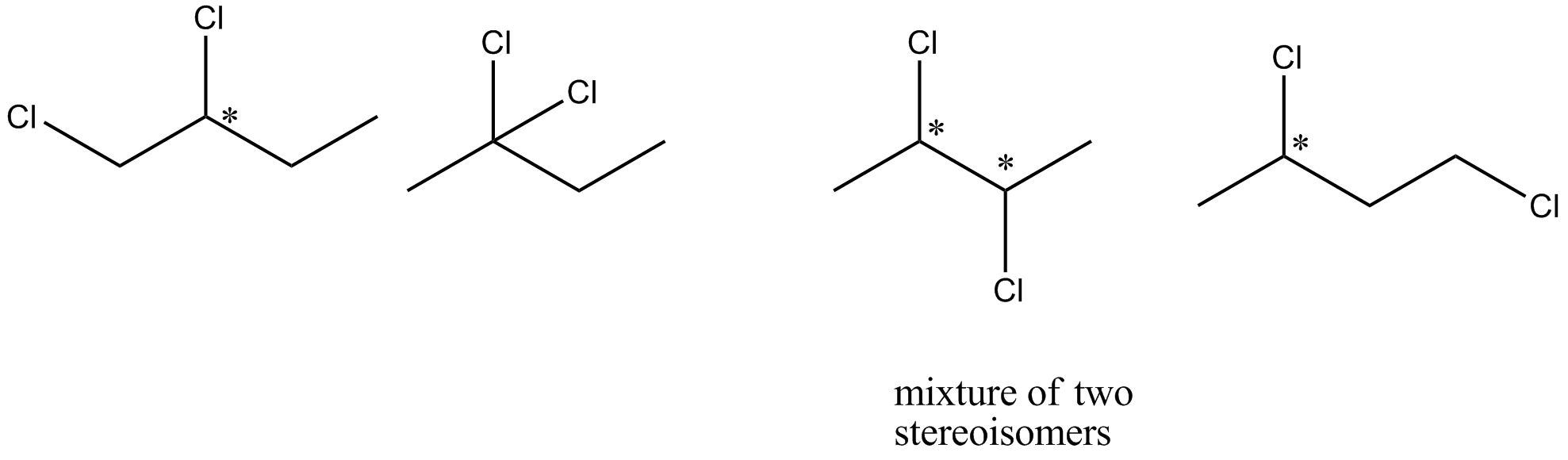
If pure (+)−2− chlorobutane is heated with chlorine gas, how many different dichlorobutanes would be formed?
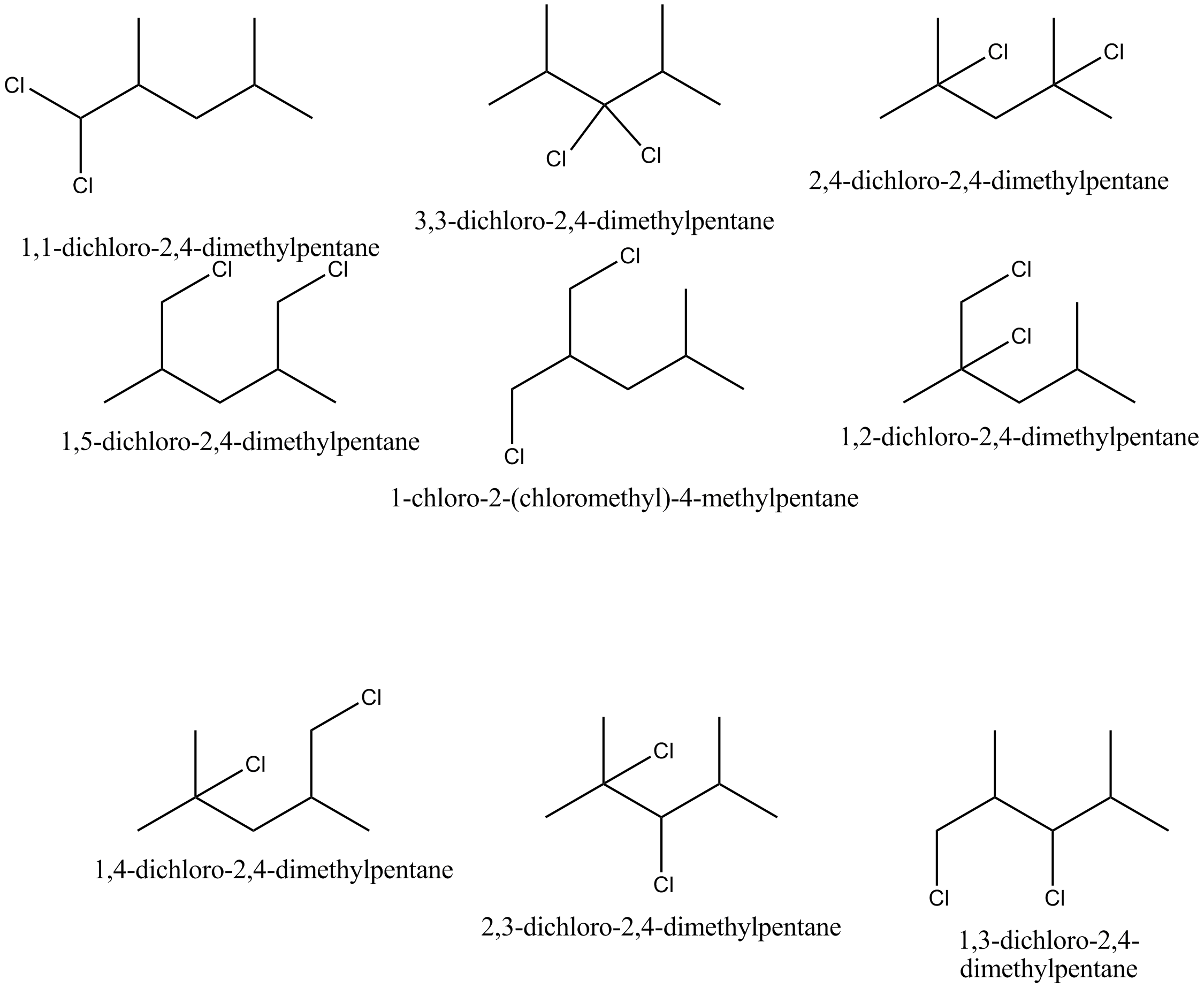
How many positional isomers would result on dichlorination of 2,4− dimethylpentane?
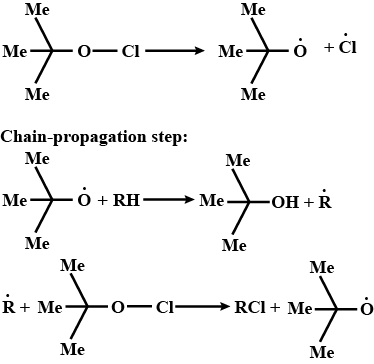
Alkanes are monochlorinated with t-butyl hypochlorite ((Me)3C−O−Cl) as a radical initiator. Give the mechanism of the reaction.
Match column.
Halogenation takes place by free-radical mechanism. The values of E for chain initiation and chain propagation steps and enthalpy of overall reaction of halogens with alkane are given. Match the values with the type of halogens.
Complete the following reactionWhat is major product?
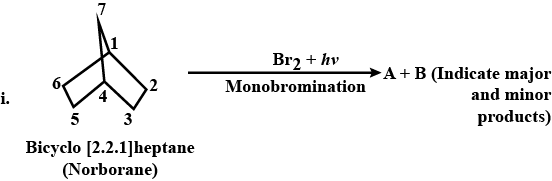
Synthesis the following:Propane to 1-fluoropropane
An experiment is conducted in the laboratory by heating the mixture of sodium acetate and sodalime. With respect to the above experiment answer the following.
(a) Name the gas evolved and write its molecular formula.
(b) Write the balanced chemical equation for the above reaction.
(c) How the gas evolved is collected?
(d) The gas liberated does not react with nitric acid. Give reason.
Identify reactant.
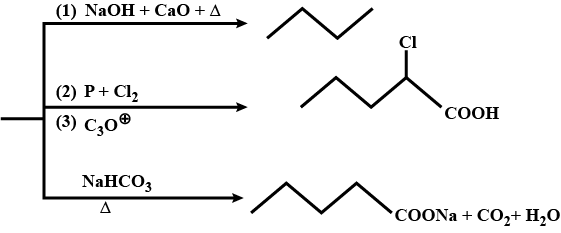
Give the IUPAC name of the following compounds.
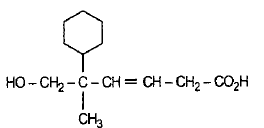
What is the major product B?
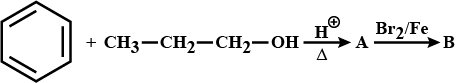
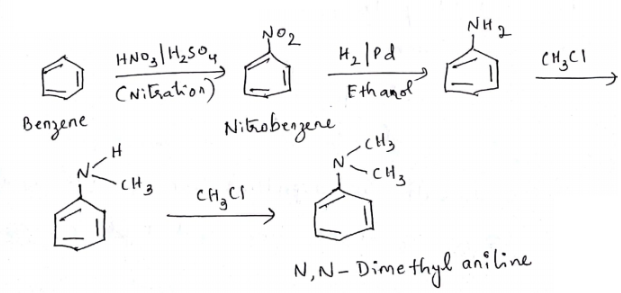
How will you convert benzene into N,N-dimethylaniline?
A hydrocarbon C5H12 gives only one monochlorination product. Identify the hydrocarbon.
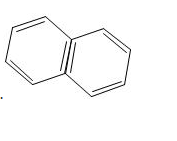
Find the number of delocalized electrons in naphthalene molecule.
IUPAC name of the above structure is ............
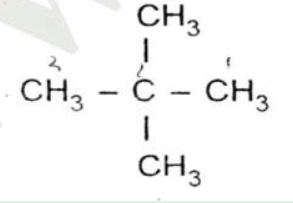
Fill in the blanks:
A compound of carbon and hydrogen used for welding purposes is ______.
Match List I with List II and select the correct answer from the given codes:
Write short notes on: Sabatier and Senderen reduction
What happens when:
Electrolysis of an aqueous solution of potassium acetate is done?
Complete the following equations :
CH3CH=CH2+CH2I2→(A).
CH3CH=CH2+CH2I2→(A).
What happens when lithium dimethyl cuprate is treated with ethyl bromide?
Sodium salt of which acid will be needed for the preparation of propane?
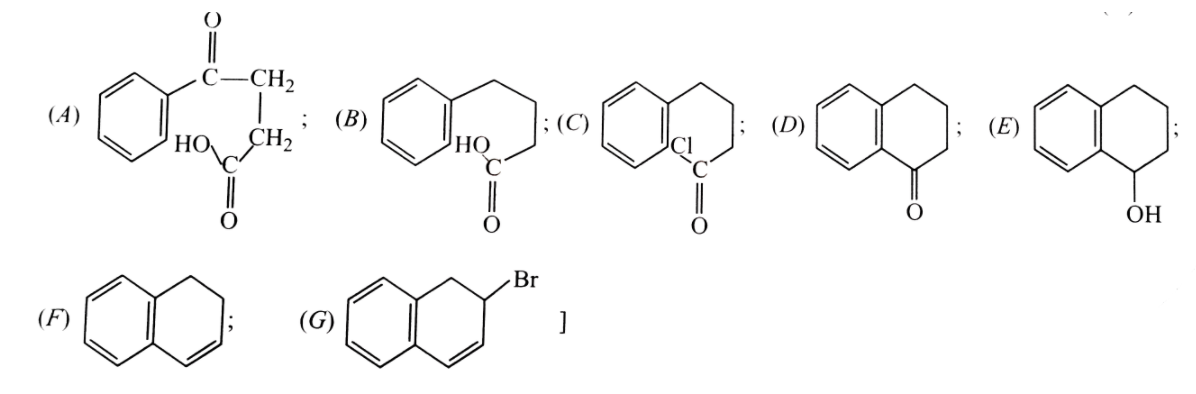
Formation of naphthalence takes place in following step.What are (A),(B),(C),(D),(E),(F) and (G) formed in between
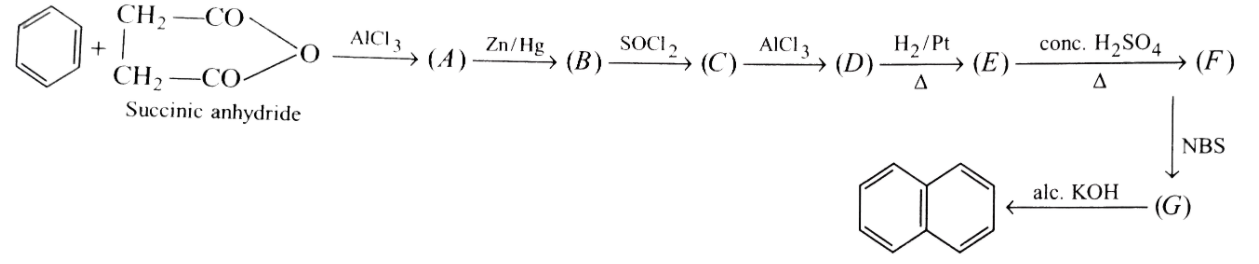
Write the structure of alkene that can be hydrogenated to form propane.
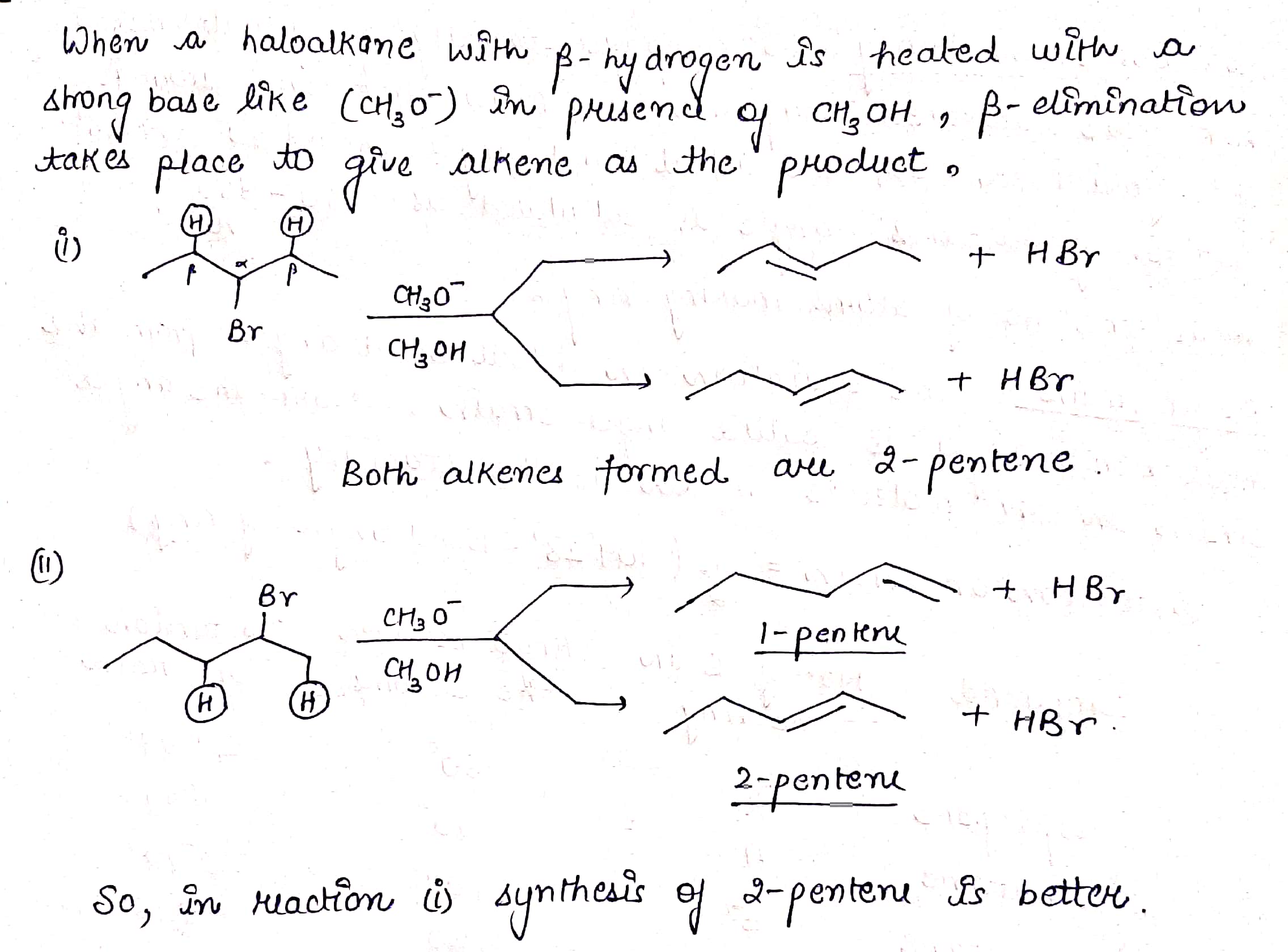
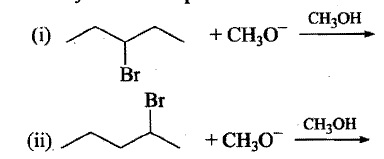
Which of the following reactions would provide a better synthesis of 2−pentene?
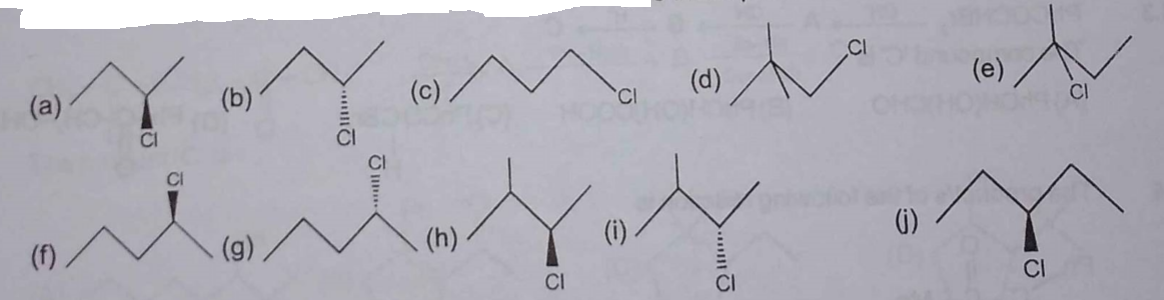
In a monochlorination experiment, some of the monochloro products obtained have been mentioned (a to j). Find the total number of alkane reactants which can give these products.
What is the action of water on the following?
Calcium carbide
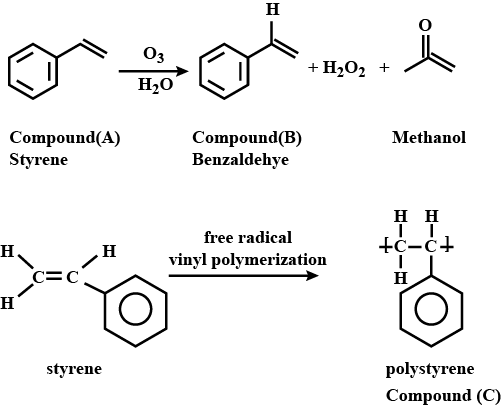
A liquid aromatic organic compound (A) containing carbon (92.3%) and hydrogen (7.7%) decolourised KMnO4, and on ozonolysis gave methanal and another compound (B). The molecular mass of (A) is 104. On treatment with a suitable catalyst, (A) gave high molecular mass solid product (C) having the same empirical formula as that of compound (A). Compound (C) is used in making toys and household goods. Identify (A), (B), and (C), and explain the reactions
How will you bring about the following conversions in not more than two steps?
Toluene to benzyl alcohol
An alkane (A) C5H12on chlorination at 3000C gives a mixture of four different monochlorinated derivatives, (B),(C),(D) and (E).Two of these derivatives give the same stable alkene (F) on dehydrohalogination. On oxidation with hot alkaline KMnO4 followed by acidification,(F) gives two product (G) and (H).Give the structures of (A) to (H) with proper readoning.
Name the organic compound prepared by each of the following reactions:
C2H5COONa+NaOH→
Molecular formula of some compounds are given in the box.
C2H4 , C6H14, CH3−CH2−Cl , CH3−COOH, C6H6
Which is an aromatic compound?
Which is an aromatic compound?
How will you prepared 3−methylbut−1−yne by starting with ethyne?
Give reasons for the following:
Normally benzene gives electrophilic substitution reaction rather than electrophilic addition reaction although it has a double bond.
What are paraffins and olefins? Give one example of each and write chemical reaction to differentiate them.
Write the equations for the following preparations:
Preparations: Ethane from sodium propionate Solution
What are alkanes? Discuss briefly the various methods used for the preparation of alkenes. Describe with a labelled diagram the laboratory preparation of ethene from ethanol.
What are arenes? How are they classified? Discuss briefly the isomerism and nomenclaure of arenes.
How will you prepared (i) cis−pent−2−ene and trans−pent−2−ene by starting with ethane.
Give reason for the following:
The melting point of cis−2−butane is lower than that of trans−2−butane.
Explain the directive influence of various substituents and their effect on reactivity of arenes.
Give reason for the following:
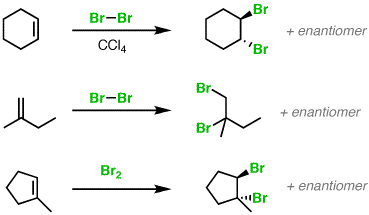
Addition of Br2 to cyclohexane gives only trans-addition product.
The real structure of benzene is a ....of two.....structures.
How will you bring about the following conversion in not more than two steps?
propene to propyne
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions