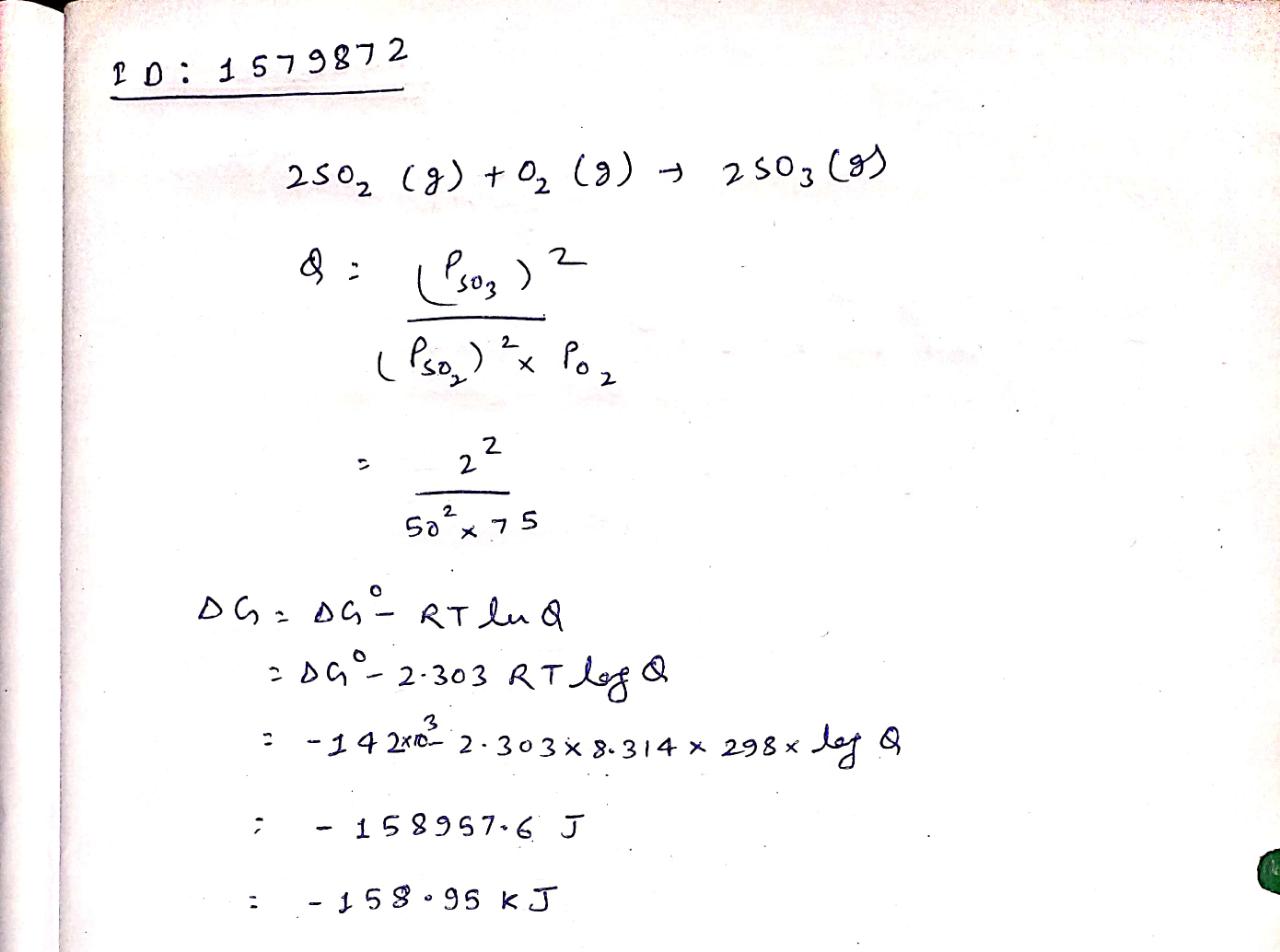Thermodynamics - Class 11 Medical Chemistry - Extra Questions
The standard heat of formation (ΔfH0298) of ethane (in kJ/mol), if the heat of combustion of ethane, hydrogen and graphite are −1560,−393.5 and −286 kJ/mol, respectively is ______.
The surface of copper gets tarnished by the formation of copper oxide. N2 gets was passed to prevent the oxide formation during heating of copper at 1250 K. However, the N2 gas contains 1 mole % of water vapour as impurity. The water vapour oxidises copper as per the reaction given below:
2Cu(s)+H2O(g)→Cu2O(s)+H2(g)
ρH2 is the minimum partial pressure of H2 (in bar) needed to prevent the oxidation at 1250 K. The value of ln(ρH2) is _________.
Given: Total pressure =1 barR (universal gas constant) =8 JK−1mol−1ln(10)=2.3Cu(s) and Cu2O(s) are mutually immiscible
At 1250 K:2Cu(s)+1/2O2(g)→Cu2O(s); ΔG∘=−78,000 J mol−1 H2(g)+1/2O2(g)→H2O(g); ΔG∘=−1,78,000 J mol−1[Upto one decimal point and take modulus of the answer]
Total pressure =1 bar
R (universal gas constant) =8 JK−1mol−1
ln(10)=2.3
Cu(s) and Cu2O(s) are mutually immiscible
At 1250 K:
At 1250 K:
2Cu(s)+1/2O2(g)→Cu2O(s); ΔG∘=−78,000 J mol−1 H2(g)+1/2O2(g)→H2O(g); ΔG∘=−1,78,000 J mol−1
[Upto one decimal point and take modulus of the answer]
The degree of freedom of a three atomic molecule can not be 5. true=1, false=0
What is meant by an equilibrium?
The specific heat of a gas is 0.125 and 0.075 cal/g. The value of its molar mass is ______.
Thermochemical equations can be reversed by changing the sign of ΔH.
If true enter 1, else enter 0.
If true enter 1, else enter 0.
ΔG=ΔH−TΔS.
If true enter 1, else enter 0.
If true enter 1, else enter 0.
Which has more heat capacity: a bucket full of water or a mug full of water?
Given equal masses of aluminium and copper, which would require more heat if both are heated to the same temperature. Justify your answer.
[Specific heat capacity of Al=899Jkg−1C−1 and Cu=387Jkg−1C−1]
Differentiate between heat capacity and specific heat capacity.
Why is water used as a coolant in car radiations and factories to keep the engine and other parts cool?
What is the quantity of heat released per kg of water per 1oC fall in temperature?
Calculate the heat energy that will be released when 5.0 kg of steam at 100oC condenses to form water at 100oC. Express your answer in S.I. unit (specific heat of vapourisation of steam is 2268 kJ/kg)
Discuss the change in energy and arrangement of molecules on increasing the temperature of ice from -5oC to 10oC at 1 atm pressure.
Water falls from a height of 50 cm. Calculate the rise in temperature of water when it strikes the bottom. (g=10ms−2, sp. heat capacity of water =4200J/kgoC)
Explain why
(a) Two bodies at different temperatures T1 and T2 if brought in thermal contact do not necessarily settle to the mean temperature (T1 +T2)/2
(b) The coolant in a chemical or a nuclear plant (i.e. the liquid used to prevent the different parts of a plant from getting too hot) should have high specific heat
(c) Air pressure in a car tyre increases during driving
(d) The climate of a harbour town is more temperate than that of a town in a desert at the same latitude
For the reaction,
2 A(g)+ B(g) → 2D(g), △U⊖=−10.5KJ and △S⊖=−44.1JK−1
Calculate △G⊖ for the reaction and predict whether the reaction may occur spontaneously.
Calculate △G⊖ for the reaction and predict whether the reaction may occur spontaneously.
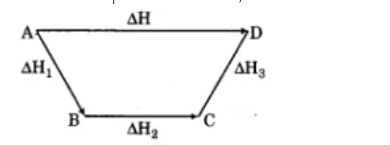
State Hess's law of constant heat summation and explain it with an example.
State Hess's law.
1 mole of Fe2O3 and 2 moles of Al are mixed at temperature 25oC and the reaction is completed to give:
Fe2O3(s)+2Al(s)⟶Al2O3(s)+2Fe(l);ΔH=−850kJ
The liberated heat is retained within the products, whose combined specific heat over a wide temperature range is about 0.8Jg−1K−1. The melting point of iron is 1530oC. Show that the quantity of heat liberated is sufficient to raise the temperature of the product to the melting point of iron in order to get it welded.
A gas expands from a volume of 3.0 dm3 to 5.0 dm3 against a constant pressure of 3.0 atm. The work done during expansion is used to heat 10.0 mole of water of temperature 290.0K. Calculate the final temperature of water (specific heat of water =4.184 J K−1g−1)
A gas contained in a cylinder is filled with a frictionless piston expands against a constant pressure 1 atmosphere from a volume of 4 litre to a volume of 14 litre. In doing so, it absorbs 800J thermal energy from surroundings. Determine ΔU for the process.
5 mole of oxygen are heated at constant volume from 10oC to 20oC. What will be the change in the internal energy of gas? The molar heat of oxygen at constant pressure, CP=7.03cal mol−1deg−1 and R=8.31Jmol−1deg−1.
Determine the heat of transformation of
C(diamond)→C(graphite) from the following data
C(diamond)+O2(g)→CCO2(g);(ΔH=−94.5kcal)
C(graphite)+O2(g)→CCO2(g);(ΔH=−94.0kcal)
The equilibrium constant at 25oC for the process:
Co3+(aq.)+6NH3(aq.)⇌[Co(NH3)6]3+(aq.) is 2×107
Calculate the value of ΔGo at 25oC. (R=8.314JK−1mol−1)
For the equilibirium,
PCl5(g)⇌PCl3(g)+Cl2(g)
Kc=1.8×10−7
Calculate ΔGo for the reaction (R=8.314JK−1mol−1)
For the reaction
2NO(g)+O2(g)⟶2NO2(g)
Calculate ΔG at 700 K when enthalpy and entropy changes are −113 kJmol−1 and −145 JK−1mol−1 respectively.
Zinc reacts with dilute hydochloric acid to give hydrogen at 17oC. The enthalpy of the reaction is −12.55kJmol−1 of zinc and entropy change equals 5.0JK−1mol−1 for the reaction. Calculate the free energy change and predict whether the reaction is spontaneous or not.
In the reaction
A++B⟶A+B+,
there is no entropy change. If enthalpy change is 22kJ of A+, calculate ΔG for the reaction.
ΔH and ΔS for the system
H2O(l)⇌H2O(g)
at 1 atmospheric pressure are 40.63 kJmol−1 and 108.8 JK−1mol−1 respectively. Calculate the temperature at which the rates of forward and backward reactions will be the same. Predict the sign of free energy for this transformation above this temperature.
For the reaction
SOCl2+H2O⟶SO2+2HCl
the enthalpy of reaction is 49.4kJ and the entropy of reaction is 336JK−1. Calculate ΔG at 300K and predict the nature of the reaction.
ΔH and ΔS for the reaction,
Ag2O(s)⟶2Ag(s)+12O2(g)
are 30.56kJmol−1 and 66.0JK−1mol−1 respectively. Calculate the temperature at which free energy change for the reaction will be zero. Predict whether the forward reaction will be forward above or below this temperature.
Calculate the temperature at which liquid water will be in equilibrium with water vapour.
ΔHvap=40.73 kJmol−1 and ΔSvap=0.109 kJmol−1K−1
For the reaction, Ag2O(s)⇌2Ag(s)+12O2(g)
ΔH,ΔS,ΔT are 40.63 kJ mol−1,1.808 JK−1mol−1 and 373.4 K respectively. Free energy change ΔG of the reaction will be:
For the reaction,
4C(graphite)+5H2⟶nC4H10;ΔHo=−124.73kJmol−1
4C(graphite)+5H2⟶iso−C4H10;ΔSo=−365.8JK−1mol−1
ΔHo=−131.6kJmol−1
ΔSo=−381.079JK−1mol−1
Indicate whether normal butane can be spontaneously converted to iso-butane or not.
All the energy released from the reaction X→Y,△rG∘=−193 kJ mol−1 is used for oxidising M+ as M+→M3++2e−,E∘=−0.25 V. Under standard conditions, the number of moles of M+ oxidised when one mole of X is converted to Y is: (F=96500 C mole−1)
Zn(s)+2AgCl(s)⇌ZnCl2(0.555 M)+2Ag(s)
E0=1.015 volt(dEdT)P=−4.02×10−4volt per degree.
Find △G,△S.
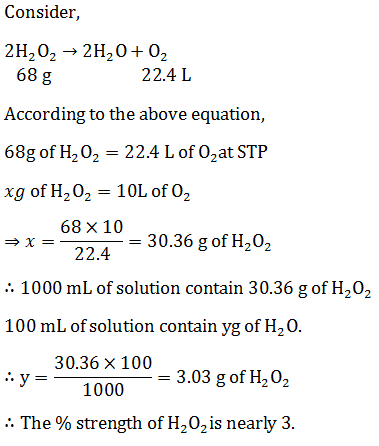
Commercial sample of H2O2 is labeled as 10 Volume. Its % strength is nearly:
The following data is known about the melting of KCl:
ΔH=7.25kJmol−1;ΔS=+0.007KJK−1mol−1
Calculate its melting point.
Show that the reaction,
CO(g)+12O2(g)⟶CO2(g)
at 300 K is spontaneous and exothermic, when the standard entropy change is −0.094 kJ mol−1K−1. The standard Gibbs free energies of formation for CO2 and CO are −394.4 and −137 kJ mol−1 respectively.
For the reaction,
Ag2O⇌2Ag(s)+12O2(g)
Calculate the temperature at which free energy change is zero. At a temperature lower than this, predict whether the forward of the reverse reaction will be forward. Give reason
(ΔH=+30.56kJ;ΔS==+0.066kJK−1mol−1)
Show that the internal energy of the air (treated as an ideal gas) contained in a room remains constant as the temperature changes between day and night. Assume that the atmospheric around remains constant and the air in the room maintains this pressure by communicating with the surrounding through the windows etc.
Calculate the standard internal energy change for the reaction
C(graphite)+H2O(g)→CO(g)+H2O(g)
Δof at 25o; H2O(g)=−241.8kJmol−1
CO(g)=−110.5kJmol−1
R=8.314JK−1mol−1
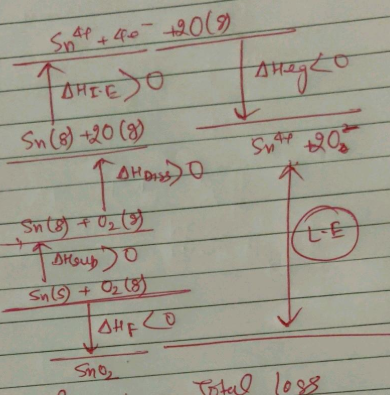
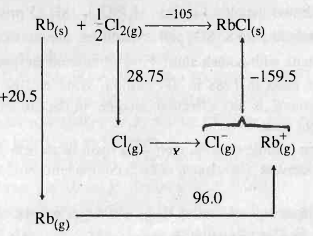
The Born-Haber cycle for formation of rubidium chloride (RbCl)is given below (the enthalpies are in kCal mol−1).
Find the value of X.

Determine whether the following reaction will be spontaneous or nonspontaneous under standard conditions.
Zn(s)+Cu2+(aq)→Zn2+(aq)+Cu(s), ΔH0=−219kJ, ΔS0=−21JK−1.
Calculate the heat change during the reaction 24g C and 128g S following :the change C+S2→CS2; ΔH=22K cal.
For a chemical reaction, ΔH and ΔS are negative. State, giving reason, under what conditions this reaction is expected to occur spontaneously.
For the reaction, 2A(g)+B(g)\longrightarrow 2D(g),\Delta { U }=-10.5KJ and \Delta { S }=-44.10J{ K }^{ -1 }. Calculate \Delta { G } for the reaction and reaction and predict whether the reaction may occur spontaneously.
For a reaction both enthalpy change and entropy change are positive. Under what conditions the reaction will be spontaneous?
For the reaction:
2A(g)+B(g)\rightarrow 2D(g),
\Delta U^{\ominus}=-10.5 kJ and \Delta S^{\ominus}=-44.1 JK^{-1}.Does the reaction occur spontaneously?
[Give your answer in terms of Yes or No]
\Delta U^{\ominus}=-10.5 kJ and \Delta S^{\ominus}=-44.1 JK^{-1}.Does the reaction occur spontaneously?
How do you define specific heat ? What is specific heat of water ?
Explain Third law of thermodynamics.
For oxidation of iron,
4Fe\left( s \right) +{ 3O }_{ 2 }\left( g \right) \rightarrow 2{ Fe }_{ 2 }{ O }_{ 3 }\left( s \right) entropy change is - 549.4 { JK }^{ -1 }{ mol }^{ -1 } at 298 K. In spite of negative entropy change of this reaction, why is the reaction spontaneous?(\triangle ,{ H }^{ \circleddash } for this reaction is -1648\times { 10 }^{ 3 }J\quad { mol }^{ -1 })
Many of the photochemical changes have positive sign of \delta G, yet they are spontaneous. Why?
What is specific heat?
If 1.0 kcal of heat is added to 1.2 L of { O }_{ 2 } in a cylinder at constant pressure of 1 atm, the volume increase to 1.5 L. Calculate \Delta U and \Delta H of the process (1 L-atm=100 J)
Identify the correct statement regarding a spontaneous process
1) For a spontaneous process in an isolated system, the change in entropy is positive
2) Endothermic process are never spontaneous
3) Exothermic processes are always spontaneous (4) Lowering of energy in the reaction process is the only criterion for spontaneity
State and explain Hess's law of constant heat summation? Give its application.
Derive relationship between H and U.
Write the importance of Hess's law of constant heat summation.
A gas cylinder contains 14.2 Kg butane gas. it is consumption of energy in a family is 10000 KJ for cooking purposes how long this cylinder will be sufficient to supply. If the enthalpy of combustion of butane is 2658 KJ mol
How it is possible to find the value of \Delta U and not the value of U
Calculate \text E_{cell} and \Delta G for the following 28 ^ { \circ } \mathrm { C } :\mathrm { Mg } _ { ( s ) } + \mathrm { Sn } ^ { 2 + } ( 0.04 \mathrm { M } ) \rightarrow \mathrm { Mg } ^ { 2 + } ( 0.06 \mathrm { M } ) + \mathrm { Sn } _ { ( s ) }
E _ { \text { cell } } ^ { \circ } = 2.23 \mathrm { V }
Is the reaction spontaneous?
The reaction of nitrogen with hydrogen to make ammonia has \Delta H=-92 kJ N_2(g)+3H_2(g)\rightarrow2NH_3(g). What is the value of \Delta U (in kJ) if the reaction is carried out at a constant pressure of 40\ bar and the volume change is -1.25\ litre?
1g of graphite is burnt in a bomb calorimeter in excess of oxygen at 298\ K and 1 atmospheric pressure according to the equation.
C (graphite) + O_{2}(g) \rightarrow CO_{2}(g)
During the reaction, temperature rises from 298 k to 299 k. If the heat capacity of the bomb calorimeter is 20.7 kJ/K. What is the enthalpy change for the above reaction at 298 K and 1 atm?
Calculate the \Delta_{r}H^{o} at 298\ K for the reaction:
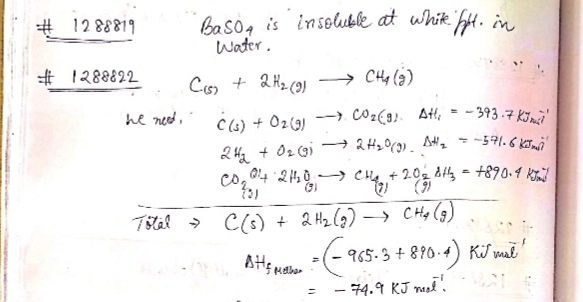
C(graphite)+2H_{2}(g)\rightarrow CH_{4}(g)
from the following date:
C(graphite)+O_{2}(g)\rightarrow CO_{2}(g) \\ \Delta_{r}H^{o}=-393.5\ kJ\ mol^{-1} \\ H_{2}(g)+\dfrac {1}{2}O_{2}(g)\rightarrow H_{2}O(l)\\ \Delta_{r}H^{o}=-285.8\ kJ\ mol^{-1}
CO_{2}(g)+2H_{2}O(l)\rightarrow CH_{4}(g)+2O{2}(g)\\ \Delta_{r}H^{o}=+890.3\ kJ\ mol^{-1}
Water can be lifted into the water tank at the top of the house with the help of a pump. Then why is it not considered to be spontaneous?
A chemical reaction is spontaneous at 298K and non-spontaneous at 350K. Which of the following is correct for the reaction?
| s.no | \Delta G | \Delta H | \Delta S |
| A) | - | - | + |
| B) | + | + | + |
| C) | - | + | - |
| D) | + | - | + |
| E) | - | - | - |
When a substance having mass 3 kg receives 600 cal of heat, its temperature increases by 10^0C. What is the specific heat of the substance?
Calculate \triangle G for the reaction at 298 K2SO_2(g)+O_2(g)\longrightarrow 2SO_3(g), \triangle { G }^{ o }=-142 kJ{ mol }^{ -1 }
If partial pressures of SO_2, O_2 and SO_3 are 50 atm 75 atm and 2 atm respectively at 298 K
If partial pressures of SO_2, O_2 and SO_3 are 50 atm 75 atm and 2 atm respectively at 298 K
Explain why:
Ice at zero degrees centigrade has a greater cooling effect than water at 0 ^\circ C.
What is the need to heat solution ?
At 298 \mathrm{K}. \mathrm{K}_{\mathrm{p}}
for the reaction N_{2} O_{4}(g) \rightleftarrows 2 \mathrm{NO}_{2}(g) is
0.98 . Predict whether the reaction is spontaneous or not.
Given that \Delta H=0 for mixing of two gases. Explain whether the diffusion of these gases
into each other in a closed container is a spontaneous process or not?
Explain the meaning of thermochemical equation for the reaction:
2H_{2}(g)+O_{2}(g)\rightarrow 2H_{2}O(l)+136kcal.
What is spontaneous process? Give example.
Justify the following statements:
(a) An exothermic reaction is always thermodynamically spontaneous.
(b) The entropy of a substance increases on going from liquid to vapour state at any temperature.
Evaporation of water is an endothermic process but spontaneous. Explain.
What is meant by entropy driven reaction? How can a reaction with positive changes of enthalpy and entropy be made entropy driven?
Define non-spontaneous process.
What are the applications of Hess's law of constant heat summation?
What is meant by the free energy of a system? What will be the direction of chemical reaction when
(i) \Delta G=0
(ii) \Delta G>0
(iii) \Delta G<0
Give Hess's Law of constant Heat summetion and write its uses.
What will be the sign of G for melting of ice at 267 K and 276 K ? (Melting point of ice= 272 K ).
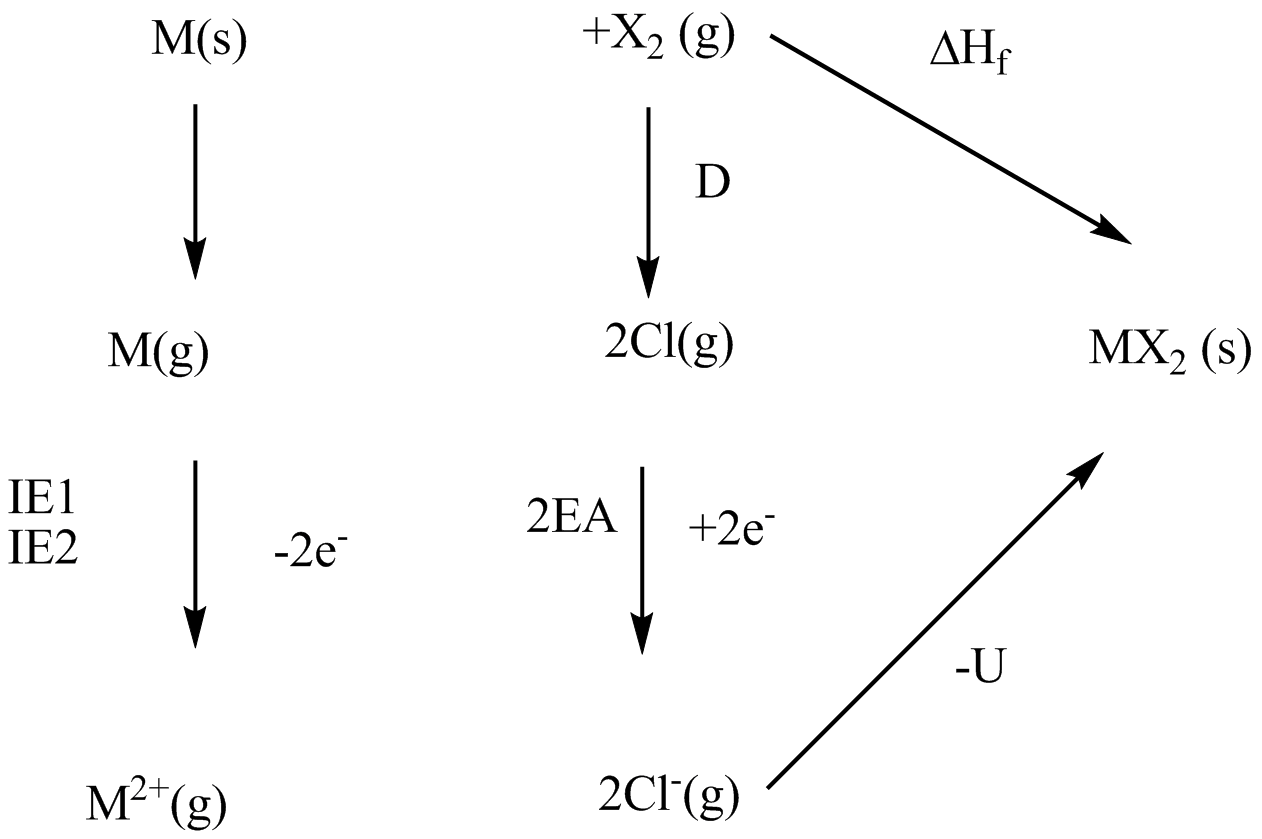
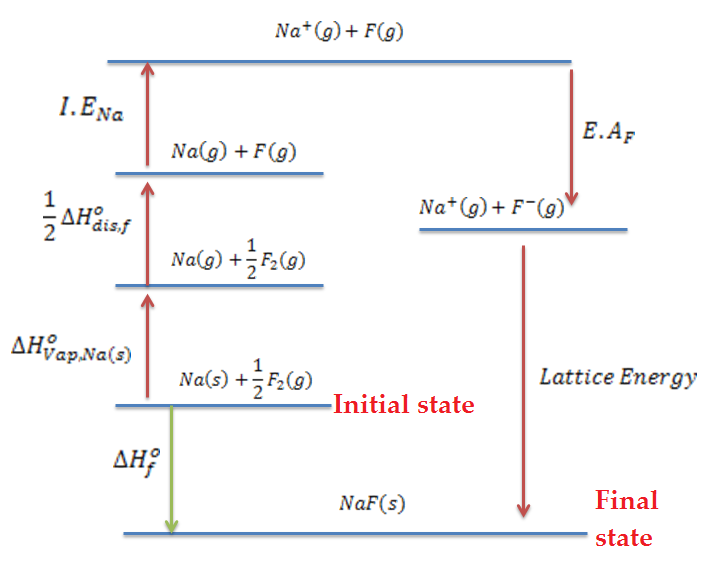
Explain the steps involved in Born Haber cycle for the formation of NaCl.
Why most of the exothermic processes (reactions) are spontaneous?
In a constant volume calorimeter, 3.5 g of a gas with molecular weight 28 was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to the combustion process. Given that the heat capacity of the calorimeter is 2.5 kJ K^{-1}, the numerical value for the enthalpy of combustion of the gas in kJmol^{-1} is:
200 ml of 1M HCl is mixed with 400 ml of 0.5M NaOH. The temperature rise in the calorimeter was found to be 4.0^{o}C. Water equivalent of calorimeter is 25g and the specific heat of the solution is 1 cal/mL/degree. If the theoritical heat of neutralization of a strong acid and strong base is -13.5 kcal, then the percentage error (as nearest integer) incurred in this experiment while calculating the heat of neutralization is
It is observed that the functioning of neuronal circuits in our brain is driven by the energy available from the combustion of glucose. Calculate the amount of glucose (in gm) which should be burnt per hour to produce sufficient energy for the brain, which operates at \dfrac{128}{9} watts.
Given : |\Delta H^{0}| of combustion of glucose at 400 K = 3000 kJ/mol
|\Delta S^{0}| of combustion of glucose at 400 K= 180 J/mol K
|\Delta S^{0}| of combustion of glucose at 400 K= 180 J/mol K
From the following data at \displaystyle 25^{\circ}C, The standard enthalpy of formation of FeO(s) is X, then 0.3-X is:
Reaction \displaystyle \Delta _{r}H^{\circ} ( kJ / mole ) \displaystyle Fe_{2}O_{3}(s)+3C (graphite)\rightarrow 2Fe(s)+3CO(g) 492.6 \displaystyle FeO(s)+C (graphite)\rightarrow Fe(s)+CO(g) 155.8 \displaystyle C (graphite)+O_{2}(g)\rightarrow CO_{2}(g) \displaystyle -393.51 \displaystyle CO(g)+1/2\: O_{2}(g)\rightarrow CO_{2}(g) \displaystyle -282.98
| Reaction | \displaystyle \Delta _{r}H^{\circ} ( kJ / mole ) |
| \displaystyle Fe_{2}O_{3}(s)+3C (graphite)\rightarrow 2Fe(s)+3CO(g) | 492.6 |
| \displaystyle FeO(s)+C (graphite)\rightarrow Fe(s)+CO(g) | 155.8 |
| \displaystyle C (graphite)+O_{2}(g)\rightarrow CO_{2}(g) | \displaystyle -393.51 |
| \displaystyle CO(g)+1/2\: O_{2}(g)\rightarrow CO_{2}(g) | \displaystyle -282.98 |
For the ionic solid MX_{2},where X is monovalent ,the enthalpsy of formation of the solid from M(s) and X_{2}(g) is 1.5 times the electron gain enthalpsy of X(g).the first and second ionisation enthalpsy of the metal(M) are 1.2 and 2.8 times of the enthalpsy of sublimation of M(s).the bond dissociation enthalpy of X_{2}(g) is 0.8 times the first ionisation enthaply of metal and it is also equal to one-fifth of the magnitude of lattice enthalpy of MX_{2}. If the electron gain enthalpy of X(g) is 96Kcal/mol, find the enthalpy of sublimation of metal(M) in Kcal/mol.(Round off answer to integer).
If X is the lattice entahlpy of Ca{Cl}_{2} ,find X. Given that the enthalpy of
(i) sublimation of Ca is 121kJ{ mol }^{ -1 }
(ii) dissociation of {Cl}_{2} to Cl is 242.8kJ{ mol }^{ -1 };
(iii) ionization of Ca to {Ca}^{2+} is 2422kJ{ mol }^{ -1 };
(iv) electron gain enthalpy for Cl to {Cl}^{-} is -355kJ{ mol }^{ -1 };
(iv) { \Delta }_{ f }H overall is -795kJ{ mol }^{ -1 }.
Calculate the lattice enthalpy(nearest integer value) in kJ{ mol }^{ -1 } of LiF, given that enthalpy of,
(i) sublimation of lithium is 155.3kJ { mol }^{ -1 };
(ii) dissociation of 1/2 mole of {F}_{2} is 75.3kJ;
(iii) ionization enthalpy of lithium is 520kJ { mol }^{ -1 };
(iv) electron gain enthalpy of 1 mole of F(g) is -333kJ;
(v) { \Delta }_{ f }H overall is -594kJ { mol }^{ -1 }.
The standard enthalpy of formation of FeO and Fe_2O_3 is -65 \: kcal \: mol^{-1} and -197 \: kcal \: mol^{-1} respectively. If a mixture containing FeO and FeO_3 in 2 : 1 mole ratio on oxidation is changed into 1 : 2 mole ratio, thermal energy in kcal released per 6 mole of mixture will be (nearest integer)____________.
The endothermic reaction of carbon (graphite) in super heated steam at 500 K [i.e., C_{graphite} +H_2O(g)\longrightarrow CO(g)+ H_2 (g)]
is made by providing heat by burning a part of carbon (graphite) in O_2(g) followed by an exothermic reaction :[C_{graphite} +O_2(g)\longrightarrow CO_2(g);\:\Delta H= -393.7 \: kJ \: mol^{-1} \: at \: 500K].
If a total of 1.34 mole of graphite is required for the production of 1 mole of H_2 at 500 K, the heat of reaction for carbon (graphite) with H_2O in kJ is ________.
6.80 g of NH_3; is passed over heated CuO. The standard heat enthalpIes of NH_{3}, CuO(s) and H_20(l) are -46.0, -155.0 and -285 0 \: kJ \: mol^{-1} respectively and the change is.
NH_3 +(3/2)CuO\longrightarrow \frac{1}{2}N_2(g)+ (3/2)H_2O(l) +(3/2)Cu(s).
If the enthalpy change is x kJ then -\dfrac{x}{15} (nearest integer) is___________.
The dissolution of 1 mole of NaOH(s) in 100 mole of H_2O(l) give rise to evolution of heat as -42.34 kJ. However, if 1 mole of NaOH(s) is dissolved in 1000 mole of H_2O(l) the heat given out is 42.76 kJ. If the enthalpy change when 900 mole of H_2O(l) are added to a solution containing 1 mole of NaOH(s) in 100 mole of H_2O is x kJ then the value of -100x is _____.
Calculate the resonance energy of C_6H_6, using Kekule formula for C_6H_6 from the following data: (i) \Delta H^{\circ} for C_6H_6 = -358.5 \: kJ \: mol^{-1} (ii) Heat \: of \: atomisation \: of \: C = 716.8\: kJ \: mol^{-1} (iii) Bond energy of C-\! \! \! -H, \;C-\! \! \! -C, \:C=\! \! =C and H-\! \! \! -H are 490, 340, 620 and 436.9 \: kJ \: mol^{-1}. Report your answer in kj (only value).
2C_4H_{10}\longrightarrow C_8H_{18}+H_2. Given that bond energy of C -C,\:C -H are 347.3 and 414.2 kJ mol^{-1} and the heat of formation of hydrogen atom is 217.55 kJ mol^{-1}. Find \Delta H for the above reaction in kJ mol^{-1}.(If \Delta H=x, input the answer to the nearest integer as \dfrac{x}{9}).
The heat of combustion of acetylene is 312 kcal. If heat of formation of CO_2 and H_2O are 94.38 and 68.38 kcal respectively, C \equiv\!\! \equiv C \: bond energy is x \:kcal . Given that heat of atomisation of C and H are 150.0 and 51.5 kcal respectively and C-\! \! \! -H bond energy is 93.64 kcal.
Value of x (nearest integer value) is _______.
Value of x (nearest integer value) is _______.
Exactly 3.0 g of carbon was burned to \displaystyle { CO }_{ 2 } in copper calorimeter. The mass of calorimeter is 1.5kg and the mass of water in calorimeter is 2kg. The initial temperature was \displaystyle { 20 }^{ o }C and the final temperature was \displaystyle { 31.3 }^{ o }C. If specific heat of copper is \displaystyle 0.0826\,cal/g-K, the heat value to closest integer of carbon in kcal/g is
A new element E forms a compound with chlorine which contains 1.455 g \displaystyle Cl per \displaystyle g\ E. The specific heat of E was found 0.05\ cal/g . If eq. weight of element is 24, then the value of \displaystyle x in \displaystyle { ECl }_{ x } is:
The enthalpy changes of some process are given below :
\alpha\! -\! D\! -\! glucose_{(s)}+ H_2O\longrightarrow \alpha\! -\! D\! -\! glucose_{(aq)}; \quad \Delta H_{dissolution}= 10.84 \:kJ.
\beta \! -\! D\! -\! glucose_{(s)}+ H_2O\longrightarrow \beta \! -\! D\! -\! glucose_{(aq)}; \quad \Delta H_{ dissolution}= 4.68 \:kJ.
\alpha\! -\! D\! -\! glucose_{(aq)}\longrightarrow \beta \! -\! D\! -\! glucose_{(aq)}; \: \quad \Delta H_{mutarotation} = -1.16 \:kJ
The \Delta H^{\circ} for \alpha\! -\! D\! -\! glucose\longrightarrow \beta \! -\! D\! -\! glucose is :
\beta \! -\! D\! -\! glucose_{(s)}+ H_2O\longrightarrow \beta \! -\! D\! -\! glucose_{(aq)}; \quad \Delta H_{ dissolution}= 4.68 \:kJ.
\alpha\! -\! D\! -\! glucose_{(aq)}\longrightarrow \beta \! -\! D\! -\! glucose_{(aq)}; \: \quad \Delta H_{mutarotation} = -1.16 \:kJ
The \Delta H^{\circ} for \alpha\! -\! D\! -\! glucose\longrightarrow \beta \! -\! D\! -\! glucose is :
If \displaystyle \Delta G and \displaystyle \Delta S for the reaction: \displaystyle A(g)+B(g)\rightarrow P(g), at 300 K are \displaystyle -600 cal and \displaystyle -10\;cal/K. Then \displaystyle \Delta H for the reaction in kcal is ______.
\Delta H^{\circ}_f of Fe_2O_3 and Al_2O_3 are -189 kJ and -405 kJ respectively. How much heat (in kJ) is given out during reaction of 1 g Al according to
2Al +Fe_2O_3\longrightarrow Al_2O_3 +2Fe ?
KCl_{(s)} \longrightarrow K^ + + Cl^-
The heat evolved in kcal for the process at 250^{\circ}C of dissolving 1.0 mole of KCl in excess of water is __________. [Given: \Delta H^{\circ}_f of K^+_{(aq)}, \:Cl^-_{(aq)} and KCl_{(s)} are -60, -40 and -104 kcal/mole]
[Given: \Delta H^{\circ}_f of K^+_{(aq)}, \:Cl^-_{(aq)} and KCl_{(s)} are -60, -40 and -104 kcal/mole]
If heat of formation of CaCl_2 and NaCl are 191 and 97.5 kcal, the heat of reaction for CaCl_2 + 2Na \longrightarrow 2NaCl + Ca is:
Match the Following:
The enthalpies of neutralisation of NaOH and {NH}_{4}OH by HCl are -13680 calories and -12270cal respectively. What would be the enthalpy change of one gram equivalent of NaOH is added to one gram equivalent of {NH}_{4}Cl is solution? Assume that {NH}_{4}OH and NaCl are quantitatively obtained (only magnitude in cal).
The standard heats of formation of {CH}_{4}(g), {CO}_{2}(g) and {H}_{2}O(l) are -76.2, -398.8, -241.6kJ{mol}^{-1}. Calculate amount of heat evolved by burning 1\ {m}^{3} of methane measured under normal (STP) conditions (nearest number in MJ).
Calculate the enthalpy change when infinitely dilute solution of Ca{Cl}_{2} and {Na}_{2}{CO}_{3} mixed \Delta {H}^{o}_{r} for {Ca}^{2+}(aq), {CO}_{3}^{2-}(aq) and Ca{CO}_{3}(s) are -129.80, -161.65, -288.5 kcal {mol}^{-1} respectively (nearest number in kcal).
Calculate the electron affinity of fluorine atom using the following data (only magnitude in nearest integer in kj/mol). All the values are in kJ {mol}^{-1} at {25}^{o}C, \Delta{H}_{diss}({F}_{2})=160; \Delta {H}^{o}_{r} (NaF(s))=-571;\ I.E [Na(g)]=494, \ \Delta {H}_{vap}[Na(s)]=101. Lattice energy of NaF(s)=-894.
List equation/law (in Column I) with statement (in Column II).Match the following.
Becker and Ruth measured the heat evolved in the following processes at {20}^{o}C: (1) 1 mole of solid {({COONH}_{4})}_{2}{H}_{2}O is burned in oxygen, (2) 1 mole of solid {(COOH)}_{2}{({H}_{2}O)}_{2} is burned in oxygen, (3) 1 mole of solid {({COONH}_{4})}_{2}{H}_{2}O is dissolved in a large excess of water, (4) 1 mole of solid {(COOH)}_{2}{({H}_{2}O)}_{2} is dissolved in a large excess of water, (5) 1 mole of oxalic acid in dilute solution is neutralized with gaseous ammonia. They found (1) 189.86kcal, (2) 53.10kcal, (3) -11.47kcal, (4) -8.62kcal, (5) 43.13kcal; (1) and (2) were measured at constant volume, the others at constant pressure. The end products of (1) and (2) were nitrogen, carbon-di-oxide and water. The heat of formation for 1 mole of water from the elements had previously been determined as 68.35kcal at constant pressure and {20}^{o}C. Find the change in enthalpy when 1 mole of {NH}_{3} is formed from the elements at {20}^{o}C (only magnitude in nearest integer in kcal)
For the hypothetical reaction,
\displaystyle A_{2}g+B_{2}\left ( g \right )\rightleftharpoons 2AB\left ( g \right )
If \displaystyle \Delta _{r}G^{\circ}\: and\: \Delta _{r}S^{\circ} are 20 kj/mol and -20 \displaystyle JK^{-1}mol^{-1} respectively at 200 K
\displaystyle \Delta _{r}C_{P}\: is\: 20JK^{-1}mol^{-1} then value \displaystyle \frac{\Delta _{r}H^{\circ}}{10} of (KJ/mol) at 400 K is:
For the hypothetical reaction, \displaystyle { A }_{ 2 }\left( g \right) +{ B }_{ 2 }\left( g \right) \rightleftharpoons 2AB\left( g \right) , if \displaystyle { \Delta }_{ r }{ G }^{ o } and \displaystyle { \Delta }_{ r }{ S }^{ o } are \displaystyle 20\ { kJ }/{ mol } and \displaystyle -20 \ { JK }^{ -1 }{ mol }^{ -1 } respectively at 200 K. \Delta _rC_p is 20\ JK^{-1}mol^{-1}.
Then value \displaystyle \frac { { \Delta }_{ r }{ H }^{ o } }{ 10 } of (kJ/mol) at 400 K is:
For the reaction at 298 K, 2A+B \displaystyle \rightarrow C
\displaystyle \bigtriangleup H = 400 KJ \displaystyle mol^{-1} and \displaystyle \bigtriangleup S = 0.02 KJ \displaystyle K^{-1} \displaystyle mol^{-1}
At what temperature will the reaction become spontaneous considering \displaystyle \bigtriangleup H and \displaystyle \bigtriangleup S to be constant over the temperature range?
\displaystyle \bigtriangleup H = 400 KJ \displaystyle mol^{-1} and \displaystyle \bigtriangleup S = 0.02 KJ \displaystyle K^{-1} \displaystyle mol^{-1}
At what temperature will the reaction become spontaneous considering \displaystyle \bigtriangleup H and \displaystyle \bigtriangleup S to be constant over the temperature range?
The equilibrium constant for a reaction iswhat will be value of \displaystyle \bigtriangleup G^{\ominus } ? R =8.314 \displaystyle JK^{-1} mol^{-1} , T=300 K
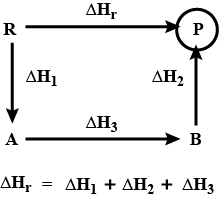
Calculate the value of { \Delta H }/{ kJ } for the following reaction using the listed thermochemical equations:
2C\left( s \right) +{ H }_{ 2 }\left( g \right) \longrightarrow { C }_{ 2 }{ H }_{ 2 }\left( g \right)
2{ C }_{ 2 }{ H }_{ 2 }\left( g \right) +5{ O }_{ 2 }\left( g \right) \longrightarrow 4C{ O }_{ 2 }\left( g \right) +2{ H }_{ 2 }O\left( I \right) { \Delta H }/{ kJ }=-2600kJ
C\left( s \right) +{ O }_{ 2 }\left( g \right) \longrightarrow C{ O }_{ 2 }\left( g \right) { \Delta H }/{ kJ }=-390kJ
2{ H }_{ 2 }\left( g \right) +{ O }_{ 2 }\left( g \right) \longrightarrow 2{ H }_{ 2 }O\left( I \right) { \Delta H }/{ kJ }=-572kJ
Calculate E_{cell} and \DeltaG for the following at 28^oC
Mg(s)+Sn^{2+}(0.025M)\rightarrow Mg^{2+}(0.06M)+Sn_{(s)}
E^0_{cell}=2.23V
Is the reaction spontaneous?
Mg(s)+Sn^{2+}(0.025M)\rightarrow Mg^{2+}(0.06M)+Sn_{(s)}
E^0_{cell}=2.23V
Is the reaction spontaneous?
Give the characteristics of free energy.
For a chemical reaction the values of \Delta H and \Delta S at 400 K are -10 K cal mol^{-1} and 20 cal. deg^{-1} mol^{-1} respectively. Calculate the value of \Delta G of the reaction.
Calculate q.w.\Delta U and \Delta H for the reversible isothermal expansion of one mole of an ideal gas at {127}^{o}C from a volume of 10\ {dm}^{3} to 20\ {dm}^{3}.
Pressure over 1000\ ml of a liquid is gradually increases from 1 bar to 1001 bar under adiabatic conditions. If the final volume of the liquid is 990\ ml, calculate \triangle U and \triangle H of the process, assuming linear variation of volume with pressure.
Explain spontaneous and non-spontaneous processes, giving two examples of each.
One mole of ethane was burnt in oxygen at constant volume to give C{O}_{2} at 298 K. The heat evolved was found to be 1554 kJ. Calculate the heat of reaction at constant pressure.
Five moles of an ideal gas at 300K, expanded isothermally from an initial pressure of 4\ atm to a final pressure of 1\ atm against a cont. ext. pressure of 1\ atm. Calculate q, w, \triangle U and \triangle H. Calculate the corresponding value of all if the above process is carried out reversibly.
The volume of a gas at STP is 2 L, 300 J heat is given to it, as a result of which volume changes to 2.5 L at 1 atm. Calculate the change in internal energy of the system.
What will be the final temperature attained if all the heat released in neutralization of 1L of 0.2M NH_4OH with 2L of 0.1M HCl increases the temperature of the final solution having density 0.95 gm/ml and specific heat capacity = 13J/g^0C, if original temperature was 27^0C? Assume weak base to be completely unionized.
The cell in which the following reaction occurs:
2{\text{F}}{{\text{e}}^{{\text{3 + }}}}\left( {{\text{aq}}} \right) + 2{{\text{I}}^{\text{ - }}}\left( {{\text{aq}}} \right) \to 2{\text{F}}{{\text{e}}^{{\text{2 + }}}}\left( {{\text{aq}}} \right) + {{\text{I}}_{\text{2}}}\left( {\text{s}} \right)\,{\text{has}}\,{\text{E}}_{\left( {{\text{cell}}} \right)}^ \odot = 0.236\,{\text{V}} at 298 K.
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
\Delta { H }^{ 0 }\quad and \quad \Delta { S }^{ 0 }\quad for\quad CC{ l }_{ 4 } are 2.5{ KJ }^{ -1 }{ mol }^{ -1 }\quad $\quad 9.99{ JK }^{ -1 }{ mol }^{ -1 } respectably at 29{ 8 }^{ 0 }K find the temperature at which solid { CCl }_{ 4 } and its liquid are in equilibrium at one atmosphere.
100kJ heat is transferred from a larger heat reservoir at 400K to another large heat reservoir at 300K. Suppose there is no change in temperature due to exchange of heat:
Find (a) \Delta {S}_{source}, (b) \Delta{S}_{sink} and (c) \Delta {S}_{total}. Comment on spontaneity of process:

Determine whether the following reaction will be spontaneous or non-spontaneous under standard conditions.Zn\left( s \right) +{ Cu }^{ 2+ }\left( a \right) \longrightarrow { Zn }^{ 2+ }\left( aq \right) +Cu\left( s \right) \triangle { H }^{ 0 }=-219kJ,\triangle { S }^{ 0 }=-21{ JK }^{ -1 }\left( Spontaneous \right)
A heat engine absorbs 760kJ heat from a source at 380K. It rejects (i) 650kJ, (ii) 560kJ, (iii) 504kJ of heat to sink at 280K. State which of these represent a reversible, an irreversible or an impossible cycle.
In the following reaction 2{H_2}{O_2}\left( {aq} \right) \to 2{H_2}O\left( \ell \right) + {O_2}\left( g \right) rate of formation of {O_2}{\text{ is }}36g{\min ^{ - 1}},
(a) What is the rate of formation of {H_2}O
(b) What is the rate of disappearance of {H_2}O.
a) The enthalpy of combustion of methane, graphite and dihydrogen at 298 K are -890.3 kJ\quad { }^{ -1 }, -393.5 kJ\quad { }^{ -1 } and -285.8 kJ\quad { }^{ -1 } respectively. Calculate the enthalpy of formation of { CH }_{ 4 }\left( g \right)
b) Write the relationship between Gibb's energy, equilibrium constant and change in enthalpy.
For the reaction NH_4Cl(S) \rightarrow NH_3(g) + HCl(g) at 25^0C, enthalpy change \triangle = +177 kJ mol^{-1} and entropy change \triangle S = +285 JK^{-1}mol^{-1}. Calculate free energy change \triangle G at 25^0C and product whether the reaction is spontaneous or not.
State and explain Hesss law of constant heat summation. What is the value of \triangle { S }^{ o }_{ surr. }] for the following reaction at 298\ K
{ 6CO }_{ 2\left( g \right) }+{ 6H }_{ 2 }{ O }_{ \left( 1 \right) }\rightarrow { C }_{ 6 }{ H }_{ 12 }{ O }_{ 6\left( g \right) }+{ 6CO }_{ 2\left( g \right) } Given
\triangle { G }^{ o }=2879kJ{ mol }^{ -1 }
\triangle { S }^{ o }=-205Jk^{-1}{ mol }^{ -1 }
Calculate the electron gain enthalpy of fluorine from the data given below. \Delta H_f of KF is - 560.8 KJ/ mol, dissociation energy of F_2 is 158.9 KJ / mol. Lattice energy of KF is 807.5 KJ/mol, ionization energy of potassium is 414.2 KJ/mol and enthalpy of sublimation of K = 87.8 KJ/ mol.
If the molar heat capacity of O_2 gas is 640Jk^{-1}mol^{-1}. Then calculate its specific heat capacity.
Calculate \Delta H_{lattice} of SnO_2, If \Delta H_f of SnO_2 is -588 KJ / mol, Enthalpy of Sublimation (Sn) = 292 KJ / mol, Enthalpy of Dissociation (O_2) = 454 \, KJ / mol. Total Electron gain enthalpy for O = 636 KJ / mol, Ionization enthalpy (Sn \rightarrow Sn^{4+}) = 8990 kJ / mol.
A gas expands from 14 liters to 19 liters against a constant pressure of 2 atm. During the process 600 J of heat flows in the gas from the surroundings. Calculate the internal energy change of its system.
Calculate w and \Delta U for the conversion of 0.5 mol of water at 100-degree celsius to steam at 1atm pressure. The heat of vaporization of water at 100degree celsius is 40670 J/mol.
During an adiabatic process, the pressure of a gas is found to be proportional to cube of its absolute temperature. Calculate the Poisson's ratio of gas.
Work done by a sample of an ideal gas in a process A double the work done in another process B. The temperature rises through the same amount in the two processes. If {C_A} and {C_B} are the molar heat capacities for the two processes, then the relation between C_A and C_B will be:
Calculate \Delta U and \Delta H in calories if 1 mole of a monoatomic ideal gas is heated at constant pressure of 1 atm from 25^oC to 50^oC.
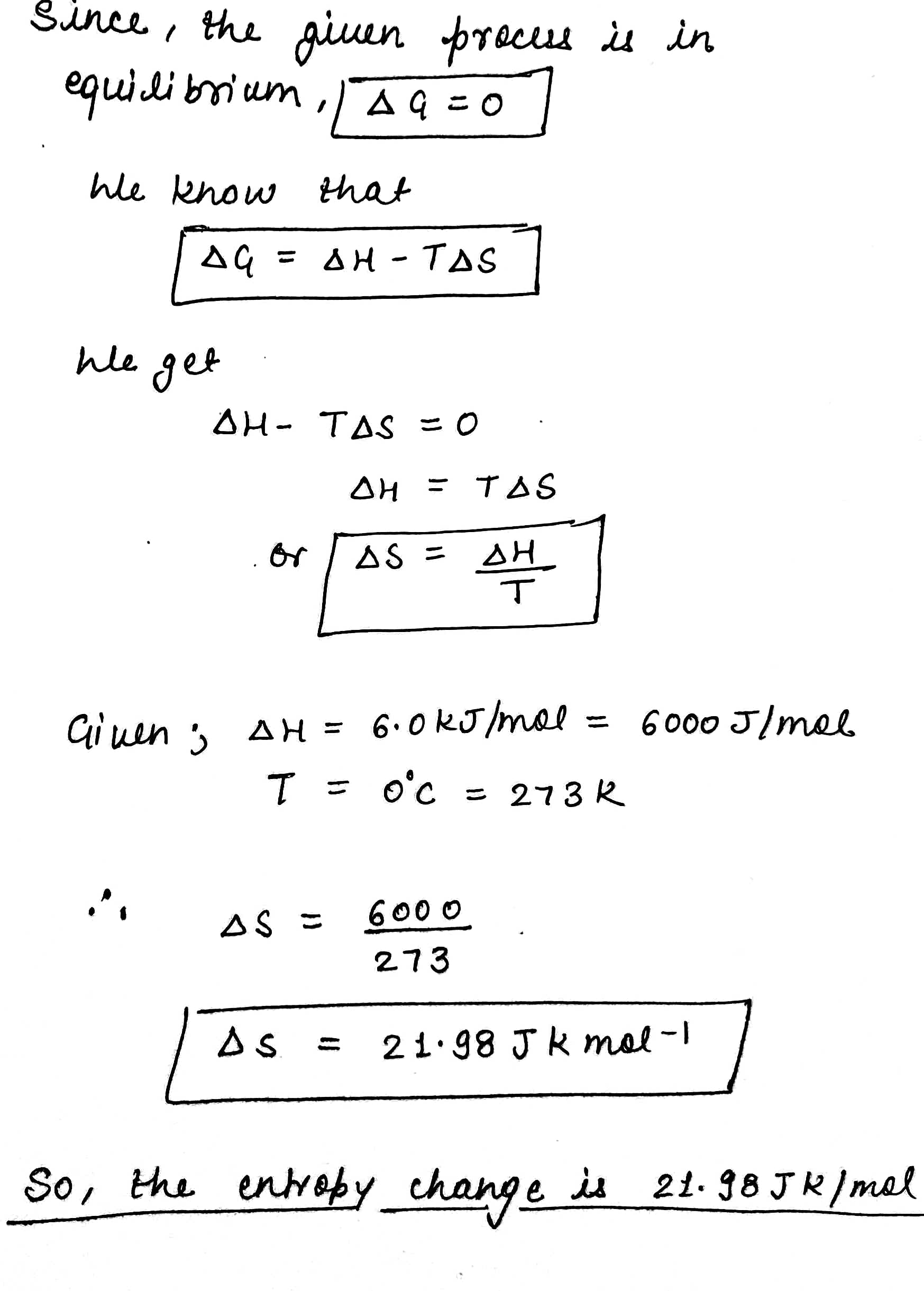
At O^oC ice and water are in equilibrium and enthalpy change for the process:
{ H }_{ 2 }{ O }_{ (S) }\rightleftharpoons { H }_{ 2 }{ O\quad (l) } is 6.0 kJ mol^{-1}
Calculate the entropy change for this conversion of ice into liquid water.
An athlete in the weight room lifts a 50kg mass through a vertical distance of 2.0m. The mass is allowed to fall through the 2.0m distance while coupled to an electrical generator. The electrical generator produces an equal amount of electrical work which is used to produce aluminium by Hall electrolytic process.
{ Al }_{ 2 }{ O }_{ 3 }(\textrm{solution})+3C(\textrm{graphite})\rightarrow 2Al(l)+3CO(g);\Delta { G }^{ o }=593kJ
How many times must the athlete lift the 50kg mass to provide sufficient Gibbs energy to produce 27g Al? \left( g=10m/{ s }^{ 2 } \right)
{ Al }_{ 2 }{ O }_{ 3 }(\textrm{solution})+3C(\textrm{graphite})\rightarrow 2Al(l)+3CO(g);\Delta { G }^{ o }=593kJ
How many times must the athlete lift the 50kg mass to provide sufficient Gibbs energy to produce 27g Al? \left( g=10m/{ s }^{ 2 } \right)
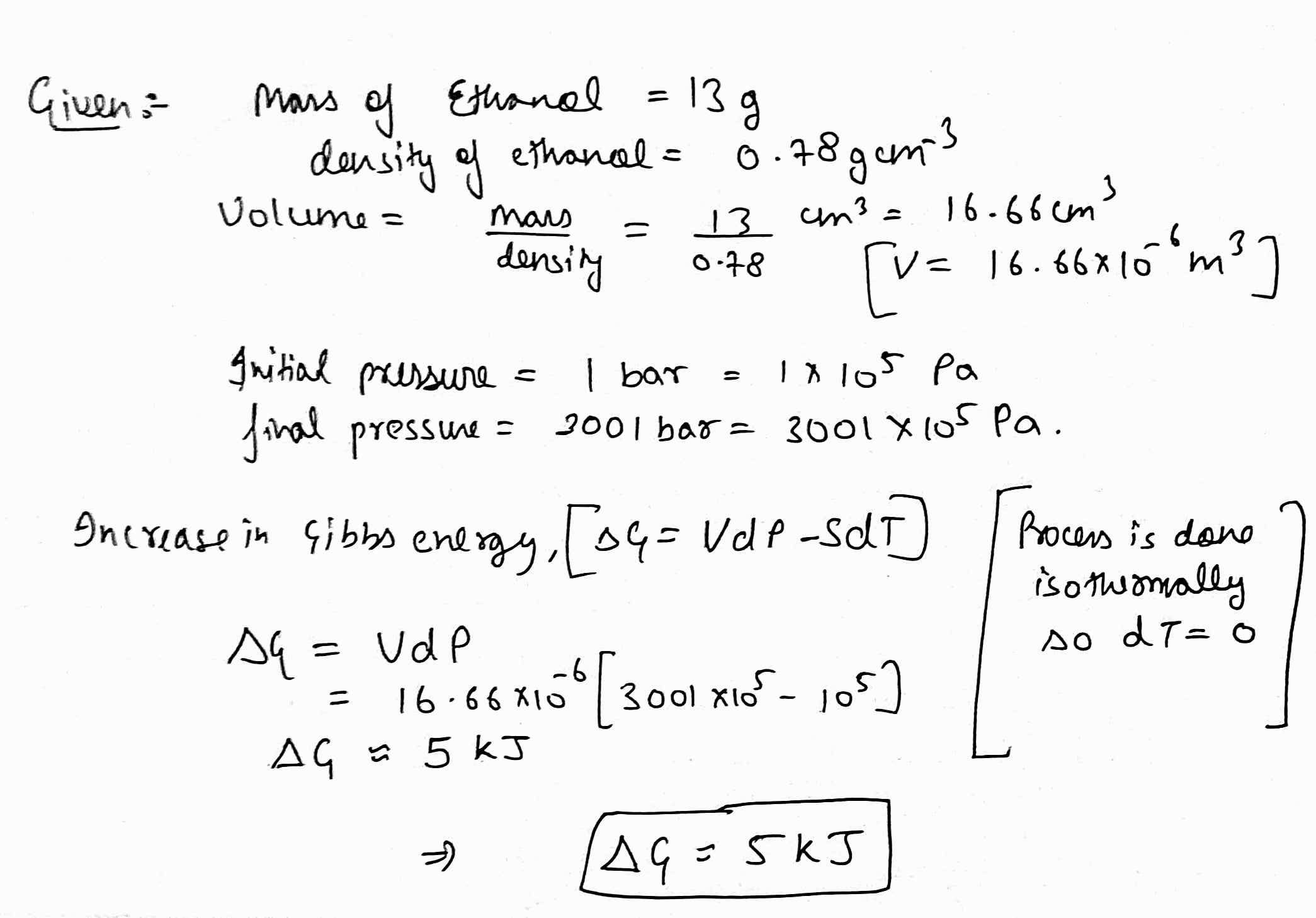
The increase in Gibbs free energy (in kJ) of 13g of ethanol (density =0.78g{cm}^{-3}), when the pressure is increased isothermally from 1 bar to 3001 bar, is _____.
State and explain Hess's law of constant heat summation.
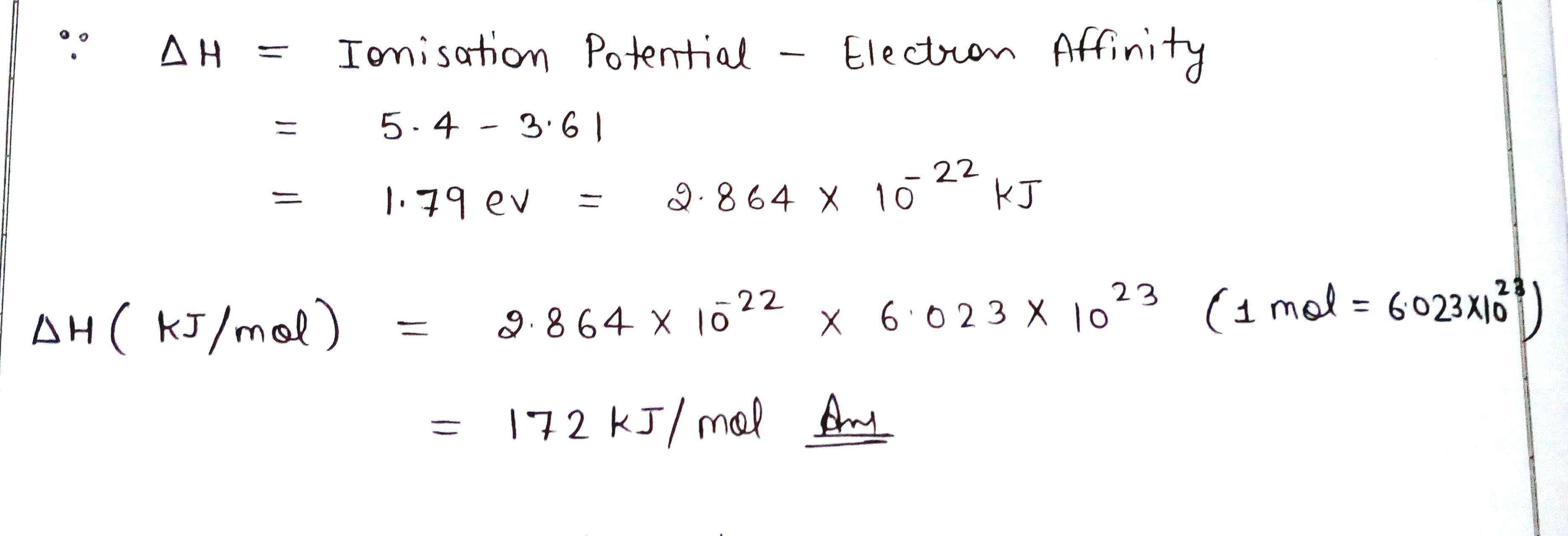
The first ionisation enthalpy of Li is 5.4 eV and the electron gain enthalpy of Cl is 3.6 eV. Calculate \triangle H in kcal mol^{-1} for the reaction,
Li(g) +Cl(g) \rightarrow Li^++ Cl^-
Carried out at such low pressure that resulting ions do not combine with each other.
Explain the term lattice energy as applied to an ionic solid. Calculate the lattic energy of caesium chloride using the following data:
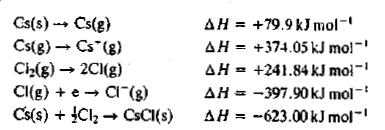
Ammonium nitrate can decompose with explosion by the following reaction :
NH_{4}NO_{3(s)}\rightarrow N_{2}O_{(g)} + 2H_2O_{(g)}; \, \Delta H = -37.0\ kJ
Calculate the heat produced when 2.50\ g of NH_{4}NO_{3} decomposes.
A cooking gas cylinder is assumed to contain 11.2 \ kg isobutane. The combustion of isobutane is given by:
C_{4}H_{10(g)} + (13/ 2)O_{2(g)} \rightarrow 4CO_{2(g)} + 5H_{2}O_{(l)} ; \Delta H = -2658\ kJ
(a) If a family needs 15000\ kJ of energy per day for cooking, how long would the cylinder last?
(b) Assuming that 30\% of the gas is wasted due to incomplete combustion, how long would the cylinder last?
C_{4}H_{10(g)} + (13/ 2)O_{2(g)} \rightarrow 4CO_{2(g)} + 5H_{2}O_{(l)} ; \Delta H = -2658\ kJ
(a) If a family needs 15000\ kJ of energy per day for cooking, how long would the cylinder last?
(b) Assuming that 30\% of the gas is wasted due to incomplete combustion, how long would the cylinder last?
For the reaction, C(s) +O_2(g) \rightarrow CO_2(g), \Delta H ^{\circ}=-393.51\ kJ/mol and \Delta S ^{\circ}=2.86\ J/mol. K at 25^{\circ}C. Does the reaction become more or less favourable as the temperature increases?
Show how the Born-Haber cycle may be used to estimate the enthalpy of the hypothetical reaction:
Ca(s)+ Cl_2(g)\to CaCl(s)
explain why CaCl(s) has never been made even though the enthalpy for this reaction is negative.
Calculate \Delta G^0 for the following reaction.
Ag^+(aq)+Cl^-(aq)\rightarrow AgCl(s)
Given: \Delta G^0_f(AgCl)=-109 kJ/mol
\Delta G^0_f(Cl^-)=-129 kJ/mol
\Delta G^0_f(Ag^+)=77 kJ/mol
Represent the given reaction in the form of a cell. Also calculate E^0 of the cell and find log K_{sp} of AgCl.
What is the relationship between \Delta G, \Delta H \ \& \ \Delta S?
From the following data of AH, of the following reactions,C_{( s)}+\dfrac{1}{2}O_2(g) \longrightarrow CO_{(g)}; \Delta H = -110 \ kJA C_{(s)} + H_2O_{(g)} \longrightarrow CO_{(g)} + H_{2(g)}; \Delta H = 132kJ
Calculate the mole composition of the mixture of steam and oxygen on being passed over coke at 1273\ K, keeping the temperature constant.
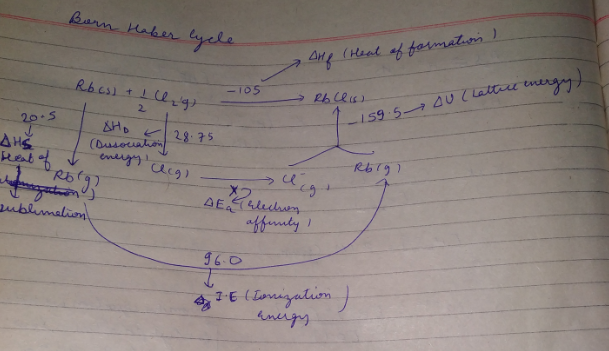
The Born-Haber's cycle for rubidium chloride (RbCl) is given above , ( the energies are in kcal \,mol^{-1} .
Find out the electron affinity of chloride in kJ\,mol^{-1} .
What is Heat capacity?
How non-spotaneous process be converted to spontaneous process?
At 0^o and 1 atm, heat absorbed by the system by melting of one mle of ice is 6.05 KJ/mol. The molar volume of ice and water are 0.0196 L and 0.0180 L respectively. Calculate \Delta H and \Delta U.
Taking a specific example show that \Delta S_{total} is a criterion for spontaneity of a change.
State and illustrate Hess's law.
What you mean by Born-Haber cycle ?
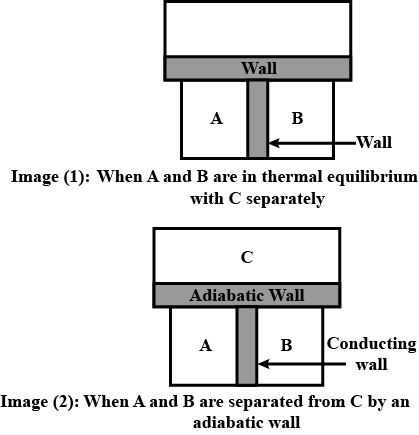
What is Thermodynamics? Explain the Zeroth law of Thermodynamics and give its importance.
Enthalpies of formation of CO(g),CO_2(g), NaO(g) and N_2O_4(g) are -110,-393.81 and 9.7 kJ \quad mol^{-1} respectively. Find the value of \Delta H for the reaction
N_2O_{3(g)} \rightarrow N_2O_{(g)}+3CO_{(g)}
For the reaction
2A_{(g)}+B_{(g)} \rightarrow 2D_{(g)}
\Delta U^o =-10.5 kJ and \Delta S^o =-44.1 JK_{-1}
Calculate \Delta G^o for the reaction and predict whether the reaction may occur spontaneously.
The reaction of cyanamide, NH_2CN(s), with dioxygen was carried out in a bomb clorimeter, and \Delta U was found to be -742.7 kJ mol^{-1} at 298 K. Calculate enthalpy change for the reaction at
298 K. NH_2CN(g) + 3/2O_2(g) \rightarrow N_2(g)+CO_2(g)+H_2O(l)
Explain heat, work and internal energy of a system.
A chemist while studying the properties of gaseous CCl_2F_2, a chlorofluorocarbon refrigerant cooled a 1.25\ g sample at constant atmospheric pressure of 1.0\ atm from 320\ K to 293\ K. During cooling, the sample volume decreased from 274 to 248\ mL. Calculate \Delta H and \Delta U for the chorofluorocarbon for this process. For CCl_2F_2, C_p \simeq 80.7\ J/(mol\ K).
In a gobar gas plant, gobar gas is obtained by bacterial fermentation of animal refuse. The main combination gas present in the gobar gas is found to be methane (80\% by weight )whose heat of combustion is 809\ mol^{-1}. How much gobar gas would have to produced per day for a village of 100 families if average consumption of a family is 20.000\ kJ per day to meet all its energy requirements.
Class 11 Medical Chemistry Extra Questions
- Chemical Bonding And Molecular Structure Extra Questions
- Classification Of Elements And Periodicity In Properties Extra Questions
- Environmental Chemistry Extra Questions
- Equilibrium Extra Questions
- Hydrocarbons Extra Questions
- Hydrogen Extra Questions
- Organic Chemistry Some Basic Principles And Techniques Extra Questions
- Redox Reactions Extra Questions
- Some Basic Concepts Of Chemistry Extra Questions
- States Of Matter Gases And Liquids Extra Questions
- Structure Of Atom Extra Questions
- The P-Block Elements Extra Questions
- Thermodynamics Extra Questions
- The S-Block Elements Extra Questions